Abstract
The aim of this study was to determine the copy number changes of chromosomes 7, 17, 3p, and Y in a non-neoplastic tubular epithelium in end-stage kidney disease (ESKD). Seventeen kidneys from 11 patients with ESKD were retrieved from the archive files. Non-neoplastic kidney tissue in these cases was examined separately. Tissues containing papillary adenomas (PA), clear (CRCC) and papillary renal cell carcinomas (PRCC), and myxoid liposarcoma (LPS) were examined using the same probes and compared with non-neoplastic tissue. Tubular changes in the kidney parenchyma were classified into three types: (1) The vast majority of tubules were entirely atrophic; (2) Several tubules were hyperplastic, i.e., tubules with undifferentiated large epithelial cells, in which it was impossible to establish the specific type of a renal tubulus; (3) Dysplastic tubules were dilated, sometimes wrinkled. The basal membranes were lined by large eosinophilic epithelial cells with polymorphic nuclei and pseudostratification. Nucleoli were clearly visible. These tubular changes were multifocal with a haphazard distribution within the atrophic parenchyma. PA were detected in nine patients, of whom eight patients also revealed an additional tumor type(s) (4x CRCC, 3x PRCC, 1x PRCC, and CRCC). One patient had a CRCC only, another had a combination of PRCC and LPS. Chromosomal abnormalities were found in the second and third group of tubular changes, i.e., in hyperplastic and dysplastic tubules. Trisomy of chromosome 7 was detected in six cases, whereas trisomy of chromosome 17 in eight cases. A combination of both trisomies was found in five cases. Loss of chromosome Y was found in two cases. Fluorescence in situ hybridization on tissues containing papillary adenomas, renal cell carcinomas, and liposarcoma revealed expected results, i.e., trisomy of chromosomes 7 and 17 in all PAs and PRCC. No gains were present in CRCC and LPS. Loss of Y was found in six PA, five PRCC, and one LPS; loss of X was found in two CRCCs. We suggest that chromosomal changes typical of the papillary renal cell lesions, i.e., trisomies of chromosomes 7 and 17, are very frequent in non-neoplastic parenchyma of the end-stage kidney, and they have a tendency to a multifocal occurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that patients with end-stage kidney disease (ESKD) have an increased risk of renal cell carcinoma. There have been several types of renal cell tumors reported in patients with ESKD [5, 6, 8, 10, 18, 19]. Papillary renal cell carcinoma (PRCC), clear renal cell carcinoma (CRCC), and papillary adenoma (PA) are the most frequent types of tumors occurring in ESKD [4, 7, 8]. Trisomy of chromosome 7 has been found in non-neoplastic proximal tubulus epithelias of normal kidney and lymphocytes infiltrating kidney tumors or surrounding parenchyma [2, 3, 11]. Little data are available on chromosomal alterations of tubular changes in ESKD. The aim of this study was to determine the copy number changes of chromosomes 7, 17, 3p, and Y in histologically non-neoplastic-looking tubular epithelium in ESKD.
Materials and methods
Seventeen kidneys from 11 patients with ESKD were retrieved from the files of the Departments of Pathology and Urology, Charles University in Plzen. Histologic sections of formalin-fixed, paraffin-embedded tissue were stained with hematoxylin and eosin. The number of blocks from each kidney varied from seven to 18 from one patient (mean 13). Both neoplastic and non-neoplastic tissues were available for analysis. Non-neoplastic renal parenchyma was examined separately from tumorous tissues. The number of examined hyperplastic and dysplastic tubules varied from two to six per one patient (mean 4).
Tissues containing papillary adenomas, renal cell carcinomas, and liposarcoma were studied separately under the same conditions.
As a negative control group, ten healthy donor kidney tissue samples and ten end-stage kidneys without any tumorous mass were examined to set the cutoff levels for fluorescence in situ hybridization (FISH) evaluation.
All patients from this control group have been dialyzed for a long-term.
FISH
FISH was carried out on 5-μm paraffin sections. Tissues were deparaffinized in xylene 3 × 5 min, washed 2 × 2 min in 100% ethanol, 1 × 2 min in 95% ethanol, and 1 × 5 min in deionized water. Then, the slides were incubated in the Epitope Retrieval Solution (DAKO, Glostrup, Denmark) for 40 min at 95°C and subsequently cooled at room temperature (RT) for 20 min in the Epitope Retrieval Solution. The slides were washed 5 min in deionized water, and tissues were covered with the Proteinase K Ready-to-Use (DAKO) solution for 3–5 min. Then, the slides were immediately washed twice for 2 min in deionized water, dehydrated in 70%, 85%, and 100% ethanol for 2 min each and air dried.
Afterward, the mixtures of CEP7, CEP17, CEP3 + TelVysion 3p (Vysis, Downers Grove, IL, USA), or SE X/Y (Kreatech, Amsterdam, Netherlands) probes were applied on the samples and covered with coverslips. Slides were denatured at 85°C for 5 min and incubated in humidified box at 37°C overnight. Then, the slides were washed for 2 min in 0.4× SSC/0.3% NP-40 at 73°C and for 1 min in 2× SSC/0.1% NP-40 at RT. After that, the slides were counterstained with DAPI II (Vysis) and examined.
Fluorescent signals from non-neoplastic tubular epithelium nuclei were counted. Only nonoverlapping intact nuclei were scored, split centromere signals were counted as one. Cutoff levels for gain and loss of a signal (chromosome) were set at 3× SD of the mean number of variants seen in normal control nuclei and were as more than 10% for an interpretation of gain of chromosomes 7 and 17 and more than 20% for loss of chromosome Y.
Results
The basic clinicopathological data are summarized in Table 1. The patients were one woman and ten men, with ages ranging from 41 to 67 years (mean, 51.9 years). All but one patient lost kidney function because of chronic glomerulonephritis, the exception being a case of renal complete insufficiency due to diabetic nephropathy. Eight patients were treated by hemodialysis and subsequent renal transplantation, while the remaining three individuals were only dialyzed. The duration of hemodialyzation ranged from 0.6 to 6 years (mean, 2.5 years). The patients had functional renal grafts from 2 to 9 years (mean, 5.75 years). Renal tumors were found in all but one patient, using ultrasonography or computed tomography (CT) during periodic clinical examinations. All pathologic findings were located in the native kidneys. No tumors were detected in renal grafts. Unusual scarring of the kidney parenchyma was misinterpreted as a tumorous mass in one patient (case 10) on CT. Unilateral nephrectomy was performed in five patients, bilateral in six patients.
Papillary adenomas were detected in nine patients, of whom eight patients also revealed an additional tumor type(s) (4× CRCC, 3× PRCC, 1× PRCC, and CRCC). All but two patients had multiple PAs. One patient had a CRCC only; another had a combination of PRCC and low-grade myxoid liposarcoma.
Tubular changes in the kidney parenchyma were present in all examined cases and were classified into three major types:
-
1.
The vast majority (>95%) of tubules were entirely atrophic.
-
2.
Several (<4%) tubules were hyperplastic, i.e., tubules with undifferentiated large epithelial cells, in which it was impossible to establish the specific type of renal tubulus (Fig. 1). Diameter of the hyperplastic tubules varied between 0.1 and 0.4 mm.
-
3.
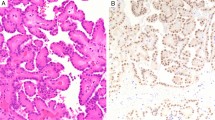
Dysplastic tubules (individual tubules). These were dilated, sometimes wrinkled. The basal membranes were lined by large eosinophilic epithelial cells with polymorphic nuclei and pseudostratification. Nucleoli were clearly visible (Fig. 2a,b). These tubular changes were multifocal with a haphazard distribution within the atrophic parenchyma. Diameter of the hyperplastic tubules varied between 0.1 and 0.8 mm.
Fig. 2 a Several tubules were dysplastic. These tubules were dilated, sometimes wrinkled. Such tubular changes were multifocal with a haphazard distribution within the atrophic parenchyma. b The basal membranes of dysplastic tubules were lined by large eosinophilic epithelial cells with polymorphic nuclei and pseudostratification. Nucleoli were clearly visible
Normal tubules and tubules with undifferentiated features (where it was impossible to establish the position in the nephron) were seen only very rarely.
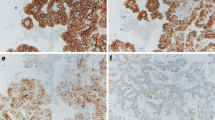
The results of the FISH analysis are summarized in Table 2. In a majority of cases, at least 100 nonoverlapping nuclei were examined. In some cases, the total number of nuclei was lower than 100 because some tubules did not contain more cells. Chromosomal abnormalities were found in the second and third group of tubular changes, i.e., in hyperplastic and dysplastic tubules (Fig. 3a,b). Trisomy of chromosome 7 was detected in six (Fig. 4), trisomy of chromosome 17 in eight cases (Fig. 5). A combination of both trisomies was found in five cases. Loss of chromosome Y was found in two cases. Then, the FISH for 3p loss detection was performed using telomeric probe for the 3p25 chromosomal region. Unfortunately, this 3p25 chromosomal region examination failed in all studied cases, especially due to a weak signal of telomeric probe. 3p25 telomeric probe worked well on a control (non-fixed metaphase lymphocytes).
FISH on tissues containing papillary adenomas, renal cell carcinomas, and liposarcoma revealed expected results (see Table 2). Papillary adenomas and papillary renal cell carcinomas showed both trisomies of chromosomes 7 and 17. Some papillary adenomas were not analyzable because of limited volume of the paraffin tumorous material. There were no significant differences in chromosome 7 and 17 status in papillary adenomas and carcinomas. Result of analysis of chromosomes 7 and 17 was not significant in papillary adenoma in case 7 because of limited volume of analyzed tissue. No trisomy of chromosomes 7 and 17 was noted in clear RCC. Loss of chromosome Y was seen in one case of well-differentiated liposarcoma. Loss of chromosome X was seen in two cases of clear RCC (one female patient and one male patient).
Discussion
In our study we found that (1) chromosomal changes such as gains of chromosomes 7 and 17 and loss of Y chromosome may be found in non-neoplastic tissue of the end-stage kidney diseases and (2) these chromosomal abnormalities tend to have a multifocal occurrence.
Tumors in ESKD have been extensively studied by Hughson et al. The authors have proposed that papillary RCC is the most frequent type of renal cell carcinoma found in these patients. Moreover, the genetic changes of these tumors are similar to those seen in sporadic cases [7–9]. Specific and probably new types of renal tumors occurring in the setting of ESKD have recently been published. The typical features of these tumors are a frequent papillary pattern, presence of oxalate crystals, and clear to eosinophilic cytoplasm [18, 19]. We were not able to find similar tumors in our series of patients. Moreover, no significant amount of oxalate deposits was present in the kidney parenchyma in our cases.
There is no reference in the literature on premalignant lesions in ESKD. Precursors of sporadic RCC in non-atrophic kidney have been described by Mourad et al., who examined non-neoplastic tissue surrounding RCC (mainly clear RCC and sarcomatoid NOS RCC) and found dysplastic changes located mainly in the cortex or in a periglomerular distribution. However, the authors found intratubular epithelial dysplasia in 30 out of 110 patients with RCC [15].
Similar findings have been published recently. Dysplastic changes in normal kidneys have been identified in 23% of the kidneys harboring RCC. Dysplastic changes were focal and located adjacent to the tumor mass in ten cases, whereas a diffuse distribution was a rare finding [20]. On the contrary, tubular changes in our series were always multifocal with a haphazard distribution spread within the entire atrophic parenchyma.
A study dealing with chromosomal changes in tubular abnormalities in clear RCC has been published recently. Premalignant lesions and tumor tissues in this study had the same genotypic changes (LOH or microsatellite instability) in more than 85% [16].
Chromosomal changes of PA and PRCC are characterized mainly by trisomy of chromosomes 7 and 17 and loss of chromosome Y [12, 13, 17]. However, trisomy of chromosome 7 was found in majority of RCC, the combination of trisomy of chromosomes 7 and 17 and loss of Y chromosome is rather specific for papillary neoplasms [14]. We tried to identify these chromosomal changes in hyperplastic and dysplastic renal tubules. Interestingly, trisomy of chromosome 7 was detected in six, trisomy of chromosome 17 in eight cases. A combination of both trisomies was found in five cases. Loss of chromosome Y was found only in two cases.
Trisomy of chromosome 7 has been studied in non-neoplastic tissue of normal, non-atrophic kidney surrounding RCC. Cells with trisomy of chromosome 7 were mainly epithelial tubular cells [3, 11] or a combination of lymphocytes infiltrating tumor tissue and tubular cells [2]. Our study was concerned of ESKD, and all positive tubules for trisomies of chromosomes 7 and 17 were abnormal. As was mentioned above, positive tubules harbored hyperplastic or dysplastic changes. No abnormalities were noted in atrophic or undiferentiated tubules without hyperplastic or dysplastic features. However, some non-neoplastic cells showed trisomy of chromosome 7 or 17; the number of trisomies was under the cutoff level.
Brunelli et al. showed that both PA and PRCC share similar chromosomal changes, i.e., gains of chromosomes 7, 17, 16, 12, and 20 and loss of Y chromosome [1]. Loss of the Y chromosome was observed in nine of ten PA from males. It was concluded that gains of chromosomes 7, 17, 16, 12, and 20 and loss of Y chromosome occur early in the neoplastic evolution of papillary renal cell neoplasia. On the contrary, we have found only two of nine male cases with loss of Y chromosome. However, the loss of chromosome Y was found in all analyzed cases of PA and PRCC. Loss of chromosome Y was interestingly seen in one case of well-differentiated liposarcoma. Loss of chromosome X was seen in two cases of clear RCC (one female patient and one male patient).
An interesting fact is that trisomies of chromosomes 7 and 17 were noted in the kidneys with different tumors (besides papillary adenomas and papillary RCC). Our findings point to the fact that chromosomal changes typical of the papillary renal cell lesions are very frequent in non-neoplastic-looking parenchyma of the end-stage kidney and that they have a tendency to a multifocal occurrence.
References
Brunelli M, Eble JN, Zhang S, Martignoni G, Cheng L (2003) Gains of chromosomes 7, 17, 12, 16, and 20 and loss of Y occur early in the evolution of papillary renal cell neoplasia: a fluorescent in situ hybridization study. Mod Pathol 16:1053–1059
Dal Cin P, Aly MS, Delabie J, Ceuppens JL, van Gool S, van Damme B, Baert L, van Poppel H, van den Berghe H (1992) Trisomy 7 and trisomy 10 characterize subpopulations of tumor-infiltrating lymphocytes in kidney tumors and in the surrounding kidney tissue. Proc Natl Acad Sci USA 89:9744–9748
Elfving P, Aman P, Mandahl N, Lundgren R, Mitelman F (1995) Trisomy 7 in nonneoplastic epithelial kidney cells. Cytogenet Cell Genet 69:90–96
Gronwald J, Baur AS, Holtgreve-Grez H, Jauch A, Mosimann F, Jichlinski P, Wauters J-P, Cremer T, Guillou L (1999) Chromosomal abnormalities in renal cell neoplasms associated with acquired renal cystic disease. A series studied by comparative genomic hybridization and fluorescence in situ hybridization. J Pathol 187:308–312
Farivar-Mohseni H, Perlmutter AE, Wilson S, Shingleton WB, Bigler SA, Fowler JE (2005) Renal cell carcinoma and end-stage renal disease. J Urol 175:2018–2021
Hoshida Y, Nakanishi H, Shin M, Satoh T, Hanai J, Aozasa K (1999) Renal neoplasms in patients receiving dialysis and renal transplantation: clinico-pathological features and p53 gene mutations. Transplantation 68:385–390
Hughson MD, Meloni AM, Silva FG, Sandberg AA (1996) Renal cell carcinoma in an end-stage kidney of patient with a functional transplant: cytogenetic and molecular genetic findings. Cancer Genet Cytogenet 89:65–68
Hughson MD, Schmidt L, Zbar B, Daugherty S, Meloni AM, Silva FG, Sandberg AA (1996) Renal cell carcinoma of end-stage renal disease: a histopathologic and molecular genetic study. J Am Soc Nephrol 7:2461–2468
Hughson MD, Bigler S, Dickman K, Kovacs G (1999) Renal cell carcinoma of end-stage renal disease: an analysis of chromosome 3, 7, and 17 abnormalities by microsatellite amplification. Mod Pathol 12:301–309
Junker K, Thrum K, Schlichter A, Müller G, Hindermann W, Schubert J (2002) Clonal origin of multifocal renal cell carcinoma as determined by microsatellite analysis. J Urol 168:2623–2636
Knuutila S, Larramendy ML, Elfving P, el-Rifai W, Miettinen A, Mitelman F (1995) Trisomy 7 in non-neoplastic tubular epithelial cells of the kidney. Hum Gen 95:149–156
Kovacs G, Fuzesi L, Emanuel A, Kung H-F (1991) Cytogenetics of papillary renal cell tumors. Genes Chromosomes Cancer 3:249–255
Kovacs G, Akhtar M, Beckwith JB, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljunberg B, Medeiros LJ, Moch H, Reuter VE, Ritz E, Roos G, Schmidt D, Srigley JR, Storkel S, van den Berg E, Zbar B (1997) The Heidelberg classification of renal cell tumours. J Pathol 183:131–133
Moch H, Sauter G, Gasser TC, Bebendorf L, Richter J, Presti JC Jr, Waldman FM, Mihatsch MJ (1998) EGF-r gene copy number changes in renal cell carcinoma detected by fluorescence in situ hybridization. J Pathol 184:424–429
Mourad WA, Nestok BR, Saleh GY, Solez K, Power RF, Jewell LD (1994) Dysplastic tubular epithelium in “normal” kidney associated with renal cell carcinoma. Am J Surg Pathol 18:117–1124
Pehlivan S, Koyuncuoglu M, Pehlivan M, Izzetoglu S, Mater Y, Cabuk M, Kirkali Z (2004) Premalignant lesions of the kidney share the same genetics changes as conventional renal cell carcinoma. World J Urol 22:120–123
Sanjmyatav J, Rubtsov N, Starke H, Schubert J, Hindermann W, Junker K (2005) Identification of tumor entities of renal cell carcinoma using interphase fluorescence in situ hybridization. J Urol 174:731–735
Sule N, Yakupoglu U, Shen SS, Krishnan B, Yang G, Lerner S, Sheikh-Hamad D, Truong LD (2005) Calcium oxalate deposition in renal cell carcinoma associated with acquired cystic kidney disease: a comprehensive study. Am J Surg Pathol 29:443–451
Tickoo SK, dePeralta-Venturina M, Lara H, Worcester HD, Salama M, Young A, Moch H, Amin M (2006) Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol 30:141–153
Yörükoglu K, Aktas S, Mungan MU, Kirkali (1999) Tubular dysplasia and carcinoma in situ: precursors of renal cell carcinoma. Urology 53:684
Acknowledgment
The study was supported by MSM 0021620819 replacement of and support to some vital organs.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hes, O., Šíma, R., Němcová, J. et al. End-stage kidney disease: gains of chromosomes 7 and 17 and loss of Y chromosome in non-neoplastic tissue. Virchows Arch 453, 313–319 (2008). https://doi.org/10.1007/s00428-008-0661-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0661-2