Abstract
Main conclusion
TaMYB44-5A identified as a transcription factor negatively regulates drought tolerance in transgenic Arabidopsis.
Abstract
Drought can severely reduce yields throughout the wheat-growing season. Many studies have shown that R2R3-MYB transcription factors are involved in drought stress responses. In this study, the R2R3-MYB transcription factor MYB44-5A was identified in wheat (Triticum aestivum L.) and functionally analyzed. Three homologs of TaMYB44 were isolated, all of which localized to the nucleus. Overexpression of TaMYB44-5A reduced drought tolerance in Arabidopsis thaliana. Further analysis showed that TaMYB44-5A reduced the sensitivity of transgenic Arabidopsis to ABA. Genetic and transcriptional regulation analyses demonstrated that the expression levels of drought- and ABA-responsive genes were downregulated by TaMYB44-5A, and TaMYB44-5A directly bound to the MYB-binding site on the promoter to repress the transcription level of TaRD22-3A. Our results provide insights into a novel molecular pathway in which the R2R3-MYB transcription factor negatively regulates ABA signaling in response to drought stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bread wheat (Triticum aestivum L., 2n = AABBDD) is a highly adaptable cereal, providing more than one-fifth of the calories consumed by humans (International Wheat Genome Sequencing 2018). Its adaptability is attributed to the distribution of tightly assembled stress-resistance genes in its genome (Itam et al. 2022). Previous reports indicate that wheat production in drought-sensitive regions needs to increase by nearly 30% by 2025 to meet the food demands of the growing global population (Pennisi 2008; Chenu et al. 2017). Therefore, it is essential to identify the functions of drought-related genes for future wheat breeding (Paux et al. 2022). Many key genes responsive to drought stress have been identified in previous studies, including transcription factors, protein kinases, and molecular chaperones (Fujii et al. 2011; Qian et al. 2021; Wang et al. 2021a). For instance, the transcription factor WRKY13 in Vitis vinifera negatively regulates drought tolerance by modulating intracellular osmolyte metabolism (Hou et al. 2020).
ABA is a core phytohormone abundantly produced under drought conditions, where it serves a pivotal role in plants’ response to drought stress (Nakashima and Yamaguchi-Shinozaki 2013). ABA regulates the expression levels of downstream stress-responsive genes through a precise pathway. Under drought stress conditions, ABA receptors, including the pyrabactin resistance (PYR)/PYR1-like (PYL)/regulatory components of ABA receptor (RCAR), interact with 2C-type protein phosphatases (PP2Cs) to release SNF1-related protein kinase 2s (SnRK2s) from PP2C-dependent repression. Phosphorylated SnRK2s can then phosphorylate downstream ABA-responsive element-binding protein (AREB)/ABRE binding factor (ABF) or other transcription factors. Consequently, the ABA-mediated drought signal is transmitted, allowing downstream genes to directly respond to the signal or influence the upstream system through feedback mechanisms (Nakashima and Yamaguchi-Shinozaki 2013). For instance, upon activation of the ABA pathway, phosphorylated apple (Malus domestica Borkh) bZIP44 interacts with MdMYB1 to prevent the degradation of MdMYB1 by the ubiquitin-26S proteasome system, thereby enhancing the response to ABA signaling (An et al. 2018). MdMYB44-like positively regulates drought tolerance via the MdPYL8-MdPP2CA module in apple (Chen et al. 2024). Furthermore, it has been reported that AtMYB44 can interact with AtPYL8 to attenuate the transmission of early signals and suppress the response to drought stress signals (Jaradat et al. 2013). Therefore, MYB transcription factors play crucial roles in the ABA signaling pathway.
MYB transcription factors constitute the largest family of transcription factors in plants (Dubos et al. 2010). Depending on the combination and type of adjacent repeat sequences in the amino acid sequence, MYB transcription factors can be classified into four subfamilies: 1R-MYB, R2R3-MYB, 3R-MYB, and 4R-MYB (Dubos et al. 2010). Members of the MYB transcription factor family are extensively involved in regulating plant photosynthesis, biotic and abiotic stress responses, and the accumulation of plant storage substances, with members of the R2R3-MYB subfamily being the most abundant (Wu et al. 2022). R2R3-MYBs have the capability to bind MYB-binding sites and subsequently regulate the expression of downstream ABA-responsive genes (Wu et al. 2022; Gao et al. 2024). Furthermore, R2R3-MYBs have been identified as crucial components in the response to drought signaling (Jung et al. 2008; Shim et al. 2013; Piao et al. 2019b; Shi et al. 2024). Previous studies have shown that AtMYB44 overexpression confers weaker drought tolerance and lower ABA sensitivity in Arabidopsis (Huang et al. 2007; Jaradat et al. 2013; Li et al. 2014). It has been reported that in the presence of ABA, the expression level of the important ABA signaling marker gene AtRAB18 is significantly upregulated in the myb44 (T-DNA insertion mutant) (Huang et al. 2007). In addition, AtMYB44 weakens the drought tolerance in Arabidopsis by inhibiting the expression levels of downstream ABA-responsive genes, such as D-1-pyrroline-5-carboxylate synthase 1 (P5CS1) and dehydration-responsive gene 22 (RD22) (Jaradat et al. 2013). In the ABA sensitivity assays, the germination rate of P35S:MYBR1(AtMYB44) was higher, while that of mybr1 was lower, indicating that overexpression of MYB44 reduces the sensitivity of Arabidopsis to ABA (Li et al. 2014). Overexpression of BnMYBL2 (Brassica napus L.) in wheat promotes the accumulation of ABA and anthocyanins, thereby increasing wheat’s drought tolerance (Gao et al. 2024). In rice (Oryza sativa L.), OsMYB102 was previously identified as an ABA-dependent regulator of leaf senescence, and OsMYB102 overexpression reduced the tolerance of drought in transgenic rice (Piao et al. 2019a, 2019b). MsMYBH (Medicago sativa L.) can directly bind to the promoters of MsMCP1, MsMCP2, MsPRX1A, and MsCARCAB and promote their expression to enhance alfalfa’s drought tolerance (Shi et al. 2024). Nonetheless, limited studies have investigated the functions of R2R3-MYBs in the response to drought stress in wheat and the associated mechanism of direct regulation of drought tolerance.
In our study, we identified three homologs of AtMYB44 in wheat based on their evolutionary relationships. Chinese spring wheat seedlings underwent drought and ABA treatments. Nuclear-localized TaMYB44s responded to both drought and ABA stress. Overexpression of TaMYB44-5A in Arabidopsis led to reduced drought tolerance and decreased ABA sensitivity. Furthermore, the transcriptional regulation analysis revealed that TaMYB44-5A directly downregulates the transcription level of the stomatal aperture regulator TaRD22-3A. These findings suggest that TaMYB44-5A may negatively regulate the ABA signaling pathway-mediated drought response.
Materials and methods
Identification of TaMYB44-5A
The cDNA sequence of AtMYB44 (AT5G67300) was obtained from Ensembl Plants (http://plants.ensembl.org/index.html). The coding sequence (CDS) of AtMYB44 was used in a TBLASTN search against the Triticum aestivum IWGSC genome in Ensembl Plants. Based on high sequence similarity scores, TraesCS5A02G159600, TraesCS5B02G157300, and TraesCS5D02G164600 were identified as orthologs of AtMYB44 in wheat. Specific primers for these genes were designed using Primer Premier 6 (Table S1).
Plant material and growth conditions
Chinese Spring wheat (Triticum aestivum cv. Chinese Spring) was utilized in our subsequent experiments. The wheat planting method and field management mode were consistent with those described in previous studies (Wang et al. 2021a). Wheat root, shoot, leaves, and spikes were collected at specific growth stages and cryopreserved with liquid nitrogen. Arabidopsis thaliana Col-0 type plants were employed in this study. Wild-type (WT) and transgenic Arabidopsis, germinated in Murashige and Skoog (MS) medium, were placed in an incubator set at a temperature of 24 °C, a relative humidity of 55%, and a photoperiod of 14-h light/10-h dark.
Stress and hormone treatments
After disinfection with sodium hypochlorite, wheat seeds were sown on MS medium (without hygromycin B) and placed in an incubator at 25 °C with a 9-h light/15-h dark photoperiod. Wheat seedlings were grown for 20 days and then transferred to an aqueous solution containing 30% PEG6000 for drought treatment and 80 µM S-( +)-ABA for ABA treatment. The wheat seedlings were gently wiped to remove any excess root moisture and transferred directly to dry medium for drought treatment. Wheat leaves were collected from all treatments at 0, 1, 3, 6, 12, and 24 h and frozen at − 80 °C. The survival rate was calculated as the percentage of surviving seedlings 4 days after re-watering. For the drought treatment, 4-week-old Arabidopsis plants were deprived of water for 14 days, and rosette leaves were collected after 14 days of drought for subsequent experiments.
qRT-PCR analysis
Total RNA was extracted from wheat and Arabidopsis subjected to different treatments following the method outlined in a previous study (Wang et al. 2021a). The qRT-PCR reaction procedure and detection instruments were also described in the same study (Wang et al. 2021a). Expression levels were normalized to the expression of the TaActin1 gene in wheat or the AtActin2 gene in Arabidopsis. Subsequently, the relative expression levels were calculated using the least squares method. All qRT-PCR analyses were conducted using three independent biological replicates. The primers utilized in this study are listed in Table S1.
Subcellular localization
In subcellular localization experiments, TaMYB44-5A/5B/5D in the intact open reading frame were cloned into pCAMBIA3301-RFP to generate the fusion vectors TaMYB44-5A/5B/5D:RFP. Fortunately, we borrowed the TaFDL2-1A:GFP fusion vector from Wang et al. (2021a). TaMYB44s:RFP and TaFDL2-1A:GFP were co-transiently transformed into tobacco leaves via Agrobacterium-mediated transformation. All injected leaves were collected 48 h after transformation and fluorescent signals were detected using a fluorescent confocal microscope (IX83-FV1200, Olympus Corporation).
Plant transformation
The TaMYB44-5A:RFP fusion vector was used to generate transgenic Arabidopsis via Agrobacterium-mediated stable transformation, where 1/2 MS medium with 50 μg/mL hygromycin B was used to screen transgenic T1/T2 Arabidopsis plants. qRT-PCR was employed to detect the expression levels. 15 transgenic lines were obtained by us.
Drought stress tolerance assays of TaMYB44-5A transgenic Arabidopsis plants
For the drought tolerance assay, 1-week-old seedlings germinated on half-MS medium were transferred to pots containing soil. Four-week-old plants grown under normal conditions were exposed to drought stress for 14 days. The plants were then re-watered for 4 days and survival was recorded. Detached leaves were air-dried for 7 h and weighed at seven time points (0 h, 1 h, 2 h, 3 h, 4 h, 5 h, and 6 h). Water loss was calculated as the weight loss at each time point divided by the initial fresh weight. Three rosette leaves of Arabidopsis after 5 weeks of growth were taken to measure their weight and recorded as FW (fresh weight). The leaves were retained in the previous step in ddH2O to absorb water to a constant weight, and the measured weight was recorded as TW (turgid weight). The leaves retained in the previous step were put in an oven at 60 °C until they achieved a constant weight, and the measured weight was recorded as DW (dry weight). The relative leaf water content was calculated according to the formula (RWC = (FW–DW)/(TW–DW) × 100%).
Physiological measurements
The physiological parameters were measured using fully expanded leaves obtained from well-watered and drought-stressed plants. The content of malondialdehyde (MDA), proline, soluble protein and the enzymatic activities of peroxidase (POD), and catalase (CAT) were determined as previously described (Luna et al. 2005; Wang et al. 2021b, 2022). Three biological replicates were performed.
Stomatal phenotype and stomatal aperture
The leaves of Arabidopsis growing under normal conditions or without water for 6 days were used for the observation of stomatal phenotype. Stomata were photographed using a confocal microscope with an objective lens (UPLSAPO20 × NA: 0.75) under LSM observation mode. Stomatal aperture was calculated as the specific value of width compared with length (Wang et al. 2022).
Transcriptional activity assays and yeast one-hybrid assays
Similar to previous studies, the Matchmaker™ Gold Y2H System (Takara Bio, Beijing, China) was used to detect the transcriptional activity of TaMYB44-5A (Wang et al. 2021a; Luo et al. 2022). BD-empty and BD-TaMYB44-5A were constructed using the pGBKT7 vector for subsequent transcriptional activity assays. The transcriptional activities of the recombinant vectors (transformed into Y2HGold yeast strain) were tested on SD/-Trp/-His/-Ade medium. The vectors were linearized using fast endonuclease BstBI. TaMYB44-5A was fused to the C-terminus of the GAL4 activation domain of pGADT7. All vectors were co-transformed into the Y1H Gold yeast strain as shown in Fig. 7B. All yeast strains were tested on SD/-Ura/-Leu and SD/-Ura/-Leu medium containing 500 ng/mL Aureobasidin A (AbA).
Electrophoretic mobility shift assay (EMSA)
TaMYB44-5A was fused to the pGEX4T vector. Purified GST-TaMYB44-5A and GST proteins were used in subsequent experiments. A LightShift® Chemiluminescent EMSA Kit 20148 (Thermo Fisher Scientific Inc, Waltham, MA, USA) was used to detect the activities of these proteins. All biotin-labeled probes used in the EMSA (modified at the 5') were synthesized by Sangon Biotech (Shanghai) Co., Ltd. The proteins were incubated at 20 °C for 50 min. Each 20 μL reaction system contained 50 fmol of the biotin-labeled probe. The components in the reaction system were separated on a non-denaturing polyacrylamide gel and then transferred to a nylon membrane. Biotin-labeled probe migration was detected using the luminescent system in the kit.
Statistical analysis
SPSS 23 (SPSS. Inc., USA) was used to process all data obtained in this study. The Student’s t-test (**P < 0.01, *P < 0.05) was used to determine significant differences between transgenic Arabidopsis and WT. Photoshop (CS6, Adobe Systems Incorporated, San Jose, CA, USA) was used to construct all images.
Results
Identification and bioinformatics analysis of TaMYB44-5A
To identify homologous genes of AtMYB44 in wheat, we utilized the coding sequence (CDS) of AtMYB44 to conduct bioinformatics searches in the Chinese Spring wheat database available in Ensembl Plants. Subsequently, we identified three homologous genes of AtMYB44 designated as TaMYB44-5A (Gene ID: TraesCS5A02G159600), TaMYB44-5B (Gene ID: TraesCS5B02G157300), and TaMYB44-5D (Gene ID: TraesCS5D02G164600), based on their distinct positions on the chromosomes (Supplementary Fig. S1). TaMYB44-5A, TaMYB44-5B, and TaMYB44-5D encode proteins comprising 336, 349, and 348 amino acids, respectively (Supplementary Fig. S2), with a high sequence similarity of 93.35%. In addition, TaMYB44 shares 42.46% and 75.74% similarity with AtMYB44 and OsMYB102, respectively (Supplementary Fig. S2). Sequence analysis revealed highly conserved N-terminal SANT domains and a C-terminal ethylene-responsive element-binding factor-associated amphiphilic repression (EAR) motif in TaMYB44s, AtMYB44, and OsMYB102 (Supplementary Fig. S2). A previous study suggested that the LXLXL-type EAR motif may be involved in transcriptional regulation or interactions (Kagale and Rozwadowski 2011).
Surprisingly, upon analyzing the promoter sequences of TaMYB44s located 2 kb upstream of the initiation codon, we discovered numerous cis-acting elements associated with the plant drought response and ABA signaling, including ABA-responsive elements (ABRE), dehydration-response elements (DRE), and MYB-binding sites (MBSs; Fig. 1a). Therefore, our findings strongly suggest that TaMYB44s may play a significant role in drought tolerance in wheat.
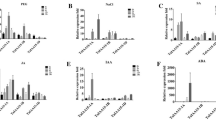
Expression analyses of TaMYB44s. a Distribution of major stress-related cis-acting elements in the 2-kb promoter regions of TaMYB44s. b–d Expression levels of TaMYB44s under drought and PEG6000 treatments. The expression levels were normalized against those at 0 h for TaMYB44s. The reference for significance analysis is the data at 0 h. **Highly significant difference (P < 0.01), *significant difference (P < 0.05). e Expression patterns of TaMYB44s. Statistical analysis was performed using the LSD method. Data represent mean values ± standard deviations (n = 3). Values labeled with different letters indicate significant differences, whereas the same letters indicate no significant differences. (P < 0.05; one-way ANOVA). The reference gene is TaActin1. The expression patterns were normalized against the expression level of TaMYB44-D at root for TaMYB44s
Expression patterns and subcellular localization of TaMYB44s
To verify whether TaMYB44s participates in the drought response, we used real-time quantitative PCR (qRT-PCR) to detect the changes in the expression levels of TaMYB44s in wheat under drought and dehydration stress (using PEG6000) (Fig. 1b–d). The results indicate that TaMYB44s are induced to higher expression levels at 1 h by drought, followed by a slight decrease in expression at 3 h (TaMYB44-5A showing a decrease at 6 h). With increasing treatment time, TaMYB44s are ultimately induced to the highest level at 24 h in a similar pattern. Under PEG6000 treatment, both TaMYB44-5A and TaMYB44-5D exhibit higher expression levels at 1 h and 24 h, while the expression level of TaMYB44-5B shows a slight increase. All these findings suggest that these three homologous genes may have similar functions in response to drought. Subsequently, we analyzed the expression of TaMYB44-5A, TaMYB44-5B, and TaMYB44-5D in different tissues, and observed that TaMYB44-5A exhibits high-level expression in both root and leaf (Fig. 1e).
To detect the possible regulatory functions of TaMYB44s, we determined their subcellular localization. The three proteins were fused to the N-terminal of red fluorescent protein (RFP) and transformed into Nicotiana benthamiana leaves. The red fluorescent signals of TaMYB44-5A:RFP, TaMYB44-5B:RFP, and TaMYB44-5D:RFP were detected in the nucleus (Fig. 2). Their co-localization with green fluorescent protein (GFP) and nuclear protein TaFDL2-1A (TaFDL2-1A:GFP) confirmed that TaMYB44-5A, TaMYB44-5B, and TaMYB44-5D may function in the nucleus. Therefore, based on the expression patterns and subcellular localizations of TaMYB44s, we selected TaMYB44-5A for further study.
Overexpression of TaMYB44-5A reduced the tolerance of drought in transgenic Arabidopsis
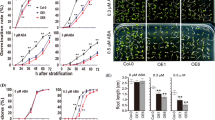
TaMYB44-5A overexpression lines were created in Arabidopsis, and then we selected TaMYB44-5A overexpression line 7 (OE7) and OE12 for the highest TaMYB44-5A expression as follow-up research objects (Fig. 3a and Supplementary Fig. S3). After drought treatment, the rosette leaves of wild type (WT), OE7, and OE12 were severely damaged, with leaf curling or yellowing (Fig. 3b). Compared with WT, the OE7 and OE12 lines exhibited greater mortality (Fig. 3c). Subsequent analysis showed that the water loss rate by the overexpression lines (OE7 and OE12) was significantly higher than that of WT (Fig. 3d). The contents of proline and soluble protein, CAT enzyme activity, and peroxidase (POD) enzyme activity were lower in the OE lines than WT, and the malondialdehyde (MDA) contents was higher than those in WT (Fig. 4c–g).
Overexpression of TaMYB44-5A reduced drought tolerance in Arabidopsis. a Identification of TaMYB44-5A overexpression lines by PCR. AtActin2 was used as a background control. b Phenotypic changes in WT and transgenic lines before and after drought stress, and after re-watering. The white scale bars represent a length of 1 cm. c Survival rates of WT and transgenic lines after drought stress. d Under normal growth conditions, the rosette leaves from WT and transgenic lines were used to measure the water loss rate. e Relative water contents of rosette leaves from WT and transgenic lines before and after drought stress. Data represent mean values ± standard deviations based on three independent replicates. **Highly significant difference (P < 0.01), *significant difference (P < 0.05)
TaMYB44-5A can alter the stomatal aperture and physiological indicators related to plant drought tolerance in transgenic Arabidopsis under drought stress. a, b Rosette leaves of WT and transgenic lines before and after drought stress, and stomata photographed at different resolutions. Stomatal aperture was calculated as the specific value of width compared with length. Data are presented as the mean ± SE based on three independent biological replicates, and 10 stomata were observed per experiment. The corresponding scale bars are shown in the figures. c CAT activities in WT and transgenic lines under normal and drought conditions. d MDA contents of WT and transgenic lines under normal and drought conditions. e Soluble protein contents in WT and transgenic lines under normal and drought conditions. f POD activities in WT and transgenic lines under normal and drought conditions. g Proline content in WT and transgenic lines under normal and drought conditions. Data represent mean values ± standard deviations based on three independent replicates. **Highly significant difference (P < 0.01), *significant difference (P < 0.05)
Leaves are the main transpiration organs of plants, so we then examined the physiological status of the rosette leaves. After drought treatment, the leaf water content was significantly higher in WT than in overexpression lines (Fig. 3e), and the stomatal aperture was maintained at a high level in the overexpression lines (Fig. 4a, b). These results indicate that the overexpression of TaMYB44-5A reduced the tolerance of drought in Arabidopsis.
ABA-induced TaMYB44-5A might decrease ABA sensitivity in Arabidopsis
The ABA signaling pathway regulates the stomatal aperture under drought conditions (Aalto et al. 2012). To determine whether TaMYB44-5A is involved in the ABA signaling pathway, we conducted qRT-PCR and ABA sensitivity assays. The expression of TaMYB44-5A in Chinese Spring wheat treated with exogenous ABA increased gradually over time (Fig. 5c), indicating that TaMYB44-5A responds to exogenous ABA treatment. Subsequently, we assessed the percentage of green cotyledons under both ABA treatment and control conditions. The number of seedlings with green cotyledons was significantly higher in the OE7 and OE12 lines compared to WT under ABA treatment, while there was no significant difference between OE7, OE12, and WT under control conditions (Fig. 5a, b). These results suggest that ABA-induced overexpression of TaMYB44-5A might reduce the sensitivity of transgenic Arabidopsis to ABA.
TaMYB44-5A attenuated the sensitivity of transgenic lines to ABA. a Seedling establishment for TaMYB44-5A overexpression and WT plants in normal, 0.25 μM ABA, and 0.75 μM ABA conditions. b Seedling greening ratios are shown for the plants reported in a. c Expression levels of TaMYB44-5A under ABA treatments. Data represent mean values ± standard deviations based on three independent replicates. The reference for significance analysis is the data at 0 h. **Highly significant difference (P < 0.01), *significant difference (P < 0.05)
To identify the function of TaMYB44-5A in the ABA signaling pathway under drought conditions, we conducted qRT-PCR to determine the expression levels of several drought- and ABA-responsive genes comprising AtP5CS1, AtRD22, AtRAB18, ABA-responsive element-binding protein 3 (AtAREB3), ABA insensitive 5 (AtABI5), and senescence-associated gene 29 (AtSAG29). The expression levels of AtP5CS1 and AtRD22 were all significantly lower in transgenic Arabidopsis compared with WT under drought treatment (Fig. 6b, c). However, except for the significant downregulation of AtSAG29 in OE7, there were no differences in the expression levels of the other genes (Fig. 6a, d, e, f). We performed a dual-luciferase assay to confirm whether TaMYB44-5A could directly regulate the transcriptional levels of AtP5CS1, AtRD22, and AtSAG29 (Supplementary Fig. S4a). However, only the transcriptional level of AtRD22 was significantly repressed by TaMYB44-5A (Supplementary Fig. S4b). These findings also indicate that TaMYB44-5A may negatively regulate drought tolerance by repressing the expression of drought- and ABA-responsive genes.
TaMYB44-5A reduced the expression of ABA-responsive genes. a–f Expression levels of AtABI5, AtRD22, AtP5CS1, AtRAB18, AtAREB3, and AtSAG29 in WT and transgenic lines under normal and drought conditions. Data represent mean values ± standard deviations based on three independent replicates. **Highly significant difference (P < 0.01), *significant difference (P < 0.05)
Transcriptional regulation assay of TaMYB44-5A
A previous study demonstrated that OsMYB102 is an R2R3-MYB transcription factor that can directly bind to the MBS element in the promoter to regulate the expression of rice (Oryza sativa L.) cyclophylin A-like protein 707-6 (OsCYP707A6) (Piao et al. 2019a). Thus, we examined the autoactivation activity of TaMYB44-5A using the yeast system. Similar to BD-empty, BD-TaMYB44-5A could grow on SD/-Trp medium but could not survive on SD/-Trp/-His/-Ade medium, indicating that BD-TaMYB44-5A could not independently activate the synthesis of His or Ade. Therefore, TaMYB44-5A does not possess autoactivation activity (Supplementary Fig. S5a and b).
Next, we performed a dual-luciferase assay to confirm that TaMYB44-5A can mediate changes in the transcription level of TaRD22-3A (the homologous gene of AtRD22 in wheat) (Liu et al. 2019). TaMYB44-5A significantly reduced the relative luciferase activity in the Reporter compared with the empty effector (Fig. 7a). Furthermore, we found two MBS motifs in the promoter within 1000 bp of TaRD22-3A (Fig. 7c). Therefore, we speculated whether TaMYB44-5A regulates the transcription level of TaRD22-3A by directly binding to the MBS motif.
Transcriptional regulation assay for TaMYB44-5A. a The promoter of TaRD22-3A was fused upstream of the luciferase gene in the pGreenII 0800-LUC vector. TaMYB44-5A was fused into the pGreenII 62-SK vector and empty pGreenII 62-SK was used as a control. b Three consecutive copies of AACNG (MBS) and mutant taattc (mbs) were fused to the pAbAi vector. TaMYB44-5A was fused to the pGADT7 vector and the unmodified pGADT7 was used as a control. The corresponding vectors were co-transformed into the Y1H Gold strain. c Distribution of MBS within 1 kb upstream of TaRD22-3A promoter. d 1–4 correspond to MBS1-4. The yeast suspension was dropped on SD/-Ura/-Leu and SD/-Ura/-Leu + AbA (500 ng/mL) medium at two concentrations. e The probes used in EMSA are indicated by green columns. Purified GST-TaMYB44-5A protein and partial fragment promoter of TaRD22-3A. “ + ” and “–” indicate that the components were added or not added; “ × 10,” “ × 50,” and “ × 200” denote tenfold, 50-fold, and 200-fold molar excesses of unlabeled probe relative to biotin probe, respectively. Data represent mean values ± standard deviations based on three independent replicates. **Highly significant difference (P < 0.01)
Therefore, we performed yeast one-hybrid assays (Fig. 7b). In SD/-Ura/-Leu/ + AbA medium, empty pGADT7 was significantly nonviable whereas the other pAbAi-MBS strains grew very well, although the AACAG-type strain was slightly less viable (Fig. 7d).
Subsequent analysis by EMSA confirmed our speculation. The migration band exhibited a decrease trend in specificity only in group P1 as the unlabeled probe concentration increased (Fig. 7c, e). Our results indicate that TaMYB44-5A can directly mediate the transcription level of TaRD22-3A, and the changes in its transcription level were consistent with the expression level of homologous AtRD22 in transgenic Arabidopsis with reduced drought tolerance.
Discussion
Drought can severely damage sessile plants but plants have also evolved molecular mechanisms to cope with drought stress (Zhu 2016). These molecular mechanisms typically involve transcriptional activators or repressors induced by drought. Therefore, identifying drought-associated transcription factors is crucial for modern molecular breeding (Paux et al. 2022; Han et al. 2023). For example, wheat (Triticum aestivum L.) N-acetylcysteine 071-A (TaNAC071-A) promotes the expression of genes related to water use efficiency to improve the drought tolerance of transgenic wheat (Mao et al. 2022). Similarly, the expression level of OsMYB2 was significantly increased in OsWRKY5-knockout transgenic rice to enhance tolerance of drought (Lim et al. 2022). The R2R3-MYB transcription factor in sessile plants is considered a good candidate gene for improving the drought tolerance of plants (Dubos et al. 2010). In the present study, we found that TaMYB44-5A (R2R3-MYB) overexpression reduced drought tolerance by repressing the genes associated with the ABA response in transgenic Arabidopsis (Figs. 3b and 6).
Physiological indexes related to drought combined with the identification test of drought tolerance can more comprehensively study the specific biological functions of target genes (Luna et al. 2005; Chenu et al. 2017; Langridge and Reynolds 2021; Li et al. 2022; Yao et al. 2022). Wheat accumulates a large amount of hydrogen peroxide under drought conditions, and CAT and POD can remove the accumulated hydrogen peroxide in wheat (Luna et al. 2005). Overexpression of CAT1/2 can reduce the accumulation rate of hydrogen peroxide in wheat under severe drought condition, to improve the drought tolerance of wheat (Luna et al. 2005). StNAC053 in potatoes can enhance drought tolerance and salt tolerance of transgenic Arabidopsis by increasing POD enzyme activity (Wang et al. 2021b). The MDA content represents the stability of the cell membrane after the plant is subjected to drought stress (Wang et al. 2022). Overexpression of TaFDL2-1A can attenuate the degree of drought stress damage to the wheat cell membrane system (Wang et al. 2022). Osmotic adjustment substances can help plants maintain intracellular osmotic pressure under drought stress. TaNAC071-A can increase the proline content of wheat to help wheat maintain a higher level of photosynthesis under water deficit (Mao et al. 2022). In this study, lower CAT enzyme activity, POD enzyme activity, and osmolyte content demonstrated that TaMYB44-5A negatively regulated the drought tolerance of transgenic Arabidopsis (Fig. 4c, e, f, g). Simultaneously, the higher MDA content proved that the overexpression of TaMYB44-5A reduced the stability of the transgenic Arabidopsis cell membrane system under drought stress (Fig. 4d). Therefore, the drought tolerance of Arabidopsis overexpressing TaMYB44-5A was weaker.
The ABA signaling pathway is one of the most critical pathways that allow plants to respond to drought stress (Jaradat et al. 2013; Zhu 2016; Boija et al. 2018). In this pathway, many transcription factors are induced to upregulate the expression levels of genes and then activate the downstream drought tolerance mechanism (Nakashima and Yamaguchi-Shinozaki 2013). ABA-induced soybean (Glycine max L.) WRKY16 can promote the expression of RD29A to enhance drought tolerance in transgenic plants (Ma et al. 2018). Differently, AtMYB44 can be induced to express at high levels by exogenous ABA treatment, and it inhibits the expression of downstream responsive genes in the ABA signaling pathway. Therefore, researchers believe that AtMYB44 can sustain the growth of Arabidopsis under stress conditions (Jaradat et al. 2013). In this study, we have shown that TaMYB44-5A also can be induced by ABA treatment and inhibited the ABA further response (Fig. 5). Previous studies demonstrated that RD22 is a downstream component induced by ABA in response to drought stress and it enhances drought tolerance in plants (Goh et al. 2003; Wang et al. 2012). In the present study, the transcriptional level of AtRD22 was significantly reduced (Fig. 6b). The subsequent LUC and binding assays also confirmed the role of TaMYB44-5A in the direct inhibition of TaRD22-3A (Fig. 7a–d). Thus, the mechanism that allows TaMYB44-5A to negatively regulate drought tolerance is mediated through the ABA signaling pathway.
Stomata on the leaves are important organs for controlling water loss by transpiration under drought stress. Plants have evolved many mechanisms to regulate stomatal aperture and prevent the excessive loss of water under drought conditions, such as stomatal closure controlled by the ABA signaling pathway (Goh et al. 2003; Aalto et al. 2012). TaFDL2-1A overexpression in wheat increases the sensitivity of transgenic wheat to ABA and leads to ABA-dependent stomatal closure (Wang et al. 2022). In the present study, compared with WT, the sensitivity to ABA was significantly lower in transgenic Arabidopsis (Fig. 5a, b), and drought treatment resulted in significantly increased stomatal aperture (Fig. 4a, b). In a previous study, RD22 was shown to regulate stomatal movement in Arabidopsis under drought conditions in an ABA-dependent manner (Goh et al. 2003; Wang et al. 2012). Therefore, we speculate that TaMYB44-5A may be involved in ABA-dependent stomatal movement.
Transcription factors recognize and bind to specific motifs in the promoters of downstream genes to regulate stress signal transduction and stress-response networks. Many R2R3-MYB transcription factors recognize and bind to AAC and TTG core motifs, including TAACNG (MBSI) and A/GTTGA/TT (MBSII), before then regulating the expression of downstream genes (Dubos et al. 2010; Shim and Choi 2013; Piao et al. 2019a; Fu et al. 2020). Previous studies based on protein-binding microarray analysis found that AtMYB44 can specifically bind to the AACNG motif. In addition, analysis using a random promoter fragment showed that sequences with migrating bands in EMSA experiments all contained AACNG (Jung et al. 2012). Therefore, we directly tested all types of TAACNG using the yeast one-hybrid system (Fig. 7b, d) and, as expected, the results showed that TaMYB44-5A could recognize and bind to the AACNG-specific motif. Furthermore, subsequent EMSA assays demonstrated that TaMYB44-5A could specifically bind to the MBS element in the TaRD22-3A promoter (Fig. 7e). The AACNG motif is widely distributed in promoter sequences in the wheat genome (International Wheat Genome Sequencing 2018), so TaMYB44-5A may reduce the expression of drought- and ABA-induced genes in wheat by directly binding to this motif.
In summary, we found that TaMYB44-5A encoding an R2R3-MYB could reduce the tolerance of drought and sensitivity to ABA in transgenic Arabidopsis, and it regulated the expression levels of downstream genes related to drought tolerance. Furthermore, TaMYB44-5A bound specifically to the MBS element in the promoter of TaRD22-3A to respond to ABA-mediated drought stress. Therefore, TaMYB44-5A may act as a negative regulator in response to drought stress via the ABA signaling pathway in wheat.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AbA:
-
Aureobasidin A
- CAT:
-
Catalase
- EMSA:
-
Electrophoretic mobility shift assay
- MBS:
-
MYB-binding site
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- RD22:
-
Responsive to dehydration 22
References
Aalto MK, Helenius E, Kariola T, Pennanen V, Heino P, Horak H, Puzorjova I, Kollist H, Palva ET (2012) ERD15 an attenuator of plant ABA responses and stomatal aperture. Plant Sci 182:19–28. https://doi.org/10.1016/j.plantsci.2011.08.009
An JP, Yao JF, Xu RR, You CX, Wang XF, Hao YJ (2018) Apple bZIP transcription factor MdbZIP44 regulates abscisic acid promoted anthocyanin accumulation. Plant Cell Environ 41(11):2678–2692. https://doi.org/10.1111/pce.13393
Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, Abraham BJ, Afeyan LK, Guo YE, Rimel JK, Fant CB, Schuijers J, Lee TI, Taatjes DJ, Young RA (2018) Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175(7):1842–1855. https://doi.org/10.1016/j.cell.2018.10.042
Chen C, Zhang Z, Lei Y, Chen W, Zhang Z, Li X, Dai H (2024) MdMYB44-like positively regulates salt and drought tolerance via the MdPYL8-MdPP2CA module in apple. Plant J 118(1):24–41. https://doi.org/10.1111/tpj.16584
Chenu K, Porter JR, Martre P, Basso B, Chapman SC, Ewert F, Bindi M, Asseng S (2017) Contribution of crop models to adaptation in wheat. Trends Plant Sci 22(6):472–490. https://doi.org/10.1016/j.tplants.2017.02.003
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15(10):573–581. https://doi.org/10.1016/j.tplants.2010.06.005
Fu C, Chen H, Gao H, Lu Y, Han C, Han Y (2020) Two papaya MYB proteins function in fruit ripening by regulating some genes involved in cell-wall degradation and carotenoid biosynthesis. J Sci Food Agric 100(12):4442–4448. https://doi.org/10.1002/jsfa.10484
Fujii H, Verslues PE, Zhu JK (2011) Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Natl Acad Sci USA 108(4):1717–1722. https://doi.org/10.1073/pnas.1018367108
Gao S, Xu J, Song W, Dong J, Xie L, Xu B (2024) Overexpression of BnMYBL2-1 improves plant drought tolerance via the ABA-dependent pathway. Plant Physiol Biochem 207:108293. https://doi.org/10.1016/j.plaphy.2023.108293
Goh CH, Nam HG, Park YS (2003) Stress memory in plants: a negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. Plant J 36(2):240–255. https://doi.org/10.1046/j.1365-313x.2003.01872.x
Han J, Li X, Li W, Yang Q, Li Z, Cheng Z, Lv L, Zhang L, Han D (2023) Isolation and preliminary functional analysis of FvICE1, involved in cold and drought tolerance in Fragaria vesca through overexpression and CRISPR/Cas9 technologies. Plant Physiol Biochem 196:270–280. https://doi.org/10.1016/j.plaphy.2023.01.048
Hou L, Fan X, Hao J, Liu G, Zhang Z, Liu X (2020) Negative regulation by transcription factor VvWRKY13 in drought stress of Vitis vinifera L. Plant Physiol Biochem 148:114–121. https://doi.org/10.1016/j.plaphy.2020.01.008
Huang D, Jaradat MR, Wu W, Ambrose SJ, Ross AR, Abrams SR, Cutler AJ (2007) Structural analogs of ABA reveal novel features of ABA perception and signaling in Arabidopsis. Plant J 50(3):414–428. https://doi.org/10.1111/j.1365-313X.2007.03056.x
International Wheat Genome Sequencing C (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361(6403):7191. https://doi.org/10.1126/science.aar7191
Itam MO, Mega R, Gorafi YSA, Yamasaki Y, Tahir ISA, Akashi K, Tsujimoto H (2022) Genomic analysis for heat and combined heat-drought resilience in bread wheat under field conditions. Theor Appl Genet 135(1):337–350. https://doi.org/10.1007/s00122-021-03969-x
Jaradat MR, Feurtado JA, Huang D, Lu Y, Cutler AJ (2013) Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol 13:192. https://doi.org/10.1186/1471-2229-13-192
Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146(2):623–635. https://doi.org/10.1104/pp.107.110981
Jung C, Kim YK, Oh NI, Shim JS, Seo JS, Choi YD, Nahm BH, Cheong JJ (2012) Quadruple 9-mer-based protein binding microarray analysis confirms AACnG as the consensus nucleotide sequence sufficient for the specific binding of AtMYB44. Mol Cells 34(6):531–537. https://doi.org/10.1007/s10059-012-0209-9
Kagale S, Rozwadowski K (2011) EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6(2):141–146. https://doi.org/10.4161/epi.6.2.13627
Langridge P, Reynolds M (2021) Breeding for drought and heat tolerance in wheat. Theor Appl Genet 134(6):1753–1769. https://doi.org/10.1007/s00122-021-03795-1
Li D, Li Y, Zhang L, Wang X, Zhao Z, Tao Z, Wang J, Wang J, Lin M, Li X, Yang Y (2014) Arabidopsis ABA receptor RCAR1/PYL9 interacts with an R2R3-type MYB transcription factor, AtMYB44. Int J Mol Sci 15(5):8473–8490. https://doi.org/10.3390/ijms15058473
Li W, Zhong J, Zhang L, Wang Y, Song P, Liu W, Li X, Han D (2022) Overexpression of a Fragaria vesca MYB transcription factor gene (FvMYB82) increases salt and cold tolerance in Arabidopsis thaliana. Int J Mol Sci 23(18):10538. https://doi.org/10.3390/ijms231810538
Lim C, Kang K, Shim Y, Yoo SC, Paek NC (2022) Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol 188(4):1900–1916. https://doi.org/10.1093/plphys/kiab492
Liu H, Xing M, Yang W, Mu X, Wang X, Lu F, Wang Y, Zhang L (2019) Genome-wide identification of and functional insights into the late embryogenesis abundant (LEA) gene family in bread wheat (Triticum aestivum L.). Sci Rep 9(1):13375. https://doi.org/10.1038/s41598-019-49759-w
Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH (2005) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56(411):417–423. https://doi.org/10.1093/jxb/eri039
Luo YX, Chen SK, Wang PD, Peng D, Zhang X, Li HF, Feng CZ (2022) Genome-wide analysis of the RAV gene family in wheat and functional identification of TaRAV1 in salt stress. Int J Mol Sci 23(16):8834. https://doi.org/10.3390/ijms23168834
Ma Q, Xia Z, Cai Z, Li L, Cheng Y, Liu J, Nian H (2018) GmWRKY16 enhances drought and salt tolerance through an ABA-mediated pathway in Arabidopsis thaliana. Front Plant Sci 9:1979. https://doi.org/10.3389/fpls.2018.01979
Mao H, Li S, Chen B, Jian C, Mei F, Zhang Y, Li F, Chen N, Li T, Du L, Ding L, Wang Z, Cheng X, Wang X, Kang Z (2022) Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol Plant 15(2):276–292. https://doi.org/10.1016/j.molp.2021.11.007
Nakashima K, Yamaguchi-Shinozaki K (2013) ABA signaling in stress-response and seed development. Plant Cell Rep 32(7):959–970. https://doi.org/10.1007/s00299-013-1418-1
Paux E, Lafarge S, Balfourier F, Derory J, Charmet G, Alaux M, Perchet G, Bondoux M, Baret F, Barillot R, Ravel C, Sourdille P, Le Gouis J (2022) Breeding for economically and environmentally sustainable wheat varieties: an integrated approach from genomics to selection. Biology (basel) 11(1):149. https://doi.org/10.3390/biology11010149
Pennisi E (2008) The blue revolution, drop by drop, gene by gene. Science 320(5873):171–173. https://doi.org/10.1126/science.320.5873.171
Piao W, Kim SH, Lee BD, An G, Sakuraba Y, Paek NC (2019a) Rice transcription factor OsMYB102 delays leaf senescence by down-regulating abscisic acid accumulation and signaling. J Exp Bot 70(10):2699–2715. https://doi.org/10.1093/jxb/erz095
Piao W, Sakuraba Y, Paek N-C (2019b) Transgenic expression of rice MYB102 (OsMYB102) delays leaf senescence and decreases abiotic stress tolerance in Arabidopsis thaliana. BMB Rep 52(11):653–658. https://doi.org/10.5483/BMBRep.2019.52.11.071
Qian D, Xiong S, Li M, Tian L, Qing QuL (2021) OsFes1C, a potential nucleotide exchange factor for OsBiP1, is involved in the ER and salt stress responses. Plant Physiol 187(1):396–408. https://doi.org/10.1093/plphys/kiab263
Shi K, Liu J, Liang H, Dong H, Zhang J, Wei Y, Zhou L, Wang S, Zhu J, Cao M, Jones CS, Ma D, Wang Z (2024) An alfalfa MYB-like transcriptional factor MsMYBH positively regulates alfalfa seedling drought resistance and undergoes MsWAV3-mediated degradation. J Integr Plant Biol 66(4):683–699. https://doi.org/10.1111/jipb.13626
Shim JS, Choi YD (2013) Direct regulation of WRKY70 by AtMYB44 in plant defense responses. Plant Signal Behav 8(6):e20783. https://doi.org/10.4161/psb.24509
Shim JS, Jung C, Lee S, Min K, Lee YW, Choi Y, Lee JS, Song JT, Kim JK, Choi YD (2013) AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J 73(3):483–495. https://doi.org/10.1111/tpj.12051
Wang H, Zhou L, Fu Y, Cheung MY, Wong FL, Phang TH, Sun Z, Lam HM (2012) Expression of an apoplast-localized BURP-domain protein from soybean (GmRD22) enhances tolerance towards abiotic stress. Plant Cell Environ 35(11):1932–1947. https://doi.org/10.1111/j.1365-3040.2012.02526.x
Wang B, Li L, Peng LM, Wei A, Li X (2021a) TaFDL2-1A interacts with TabZIP8-7A protein to cope with drought stress via the abscisic acid signaling pathway. Plant Sci 311:111022. https://doi.org/10.1016/j.plantsci.2021.111022
Wang Q, Guo C, Li Z, Sun J, Deng Z, Wen L, Li X, Guo Y (2021b) Potato NAC transcription factor StNAC053 enhances salt and drought tolerance in transgenic Arabidopsis. Int J Mol Sci 22(5):2568. https://doi.org/10.3390/ijms22052568
Wang B, Li L, Liu M, Peng WA, Hou B, Lei Y, Li X (2022) TaFDL2-1A confers drought stress tolerance by promoting ABA biosynthesis, ABA responses, and ROS scavenging in transgenic wheat. Plant J 112(3):722–737. https://doi.org/10.1111/tpj.15975
Wu Y, Wen J, Xia Y, Zhang L, Du H (2022) Evolution and functional diversification of R2R3-MYB transcription factors in plants. Hortic Res 9:58. https://doi.org/10.1093/hr/uhac058
Yao C, Li W, Liang X, Ren C, Liu W, Yang G, Zhao M, Yang T, Li X, Han D (2022) Molecular cloning and characterization of MbMYB108, a Malus baccata MYB transcription factor gene, with functions in tolerance to cold and drought stress in transgenic Arabidopsis thaliana. Int J Mol Sci 23(9):4846. https://doi.org/10.3390/ijms23094846
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167(2):313–324. https://doi.org/10.1016/j.cell.2016.08.029
Acknowledgements
This study was supported by the Key Program of Shaanxi Agricultural Cooperative Innovation and Promotion Alliance (No. LMZD202104), National Major Agricultural Science and Technology Project (NK2022060503), Program of Introducing Talents of Innovative Discipline to Universities (Project 111) from the State Administration of Foreign Experts Affairs (#B18042) ‘Crop breeding for disease resistance and genetic improvement’ and Chinese Universities Scientific Fund (2452022112).
Author information
Authors and Affiliations
Contributions
DP and LL conducted the study, analyzed data, and wrote the manuscript. AW, ML and YL collected the samples. LZ and BW contributed to the development of materials. XL and YX conceived and designed the study, and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, D., Li, L., Wei, A. et al. TaMYB44-5A reduces drought tolerance by repressing transcription of TaRD22-3A in the abscisic acid signaling pathway. Planta 260, 52 (2024). https://doi.org/10.1007/s00425-024-04485-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04485-0