Abstract
Key message
Overexpression of the Aux/IAA protein TaIAA15-1A from wheat improves drought tolerance by regulating the ABA signalling pathway in transgenic Brachypodium.
Abstract
Drought is a major abiotic stress that causes severe crop yield loss. Aux/IAA genes have been shown to be involved in drought stress responses. However, to the best of our knowledge, there has been little research on the molecular mechanism of the wheat Aux/IAA gene in the context of drought tolerance. In this study, we found that expression of the wheat Aux/IAA gene TaIAA15-1A was upregulated by PEG6000, NaCl, SA, JA, IAA and ABA. Transgenic plants overexpressing TaIAA15-1A showed higher drought tolerance than wild-type (WT) plants. The physiological analyses showed that the transgenic lines exhibited a higher survival rate, shoot length, and relative water content than the WT plants. The activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) were enhanced in transgenic lines, causing a reduction in the hydrogen peroxide (H2O2) and superoxide anion radical (O2−) contents. Transcriptome analysis showed that TaIAA15-1A overexpression alters the expression of these genes involved in the auxin signalling pathway, ABA signalling pathway, phenolamides and antioxidant pathways. The results of exogenous ABA treatment suggested that TaIAA15-1A overexpression increased sensitivity to ABA at the germination and postgermination stages compared to WT plants. These results indicate that TaIAA15-1A plays a positive role in plant drought tolerance by regulating ABA-related genes and improving antioxidative stress ability and has potential application in genetically modified crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cereal crops inevitably face various abiotic stresses, such as drought, heat, salinity, and cold, during growth and development processes; these stresses greatly impact field productivity (Robles et al. 2018). In the long-term evolutionary interaction between plants and environmental abiotic stresses, plants have evolved complex resistance mechanisms, such as antioxidant systems, osmotic balance, and phytohormone signal transduction (Sallam et al. 2019). To date, hundreds of genes, such as TaPYL1-1B, TaSAP5, OsIAA18, TaNAC69-1, and OsIAA6, have been shown to function in controlling drought tolerance in crops (Jung et al. 2015; Chen et al. 2016; Zhang et al. 2017; Wang et al. 2021; Mao et al. 2022).
Auxin has been reported to play an important role in plant stress defence responses (Mutka et al. 2013). In Arabidopsis thaliana, endogenous and exogenous auxin positively regulates drought resistance by modulating abiotic stress-related gene expression and reactive oxygen species (ROS) metabolism (Shi et al. 2014). A previous study showed that exogenous IAA treatment can alleviate drought stress and effectively enhance drought resistance in wheat (Liu et al. 2009). Plants regulate endogenous auxin homeostasis in response to drought stress (Du et al. 2013). As a key mediator, ABA has been shown to enhance the drought tolerance of plants via crosstalk with auxin signalling. For example, increasing evidence indicates that auxin homeostasis changes influence ABA synthesis and thus alter plant tolerance against drought stress (He et al. 2021; Zhang et al. 2021).
In plants, auxin/indoleacetic acid (Aux/IAA) proteins are a large family and have been identified to play an important role in the auxin signalling pathway; they bind to TIR1/AFB, are ubiquitinated by the E3 ubiquitin ligase complex SCFTIR/AFBs, and are then degraded by the 26S proteasome, allowing ARF-mediated transcriptional regulation (Maraschin et al. 2009). A number of Aux/IAA genes have been shown to influence drought tolerance in crops; for example, overexpression of OsIAA20 enhances transgenic rice tolerance against drought and salt stresses through the ABA pathway (Zhang et al. 2021). A previous study showed that OsIAA18 overexpression in rice plants significantly improves salt and drought tolerance by upregulating ABA-regulated gene expression levels and improving ROS scavenging capacity (Wang et al. 2021). In addition, these genes, including OsIAA6 and OsIAA9 in rice (Jung et al. 2015) and IAA5, IAA6, and IAA19 in Arabidopsis (Salehin et al. 2019), have also been reported to be involved in drought tolerance.
To date, the roles of some Aux/IAA genes in tolerance against drought stress have been widely shown; however, to the best of our knowledge, there has been little research on the molecular mechanism analysis of wheat Aux/IAA genes in the context of drought tolerance. In this study, a wheat Aux/IAA gene, TaIAA15-1A, was cloned, and the tolerance against drought stress conferred by TaIAA15-1A in transgenic Brachypodium was investigated. Expression pattern analysis showed that the expression level of TaIAA15-1A was upregulated by PEG6000, NaCl, SA, JA, IAA and ABA. Our results indicate that TaIAA15-1A enhances plant tolerance against drought stress by regulating ABA-related genes and improving antioxidative stress ability and has potential applications in crop improvement programs.
Materials and methods
Plant materials and abiotic treatments
The bread wheat (Triticum aestivum L.) cultivar “SuMai3” and Brachypodium distachyon “Bd21” were used in this study. The WT “Bd21” and TaIAA15-1A-overexpressing transgenic Brachypodium lines were grown at 22–25 °C with a photoperiod of 16/8 h in a greenhouse. The seedlings of WT and transgenic Brachypodium lines at the three-leaf and one-heart stage were stressed by withholding watering for 21 days under controlled greenhouse conditions and then rewatered for 7 days.
Cloning and transformation of TaIAA15-1A
The leaves of “SuMai3” seedlings at the three-leaf and one-heart stage were harvested and used for total RNA extraction. The RNA was reverse-transcribed into cDNA for gene amplification. The full-length sequence of TaIAA15-1A (TraesCS1A02G118400) was obtained from Ensembl plants and used to design specific primers. Then, the open reading frame (ORF) of TaIAA15-1A was cloned into the pCambia1300-35S-GFP vector. The vector was transformed into Agrobacterium and then used for genetic transformation as previously described (Vogel et al. 2008).
Expression analysis of TaIAA15-1A in various stress treatments
The three-leaf wheat leaves were harvested at 0, 6, 12, 24, 48, and 72 h after 20% (w/v) PEG 6000 treatment and at 0, 3, 6, 12, and 24 h after 200 mM NaCl, 1.5 mM SA, 200 μM MeJA, 200 μM IAA, and 200 mM ABA treatment. Total RNA was extracted from all samples using TRIzol reagent (Transgen) and then reverse-transcribed into cDNA, which was used as a template for TaIAA15-1A gene expression analysis. The expression levels of TaIAA15-1A were determined by quantitative RT‒PCR using the Roche LightCycler ®480 system (Roche, Germany). The wheat 18S rRNA gene and Brachypodium distachyon BdUBC18 gene were used as the internal references. The relative expression levels of genes under different stress treatments were calculated using the 2–ΔΔCT method. The sequence details of the primers used for qRT‒PCR are listed in Table S1.
Transcriptional analysis by RNA-sequencing
Leaves of the WT plants “Bd21” and TaIAA15-1A overexpression transgenic lines were harvested from three-week-old plants, and the total RNA was extracted from each biological replicate using RNAprep Pure Plant Kit (TIANGEN). The RNA samples were send to Metware company (Wuhan) for construction of cDNA libraries and RNA sequencing. The RNA-seq was performed using an Illumina HiSeq™2000. Then, the clean reads of RNA-seq was mapped to the Brachypodium reference genome sequences (http://plants.ensembl.org/Brachypodium_distachyon/Info/Index). Subsequently, the transcripts were assembled, and the fragments per kilobase of exon per million fragments (FPKM) values were used to measure transcript abundance. Comparisons of TaIAA15-1A transgenic lines and WT plants were used to identify differentially expressed genes (DEGs). The DESseq2 package was used to perform the DEGs analyses as previously described (Varet et al. 2016). The DEGs were identified as significantly enriched or depleted under |log2Fold Change|> = 1 and false discovery rate (FDR) < 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment analysis (https://www.genome.jp/kegg/) were performed on the DEGs.
Exogenous ABA treatments
The sterilized seeds of WT and TaIAA15-1A overexpression lines (TaIAA15-OE-1, TaIAA15-OE-2 and TaIAA15-OE-3) were placed on damp filter paper containing water or 2.5 μM ABA solution. Then, these seeds were germinated for 5 days at 25 °C with a photoperiod of 16/8 h in a growth chamber. The seed germination rates of WT and TaIAA15-1A overexpression lines were counted and assayed with three biological replicates. The seeds of WT and TaIAA15-1A overexpression lines (TaIAA15-OE-1, TaIAA15-OE-2 and TaIAA15-OE-3) that were germinated on damp filter paper containing water were transplanted to 1/2 MS medium with 2.5 mM ABA. The shoot lengths of the WT and TaIAA15-1A overexpression lines were measured after 1 week. All measurements were made with three biological replicates.
Measurements of physiological parameters
The seedlings of WT and TaIAA15-1A overexpression lines (TaIAA15-OE-1, TaIAA15-OE-2 and TaIAA15-OE-3) under control and drought treatment were collected after 21 days and used to measure physiological parameters related to abiotic stress, including relative water content (RWC), malondialdehyde (MDA), soluble total sugars, proline content, O2−, H2O2 and antioxidant enzymes. The RWC was measured by the formula RWC = (FW − DW)/(TW − DW) × 100%, where FW, DW and TW represent fresh weight, dry weight and turgid fresh weight, respectively (Zhou et al. 2014). The MDA content was measured using the thiobarbituric acid method as previously described (Heath and Packer 1968). Soluble total sugar and proline contents were measured as previously described (Spiro 1966; Bates et al. 1973). O2− and H2O2 levels were visualized by nitroblue tetrazolium (NBT) and 3-diaminobenzidine (DAB) staining (Gay et al. 1999; Tian et al. 2013). The antioxidant enzyme activity (SOD, CAT, POD) was measured as described previously (Dhindsa et al. 1981; Aebi 1984; Chance and Maehly 1955).
Measurements of IAA and ABA contents
The leaves of WT and TaIAA15-1A overexpression lines (TaIAA15-OE-1, TaIAA15-OE-2 and TaIAA15-OE-3) under normal growth conditions were collected and used to analyse IAA and ABA contents as previously described (Xiao et al. 2018). The contents of IAA and ABA were measured through (ultra performance liquid chromatography, UPLC) (ExionLC™ AD, https://sciex.com.cn/) and (Tandem Mass Spectrometry, MS/MS) (QTRAP® 6500 + , https://sciex.com.cn/). All treatments were performed in triplicate.
Results
Expression patterns of TaIAA15-1A, -1B and -1D under various stress treatments
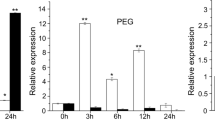
To investigate the expression patterns of TaIAA15-1A, -1B and -1D in response to stress-related signalling, we detected the expression levels of TaIAA15-1A, -1B and -1D under different stress treatments (PEG6000, NaCl, SA, MeJA, IAA, and ABA) through qRT‒PCR. The results showed that the expression levels of TaIAA15-1A and TaIAA15-1B were induced upon treatment with PEG6000 and NaCl, and the expression levels peaked at 6 h after PEG6000 treatment (Fig. 1A). NaCl stress induced TaIAA15-1A to a peak value at 24 h after treatment initiation (Fig. 1B). TaIAA15-1A expression levels were significantly higher than TaIAA15-1B, and TaIAA15-1D under PEG6000 and NaCl treatments. Then, we examined the expression levels of TaIAA15-1A, -1B and -1D under various plant hormone treatments (SA, MeJA, IAA, and ABA). As shown in Fig. 1C-F, we found that TaIAA15-1A expression was responsive to treatment with different plant hormones and exhibited approximately eightfold upregulation at 6 h after SA treatment (Fig. 1C). JA treatment induced TaIAA15-1A to a peak value at 24 h (Fig. 1D), and IAA stress induced the highest gene expression levels at 6 h after stress initiation (Fig. 1E). However, TaIAA15-1A expression remained relatively unchanged from 0 to 6 h after the initiation of ABA treatment but exhibited an approximately 1000-fold upregulation at 24 h. The expression levels of TaIAA15-1B and TaIAA15-1D were slightly altered under IAA and ABA treatments (Fig. 1F).
The expression analysis of TaIAA15-1A. TaIAA15-1A expression levels were measured with different stress treatments including 20% (w/v) PEG 6000, 200 mM NaCl, 1.5 mM SA, 100 μM MeJA, 100 μM IAA, and 100 μM ABA. The wheat gene 18SrRNA was used as an endogenous control. The relative expression levels of TaIAA15-1A was calculated using formula 2–ΔΔCT. Values are presented as mean ± SD
TaIAA15-1A enhanced drought tolerance in transgenic Brachypodium
To clarify the abiotic stress tolerance function of TaIAA15-1A in wheat, we generated transgenic TaIAA15-1A overexpression lines in the Brachypodium Bd21 background and genotyped three independent homozygous overexpression transgenic lines by monitoring GFP using fluorescence microscopy. In addition, we also confirmed the expression levels of TaIAA15-1A in three independent homozygous overexpression transgenic lines (approximately three‐fold increases in TaIAA15-1A expression were observed for each of three overexpression lines) (Fig. S1). Subsequently, we examined drought tolerance in TaIAA15-1A overexpression lines and the WT control. Under normal conditions, we did not observe significant phenotypic differences between the TaIAA15-1A overexpression lines and WT control. Under drought conditions, the TaIAA15-1A overexpression lines exhibited significantly higher tolerance to drought stress than WT plants after 21 days of drought treatment (Fig. 2A). We also found that the survival rate of WT plants was only 36%, whereas the survival rates of the three independent overexpression transgenic lines were 74, 73.9, and 74% (Fig. 2B).
TaIAA15-1A overexpression improved the drought tolerance. A The phenotype of TaIAA15-1A-overexpressing transgenic and WT plants (Brachypodium Bd21) under drought stress. The transgenic lines and WT plants were treated with drought stress for 21 days followed by 7 days of re-watering. B-D The survival rates, shoot length, and relative water content of TaIAA15-1A-overexpression seedings and WT plants under drought stress. Data are presented as mean ± SE of three biological replicates. * and ** indicate a significant difference compared with WT plants (*P < 0.05; **P < 0.01)
Then, we measured the shoot lengths and RWC between the overexpression transgenic lines and WT plants under normal and drought stress conditions. The results showed that no visible shoot length or RWC differences were observed between transgenic lines and WT plants under normal conditions, whereas transgenic lines exhibited longer shoot lengths and a higher RWC than WT plants under drought stress (Fig. 2C, D). To further explore the tolerance mechanism by which TaIAA15-1A contributes to drought stress, we compared physiological parameters between TaIAA15-1A transgenic lines and WT plants under normal and drought stress conditions. The results showed that transgenic lines had a lower MDA content (Fig. 3A). Taken together, these results suggested that TaIAA15-1A overexpression conferred drought tolerance in Brachypodium by influencing the levels of osmotic and oxidative stress tolerance-related metabolites.
Physiological and biochemical indices between WT plants and TaIAA15-1A transgenic lines. A MDA content in TaIAA15-1A-overexpression seedings and WT plants under drought stress. B-C The content of hydrogen peroxide (H2O2) and superoxide anion radical (O2.−). D–F The activity of SOD, POD, and CAT. Data are presented as mean ± SE of three biological replicates. * and ** indicate a significant difference compared with WT plants (*P < 0.05; **P < 0.01)
TaIAA15-1A overexpression influences ROS scavenging
The function of TaIAA15-1A in reducing ROS levels was examined in transgenic lines under drought treatment. First, we determined the levels of O2− and H2O2 for comparison between.
transgenic lines and WT plants. As shown in Fig. 3B, the accumulation of H2O2 was not significantly different between the transgenic lines and WT plants under normal conditions; however, the O2− contents in the transgenic lines were significantly lower than those in the WT plants (Fig. 3C). Under drought stress, we found that the levels of O2− and H2O2 in the transgenic lines were significantly lower than those in the WT plants (Fig. 3B, C). Next, the activities of some antioxidative enzymes, including SOD, POD, and CAT, were examined, and the results indicated that the SOD, POD, and CAT activities in the transgenic lines were higher than those in the WT plants (Fig. 3 D-F).
TaIAA15-1A improves drought tolerance by regulating phenolamides and antioxidant pathways
TO study genes potentially regulated by TaIAA15-1A, the transcriptome analysis was performed. The results showed that TaIAA15-1A overexpression modulated both phenolamides and antioxidant pathways. Based on the DEGs and KEGG pathways, we generated a network of signalling pathways involved in phenolamides and antioxidant pathways (Fig. 4). The results showed that some DEGs were enriched in phenolamides and antioxidant pathways, among which ornithine decarboxylase (ODC), pyrroline-5-carboxylate reductase (proC), prolyl 4-hydroxylase (P4HA), arginine decarboxylase (ARD), polyamine oxidase (PAO), glucose-6-phosphate 1-dehydrogenase (G6PD), gamma-glutamyl transpeptidase (GGT), ribonucleoside-diphosphate reductase subunit M1 (RRM1), galactose dehydrogenase (GalDH), and ascorbate oxidase (AOX) were upregulated in transgenic lines. In contrast, spermidine synthase (SPE), isocitrate dehydrogenase (ICD), glutathione peroxidase (GPX), aminopeptidase N (CD13), monodehydroascorbate reductase (NADH), (S)-2-hydroxy-acid oxidase (HAO), and catalase (CAT) were significantly downregulated in transgenic lines compared with WT plants (Fig. 4).
TaIAA15-1A overexpression altered expression of polyamine and antioxidant-related genes. The polyamine and antioxidant-related genes are list next to the arrow with green (down-regulated) and red (upregulated) color‐scale. ODC ornithine decarboxylase; proC pyrroline-5-carboxylate reductase; P4HA prolyl 4-hydroxylase; ARD arginine decarboxylase; SPE spermidine synthase; PAO, polyamine oxidase; G6PD, glucose-6-phosphate 1-dehydrogenase; ICD isocitrate dehydrogenase; GPX glutathione peroxidase; GST glutathione S-transferase; GGT gamma-glutamyl transpeptidase; CD13 aminopeptidase N; pepA leucyl aminopeptidase; RRM1, ribonucleoside-diphosphate reductase subunit M1; APX ascorbate peroxidase; VTC2 GDP-L-galactose phosphorylase; GalDH galactose dehydrogenase; AOX, ascorbate oxidase; NADH monodehydroascorbate reductase; HAO (S)-2-hydroxy-acid oxidase; CAT, catalase (color figure online)
TaIAA15-1A overexpression affects drought tolerance via the ABA signalling pathway
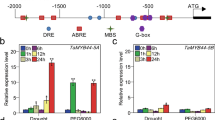
Previous transcriptome analysis showed that the ABA biosynthesis signalling pathway was influenced in TaIAA15-1A overexpression transgenic lines compared with WT plants. Based on the DEGs and KEGG pathways, we generated a network of signalling pathways involved in ABA biosynthesis (Fig. 5). The results showed that some DEGs were enriched in the ABA biosynthetic pathway, including 15-cis-phytoene synthase (ctrB), 15-cis-phytoene desaturase (PDS), zeta-carotene isomerase (Z-ISO), zeta-carotene desaturase (ZDS), prolycopene isomerase (ctrISO), lycopene beta-cyclase (LCYB), zeaxanthin epoxidase (ZEP), beta-ring hydroxylase (CYP97A3), violaxanthin de-epoxidase (VDE), 9-cis-epoxycarotenoid dioxygenase (NCED), xanthoxin dehydrogenase (ABA2), abscisic-aldehyde oxidase (AAO3), abscisic acid receptor PYR/PYL family (PYR/PYL), protein phosphatase 2C (PP2C), serine/threonine-protein kinase SRK2 (SnRK2), and ABA responsive element binding factor (ABF). qRT‒PCR analysis results supported the expression levels of genes detected from RNA-seq (Fig. S2).
Effect overview of TaIAA15-1A overexpression on ABA biosynthesis and signal transduction pathway in transgenic Brachypodium plants. The ABA-related genes are list next to the arrow with green (down-regulated) and red (upregulated) color‐scale. ctrB 15-cis-phytoene synthase; PDS 15-cis-phytoene desaturase; Z-ISO zeta-carotene isomerase; ZDS zeta-carotene desaturase; ctrISO prolycopene isomerase; LCYB lycopene beta-cyclase; ZEP zeaxanthin epoxidase; CYP97A3 beta-ring hydroxylase; VDE violaxanthin de-epoxidase; NCED 9-cis-epoxycarotenoid dioxygenase; ABA2 xanthoxin dehydrogenase; AAO3 abscisic-aldehyde oxidase; PYR/PYL abscisic acid receptor PYR/PYL family; PP2C protein phosphatase 2C; SnRK2 serine/threonine-protein kinase SRK2; ABF ABA responsive element binding factor (color figure online)
TaIAA15-1A affected seedling growth under ABA treatment
To further test the connection between TaIAA15-1A and the ABA signalling pathway, we treated TaIAA15-1A overexpression transgenic lines and WT plants with exogenous ABA solution and observed its effects on germination and shoot growth. The seeds of transgenic lines and WT plants were germinated in 1/2 MS medium containing 0 and 2.5 μM ABA. The results showed that the germination rates and shoot lengths of the transgenic lines and WT plants were similar in the absence of ABA treatment (Fig. 6). However, compared with WT plants, the germination rates and shoot lengths of the transgenic lines were significantly reduced under ABA treatment (Fig. 6B-D). The results suggested that TaIAA15-1A overexpression increased transgenic lines hypersensitive to ABA, compared with WT plants, indicating that TaIAA15-1A may positively regulate ABA signalling in Brachypodium.
ABA treatment influenced germination and growth of TaIAA15-1A overexpression transgenic lines and WT plants. A Seed germination phenotype of TaIAA15-1A overexpression transgenic lines and WT plants under normal conditions and ABA treatment. B Germination rates of TaIAA15-1A overexpression transgenic lines and WT plants under normal conditions and ABA treatment. C Growth performance of of TaIAA15-1A overexpression transgenic lines and WT plants under normal conditions and ABA treatment. D Statistical analysis of the shoot lengths and root lengths under normal conditions and ABA treatment. Values are presented as mean ± SD. (*P < 0.05; **P < 0.01, t test)
Subsequently, we measured the IAA and ABA contents in TaIAA15-1A overexpression lines and WT plants. The results showed that the IAA contents were decreased, and accumulation of high ABA contents was observed in the TaIAA15-1A overexpression lines compared with WT (Fig. S3). It is hypothesized that TaIAA15-1A overexpression enhanced drought tolerance of transgenic lines by regulating the crosstalk of ABA and IAA biosynthesis.
TaIAA15-1A overexpression alters the expression profiles of bZIP transcription factors
In plants, basic region/leucine zipper motif (bZIP) transcription factors have been reported to be involved in various processes, including flower development, tolerance against abiotic stress, and pathogen defence (Zulfiqar et al. 2016). As ABA signalling pathway genes, ABFs are a group of transcription factors that belong to the bZIP transcription factor family (Jakoby et al. 2002). In our study, we checked the expression levels of the bZIP gene family (35 members) in our RNA-seq data between TaIAA15-1A transgenic Brachypodium lines and WT plants. The results showed that all bZIP genes were DEGs (23 downregulated and 12 upregulated) (Fig. S4A). Seven bZIP genes were further selected to confirm the expression trends of RNA-seq by qRT-PCR, and the results supported the expression levels of genes detected from RNA-seq (Fig. S4B).
Discussion
Drought stress is a widespread and serious abiotic stress that causes yield and economic losses in wheat (Robles et al. 2018). At present, some studies have identified many drought tolerance genes, such as TaPYL1-1B, OsIAA18, OsIAA6, and OsIAA20 (Jung et al. 2015; Wang et al. 2021; Zhang et al. 2021; Mao et al. 2022). However, information regarding drought the tolerance mechanisms of Aux/IAA proteins is limited in wheat. Therefore, a functional study of wheat Aux/IAA genes involved in tolerance to abiotic stress is needed. In this study, we characterized the role of a wheat Aux/IAA gene, TaIAA15-1A, in response to drought stress, as this abiotic stress causes severe yield reduction. The results showed that TaIAA15-1A contributes positively to plant tolerance against drought stress by influencing ABA-related genes.
Beyond their role in plant development and growth, Aux/IAA proteins have been known to function in the plant response to abiotic stresses, and some studies have shown that overexpression of Aux/IAA proteins can improve plant resistance to abiotic stresses. For example, a previous study showed that OsIAA6 and OsIAA9 respond to drought tolerance (Jung et al. 2015). Overexpression of OsIAA20 and OsIAA18 has been shown to enhance the resistance of rice to drought and salt stresses (Wang et al. 2021; Zhang et al. 2021). In Arabidopsis, IAA5, IAA6, and IAA19 have also been reported to be involved in drought tolerance (Salehin et al. 2019). These studies suggest that Aux/IAA proteins can positively regulate plant resistance to various abiotic stresses; they are consistent with our findings in the present study, wherein we found that overexpression of a wheat Aux/IAA gene, TaIAA15-1A, improved the resistance of transgenic Brachypodium to drought stress.
Excessive ROS (particularly O2− and H2O2) accumulation accompanies abiotic stresses in plants, which results in lipid peroxidation and damage to cell membrane permeability and integrity (Baxter et al. 2013). Strong ROS scavenging ability plays an important role in improving resistance to abiotic stresses in plants. Previous studies have shown that auxin signalling and ROS might interact in plant responses to abiotic stress (Yuan et al. 2013). For example, overexpression of OsIAA18 enhanced rice tolerance to drought and salt stresses by improving antioxidant enzyme activities and reducing ROS accumulation (Wang et al. 2021). In our study, we found that TaIAA15-1A overexpression improved drought tolerance by improving antioxidant activity (SOD, CAT, and POD), thereby reducing ROS levels (H2O2 and O2−), which suggested that TaIAA15-1A regulates antioxidant-related gene expression, thereby enhancing drought tolerance. Future research will explore the tolerance mechanisms through which the interaction between auxin and ROS mediated by TaIAA15-1A regulates drought resistance in wheat.
In plants, the ABA signalling pathway has been reported to regulate multiple biological processes, including stress responses and plant growth and development (Wolters and Jürgens 2009). In Arabidopsis and rice, the positive function of ABA in altering plant resistance to abiotic stress has been recently described (Li et al. 2021; Zhang et al. 2020). For example, exogenous ABA treatments can enhance plant resistance to drought stress by improving antioxidative enzyme activities (Awan et al. 2021). In wheat, overexpression of the ABA receptor genes TaPYL4 and TaPYL1-1B confers wheat tolerance to drought stress and promotes grain production under drought conditions (Mega et al. 2019; Mao et al. 2022). The crosstalk between ABA and auxin has been reported to contribute to plant tolerance to abiotic stress. For example, the auxin biosynthesis gene AMIDASE 1 (AMI1) is known to function in coordinating the trade-off between stress responses and growth by balancing auxin with ABA homeostasis (Pérez-Alonso et al. 2021). OsIAA20 and OsIAA18 have been implicated in mediating drought stress tolerance through the ABA pathway (Wang et al. 2021; Zhang et al. 2021). In contrast, relatively little research has focused on the function of AUX/IAA genes in controlling drought resistance via the ABA pathway in wheat. In our study, we found that TaIAA15-1A was upregulated by exogenous ABA treatment and that TaIAA15-1A overexpression improved drought tolerance via the canonical ABA signalling pathway through PYR/PYL, PP2C, SnRK2, and ABFs (Fig. 5). Thus, the combined evidence from our results in concert with that from previous studies suggests that TaIAA15-1A might improve drought tolerance by activating the ABA signalling pathway. Further study is warranted to explore the regulatory tolerance mechanisms through which AUX/IAA regulates these genes in the ABA signalling pathway.
Conclusion
In this study, we characterized the function of the Aux/IAA gene TaIAA15-1A in response to drought stress in wheat. The results demonstrated that the overexpression of TaIAA15-1A significantly improved tolerance to drought stress in transgenic Brachypodium by activating the auxin signalling pathway, ABA signalling pathway, phenolamides and antioxidant pathways, resulting in a change in ABA-related gene expression and lower ROS accumulation. Our findings will deepen the understanding of drought tolerance mechanisms and have high application value in drought-tolerant wheat variety cultivation in the future.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Awan SA, Khan I, Rizwan M et al (2021) Exogenous abscisic acid and Jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol Plant 172(2):809–819
Bates LS, Waldren PR, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Baxter A, Mittler R, Suzuki NN (2013) ROS as key players in plant stress signaling. J Exp Bot 65:1229–1240
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chen D, Richardson T, Chai S et al (2016) Drought-up-regulated TaNAC69-1 is a transcriptional repressor of TaSHY2 and TaIAA7, and enhances root length and biomass in wheat. Plant Cell Physiol 57(10):2076–2090
Dhindsa RA, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 126:93–101
Du H, Liu H, Xiong L (2013) Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4:397
Gay C, Collins J, Gebicki JM (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273:149–155
He Y, Liu Y, Li M et al (2021) The Arabidopsis SMALL AUXIN UP RNA32 protein regulates ABA-mediated responses to drought stress. Front Plant Sci 12:625493
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Jakoby M, Weisshaar B, Dröge-Laser W et al (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7(3):106–111
Jung H, Lee DK, Choi YD et al (2015) OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci 236:304–312
Li X, Yu B, Wu Q et al (2021) OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet 17(8):e1009699
Liu J, Ma XL, Shang XW et al (2009) Regulation of exogenous auxin IAA on drought and salt stress during seedling stage of spring wheat (cv. Xihan No. 2). J Gansu Agric Univ 2(44):47–51
Mao H, Jian C, Cheng X et al (2022) The wheat ABA receptor gene TaPYL1-1B contributes to drought tolerance and grain yield by increasing water-use efficiency. Plant Biotechnol J 20(5):846–861
Maraschin Fdos S, Memelink J, Offringa R (2009) Auxin-induced, SCF(TIR1)-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J 59(1):100–109
Mega R, Abe F, Kim JS et al (2019) Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat Plants 5(2):153–159
Mutka AM, Fawley S, Tsao T et al (2013) Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J 74:746–754
Pérez-Alonso MM, Ortiz-García P, Moya-Cuevas J et al (2021) Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J Exp Bot 72(2):459–475
Robles P, Navarro-Cartagena S, Ferrández-Ayela A et al (2018) The characterization of arabidopsis mterf6 mutants reveals a new role for mterf6 in tolerance to abiotic stress. Int J Mol Sci 19(8):2388
Salehin M, Li B, Tang M et al (2019) Auxin-sensitive Aux/IAA proteins mediate drought tolerance in arabidopsis by regulating glucosinolate levels. Nat Commun 10(1):4021
Sallam A, Alqudah AM, Dawood MFA et al (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20(13):3137
Shi H, Chen L, Ye T et al (2014) Modulation of auxin content in arabidopsis confers improved drought stress resistance. Plant Physiol Biochem 82:209–217
Spiro RG (1966) Analysis of sugars found in glycoprotein. Method Enzymol 8:3–26
Tian F, Gong J, Zhang J et al (2013) Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot 64:1509–1520
Vain P, Worland B, Thole V et al (2008) Agrobacterium-mediated transformation of the temperate grass Brachypodium distachyon (genotype Bd21) for T-DNA insertional mutagenesis. Plant Biotechnol J 6(3):236–245
Varet H, Brillet-Guéguen L, Coppée JY et al (2016) SARTools: A DESeq2- and EdgeR-Based R pipeline for comprehensive differential analysis of RNA-Seq Data. PLoS ONE 11(6):0157022
Wang F, Niu H, Xin D et al (2021) OsIAA18, an Aux/IAA transcription factor gene, is involved in salt and drought tolerance in rice. Front Plant Sci 12:738660
Wolters H, Jürgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10(5):305–317
Xiao HM, Cai WJ, Ye TT, Ding J, Feng YQ (2018) Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal Chim Acta 1031:119–127
Yuan HM, Liu WC, Jin Y et al (2013) Role of ROS and auxin in plant response to metal-mediated stress. Plant Signal Behav 8(7):24671
Zhang N, Yin Y, Liu X et al (2017) The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant Physiol 175(4):1878–1892
Zhang H, Liu D, Yang B et al (2020) Arabidopsis CPK6 positively regulates ABA signaling and drought tolerance through phosphorylating ABA-responsive element-binding factors. J Exp Bot 71(1):188–203
Zhang A, Yang X, Lu J et al (2021) OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci 308:110903
Zhou SM, Sun XD, Yin SH et al (2014) The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol Biochem 84:213–223
Zulfiqar A, Samara SS, Ihsan K et al (2016) Functions of plant’s bzip transcription factors. Pak J Agric Sci 53:303–314
Acknowledgements
This work was funded by Natural Science Foundation of Shandong Province (ZR2022QC129); Doctoral research start-up funds, Liaocheng University (318052018); State Key Laboratory of Crop Biology, Shandong Agricultural University (2021KF03).
Author information
Authors and Affiliations
Contributions
PS and SG conceived and designed the experiments; PS performed most experiments; CS performed exogenous ABA treatments; YJL performed expression analysis. KW, HS, SHW, XQL performed physiological and biochemical indice measures; PS wrote and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Zheng-Yi Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


299_2022_2965_MOESM2_ESM.jpg
Supplementary Fig S2. The expression levels of ABA biosynthesis and signal transduction pathway genes in TaIAA15-1A transgenic lines and WT plants file2 (JPG 2078 KB)

299_2022_2965_MOESM3_ESM.jpg
Supplementary Fig. S3. Effect overview of TaIAA15-1A overexpression on bZIP transcription factors. (A) Heatmap of bZIP transcription factors based on RNA‐seq. (B) Expression levels of bZIP transcription factors in TaIAA15-1A transgenic lines and WT plants file3 (JPG 58 KB)

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, P., Sui, C., Li, J. et al. The Aux/IAA protein TaIAA15-1A confers drought tolerance in Brachypodium by regulating abscisic acid signal pathway. Plant Cell Rep 42, 385–394 (2023). https://doi.org/10.1007/s00299-022-02965-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-022-02965-9