Abstract
Main conclusion
Fructan accumulation and remobilization to grains under salinity can decrease dependency of the wheat tolerant cultivar on current photosynthesis and protect it from severe yield loss under salt stress.
Tolerance of plants to abiotic stresses can be enhanced by accumulation of soluble sugars, such as fructan. The current research sheds light on the role of stem fructan remobilization on yield of bread wheat under salt stress conditions. Fructan accumulation and remobilization as well as relative expression of the major genes of fructan metabolism were investigated in the penultimate internodes of ‘Bam’ as the salt-tolerant and ‘Ghods’ as the salt-sensitive wheat cultivars under salt-stressed and controlled conditions and their correlations were analyzed. More fructan production and higher efficiency of fructan remobilization was detected in Bam cultivar under salinity. Up-regulation of sucrose: sucrose 1-fructosyltransferase (1-SST) and sucrose: fructan 6-fructosyltransferase (6-SFT) (fructan biosynthesis genes) at anthesis and up-regulation of fructan exohydrolase (1-FEH) and vacuolar invertase (IVR) genes (contributed to fructan metabolism) during grain filling stage and higher expression of sucrose transporter gene (SUT1) in Bam was in accordance with its induced fructan accumulation and remobilization under salt stress. A significant correlation was observed between weight density, WSCs and gene expression changes under salt stress. Based on the these results, increased fructan production and induced stem reserves remobilization under salinity can decrease dependency of the wheat tolerant cultivar on current photosynthesis and protect it from severe yield loss under salt stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a major limiting factor for plant production worldwide. Plant growth responds to salinity in two phases: a rapid, osmotic phase and a slower, ionic phase (Munns and Tester 2008). Salt-tolerant varieties with higher grain yield in saline soils use diverse mechanisms to tolerate salinity. These genotypes perform osmotic adjustment (OA) by accumulating inorganic ions (K+, Na+ and Cl−) from the soil solution and/or organic solutes such as water soluble carbohydrates (WSCs) and amino acids to maintain continuous water absorption at low soil water potentials (Hasegawa et al. 2000). They effectively control the entrance of sodium into the roots by sodium exclusion or compartmentation in vacuoles to prevent injury of the photosystems (Munns and Tester 2008). Tolerant genotypes usually accumulate more WSCs than sensitive ones. WSCs have been introduced as markers for selection of tolerant genotypes under salt stress conditions (Kerepesi and Galiba 2000).
The increase in WSCs for OA and greater carbohydrates reserves in wheat stems have been well established in salt-tolerant varieties. WSCs such as fructan have been found to protect biological macromolecules against detrimental effects of salinity by membrane stabilization and as stress tolerance mediators (Livingston et al. 2009). The significant role of WSCs in tolerance to abiotic stresses through OA and their direct interactions with lipid bilayers have also been reported (Hincha et al. 2006). In addition, the solubility of fructans makes them natural accumulators to change environmental conditions by variation in the degree of polymerizations during OA (Pilon-Smits et al. 1995). They contribute in scavenging reactive oxygen species (ROS) in the vicinity of organellar membranes (Peshev et al. 2013). These reactions can help tolerant genotypes to maintain their photosynthesis under stress conditions.
Fructans form the major portion of WSCs and increase considerably under stress conditions in the vegetative parts of temperate grasses (Pollock 1986; Pollock And Cairns 1991; Blum 1998). They are biosynthesized by fructosyl transferases. Sucrose: sucrose 1-fructosyltransferase (1-SST) and sucrose: fructan 6-fructosyltransferase (6-SFT) play a key role in fructan biosynthesis in wheat and barley by formation of β(2-1) and β(2-6) linkages, respectively (Sprenger et al. 1995; Nagaraj et al. 2004). Up-regulation of 1-SST and 6-SFT by osmotic stress has been reported in wheat stems (Xue et al. 2008). Long-term storage of fructan occurs in stem internodes as major storage sites until approximately the mid-grain filling period (Schnyder 1993). The importance of the remobilization of these stem reserves is revealed under drought and heat stresses when the photosystems lose their efficiency during the grain filling period (Blum 1998).
When the demand for grain filling is high and sucrose is limited, fructan degrades to release more fructose and sucrose. The mobilization of stored carbohydrates requires the hydrolysis of fructan, which is catalyzed by fructan exohydrolases (FEHs), mainly fructan 1-exohydrolases (1-FEHs). Wheat FEHs and fructosyl transferases are presumably controlled at the transcriptional level (Van Laere and Van den Ende 2002; Zhang et al. 2009). Zhang et al. (2009) stated that 1-FEH w3 is up-regulated at around 20–25 days post anthesis in wheat stems under terminal drought. According to Zhang et al. (2015), the 1-FEH w3 gene was shown to be the major contributor in the stem fructan remobilization process and a cleaved amplified polymorphic marker of 1-FEH w3 has been introduced as a useful marker for the selection of high stem fructan remobilization in wheat breeding under terminal drought.
Vacuolar invertase (IVR) also appears to vary inversely with fructan content (Xue et al. 2008). Invertases play a major role in sucrose partitioning and long-distance transport by modulating the sucrose gradient between the phloem and unloading tissues, and in the control of the relative sink strength of plant tissues (Godt and Roitsch 1997; Roitsch 1999). This latter function could be essential for grain filling. Invertase regulation also occurs at both transcriptional and post-transcriptional levels (Trouverie et al. 2004).
To mediate carbon partitioning, plasma membrane sucrose transporters (SUTs) translocate sucrose from the source to sink tissues through the phloem (Lalonde et al. 2003). They are regulated at transcriptional, translational and post-translational levels (Shiratake 2007).
Photosynthesis capacity, carbon use efficiency and maintenance respiration make differences in the accumulation of WSCs in wheat stem among genotypes (Xue et al. 2008). In this way, tolerant wheat varieties can harmonize the relationship between CO2 assimilation (source) and grain yield (sink) under stress using carbon remobilization as a second source (Schnyder 1993; Blum 1998; Gebbing and Schnyder 1999). Different studies have shown that after anthesis, the hydrolysis of fructan to sucrose and fructose acts as a buffer to maintain a steady rate of grain filling, especially when current photosynthesis is seriously impaired under water stress.
A complex regulation of fructan metabolic genes and the overall sugar status of the plant have been observed under drought stress (Yang et al. 2004). An increase in WSCs remobilization during the grain filling period has been confirmed under drought stress (Ehdaie et al. 2006b), but no evidence of fructan remobilization has been proposed under salt stress. A better understanding of the physiological and molecular responses of tolerant versus sensitive varieties to salt stress would be helpful in designing strategies for development of salt-tolerant wheat genotypes in breeding programs. In the current research, the expression pattern of the key genes in fructan metabolism was evaluated at five time points post-anthesis in a salt-tolerant and a salt-sensitive cultivar. WSCs and fructan content, dry matter remobilization and its efficiency were measured in total stem as well as peduncle, penultimate and lower internodes. The impact of fructan accumulation and remobilization on yield maintenance under salt stress were investigated.
Materials and methods
Plant materials and experimental treatments
Seeds of wheat (Triticum aestivum L.) cultivars (Bam and Ghods) were obtained from the Seed and Plant Improvement Institute (SPII, Karaj, Iran) and landraces (No. 14 and No. 49) kindly received from Dr. B. Ehdaie (Department of Botany and Plant Sciences, University of California, Riverside, USA). Bam maintain higher yield compared to other varieties in low rainfall and salt-affected regions of Iran (~4.00 t ha−1). Ghods is considered to be salt-sensitive, but highly productive under well-watered conditions (~6.00 t ha−1) (Poustini and Siosemardeh 2004). The two landraces, No. 14 and No. 49, originating from southwestern and central eastern regions of Iran, respectively, differ significantly in remobilization capacity under terminal drought conditions (Bazargani et al. 2011). No. 14 and No. 49 are considered as semi-salt-tolerant and salt–tolerant, respectively, and have lower yields (2.00 t ha−1) in Iran (Ehdaie et al. 2006a; Sharbatkhari et al. 2013).
Stem reserve carbohydrates were examined under two experiments. Experiment 1 was carried out in 2011 and tested the Bam and Ghods cultivars and No. 14 and No. 49 landraces for stem weight changes post-anthesis and WSCs remobilization under different water conditions. In experiment 2 in 2012, Bam and Ghods were compared for fructan content and remobilization capacity at the molecular level under different salinity treatments. In both experiments, the plants were grown in the greenhouse facilities at the Agricultural Biotechnology Research Institute of Iran (ABRII, Karaj, Iran).
Seeds were surface sterilized by 0.5 % sodium hypochlorite (10 % commercial bleach) for 10 min and washed thoroughly three times. Seeds were soaked for 12 h and pre-germinated by incubation at room temperature (25 °C) for 3 days. Seven pre-germinated seeds were planted in each 3.5 L pots with drainage filled with potting mix. The potting mix was 2 parts silt–clay soil (experiment 1) and silt-loam soil (experiment 2), one part organic fertilizer, and one part river sand. The pots were irrigated with tap water until the third leaf emerged. The genotypes were then compared at two salinity levels (control EC water: 0.5 dS m−1, stress EC water: 12 dS m−1). The salt treatment was applied by irrigation with saline water; around 350 g NaCl was solved in 50 L tap water and EC was checked for 12 dS m−1 by EC meter. The control pots were kept well watered following the same regime as before until maturity. The treatments were replicated three times (eight pots for each replicate) in a completely randomized factorial design. The pots were translocated every other day to ensure that all plants received equal radiation. Greenhouse temperature was set at 20 and 25 °C for night and day, respectively. Relative humidity was also set at 65 ± 5 %.
At anthesis, when 50 % of the spikes had extruded anthers, the main stem of the plant was marked. Stem samples were collected from well-watered and stressed plants 5 times at intervals of 7 days (experiment 1) and 10 days (experiment 2) from anthesis and oven-dried at 72 °C for 48 h. Leaf samples were taken from the flag leaf at 8 weeks after salt treatment. In experiment 2, only penultimate internodes were collected placed in liquid N, and stored at −80 °C for RNA extraction. In all these sampling cases, three biological replicates were collected (each replicate pooled from at least 5 plants).
Physiological and agronomic traits measured in experiment 1 (2011)
To analyze the sodium and potassium content of the flag leaf, leaf samples were collected 8 weeks after salt treatment and dried at 70 °C for 48 h. Dried samples were ground and 100 mg of the ground leaves were digested in 5 mL 0.1 N nitric acid for 2 h at 95 °C, the tubes were incubated overnight at room temperature, and then filtered manually. Samples were diluted with 20 mL double-distilled water and filtered through Whatman paper No. 4, assayed for Na+, K+ content by flame photometry (Jenway PFP-7, Essex, UK) and reported as mg g−1 dry weight.
Stem WSCs content was assayed spectrophotometrically at 485 nm by phenol–sulfuric acid method. WSCs were extracted from 100 mg of oven-dried powdered stem (the leaf sheath was attached) with 10 mL of 80 % (v/v) ethanol at 80 °C followed by two extractions of the same volume of water at 60 °C. The water and ethanol extracts were evaporated at 50 °C to a solid which was re-dissolved in 1 mL distilled water. From each sample, 50 µL were used for evaluating the WSCs level in the combined extracts using phenol–sulfuric acid (Yemm and Willis 1954). WSCs remobilization was calculated by subtraction of maximum and minimum of WSCs content. In this method, WSCs used for respiration are also included (Zhang et al. 2014).
The main stem was divided into 3 segments: peduncle (first internode below the spike), penultimate (the internode below the peduncle), and the lower internodes. The length and weight of the oven-dried segments were measured. The weight density (linear density) was calculated as the ratio of the weight to the length of each internode. The remobilization was estimated as the difference between post-anthesis maximum and minimum weight densities. Mobilization efficiency of dry matter at each internode segment was estimated as the proportion (%) of mobilized dry matter to the maximum post-anthesis weight. The grain was harvested at physiological maturity and the yield was reported as g per plant. The contribution of remobilization to yield production was calculated as the proportion (%) of stem weight remobilization to grain weight per plant.
Physiological and agronomic traits measured in experiment 2 (2012)
Osmotic adjustment and sodium and potassium content were measured on the flag leaf. Canopy temperature (CT) measurements were taken under a clear sky at noon during the seed filling period using an infrared thermometer (IVN 770-P). The OA was determined 8 weeks after salt treatment as: OA = ΨFT (ww) – ΨFT (ss) where ΨFT (ww) is the osmotic potential at full turgor of the unstressed plants and ΨFT (ss) is the osmotic potential at full turgor of the salt-stressed plants (Blum 1989). Osmotic potential was measured by osmometer (Wescor C5022).
The fructan content was analyzed at 10 days intervals in the penultimate of the cultivars. After WSCs extraction and the water and ethanol evaporation at 50 °C, the pellet was re-dissolved in 1 mL distilled water, filtered and used for HPLC analysis. Fructose, glucose and sucrose content were determined by HPLC using a EURO Kat H column and RI detector, as described by Rahman (2008) with some modifications. Sucrose and fructan were hydrolyzed to glucose and fructose using 0.4 N perchloric acid at 60 °C for 1 h. The glucose and fructose were determined by the HPLC method. The fructan content was measured as: Fructose after hydrolysis − (fructose before hydrolysis + sucrose before hydrolysis) (Goggin and Setter 2004).
Aqueous solutions (1 to 16 mM) of commercially available carbohydrates (sucrose, glucose and fructose, Sigma) were used as references. All aforementioned agronomic traits were analyzed again in experiment 2.
RNA extraction and cDNA synthesis
Total RNA was extracted from the salt-stressed and control penultimate tissues of the wheat cultivars (Bam and Ghods) using TRIzol reagent (Life Technology, Invitrogen). Three biological replicates were examined. DNase treatments were done using RNase-free DNase I (Promega) to assure the removal of DNA. The cDNA strand was synthesized using iScript cDNA Synthesis Kit (Bio-Rad Laboratories Inc.).
Real-time PCR
The wheat sequence of 5 genes involved in fructan remobilization (1-SST, 6-SFT, 1-FEH w3, IVR and SUT1) and a housekeeping gene i.e. PHG as the internal control (Paolacci et al. 2009) were derived from NCBI (http://www.ncbi.nlm.nih.gov/). The specific primer pairs were designed using Oligo ver. 5.1 software (Table 1). It is noteworthy that due to the high sequence similarity of SUT1 A, SUT1 B and SUT1 D genes (96 % homology) with the same functions, it was not possible to design specific primers for each one, so that the designed primer pair could amplify all the transcripts (Table S1; Fig. S1). Primer amplification efficiency for all the genes investigated in the present study was around 100 % (100 ± 5). The quantitative PCR was performed in an iCycler iQ thermocycler (Bio-Rad) using the iQ Syber Green Supermix kit (Bio-Rad). Transcript levels of the genes were calculated by 2−∆∆CT (Pfaffl 2001) and reported relative to the expression of normal treatment of Bam.
Statistical analysis
Analysis of variance was performed for each character and calculated for each year. The data was analyzed using the SAS (version 9.0) statistical package. Mean comparisons were done using the least significant difference (LSD) test (P < 0.05). Associations between characters were examined by Pearson’s correlation test.
Results
Genotypic variation in fructan content, remobilization and efficiency of remobilization
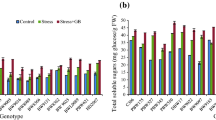
Stem reserve carbohydrates were studied in two experiments. Experiment 1 was a preliminary experiment using cultivars Bam and Ghods and landraces No. 14 and No. 49 with significant differences in remobilization capacity under terminal drought (Bazargani et al. 2011). Bam and No. 49 are salt-tolerant genotypes and had lower Na+/K+ ratio compared to Ghods and No. 14 under salinity (Table S2). The WSCs content was significantly increased under salinity in all genotypes except for Ghods (Table S2).
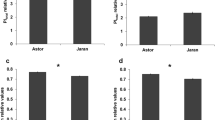
The highest grain yield, remobilization (based on weight density) and contribution of remobilization to yield under salinity stress were recorded for Bam, while Ghods showed the greatest yield loss (88 %). Additional data are given in Suppl. Table S2. The highest remobilization efficiency among the internodes was detected in the penultimate (32 %).
After anthesis, the genotypes responded differently according to their stem reserve carbohydrates and WSC remobilization (Table S2). The contribution of stem reserves to grain yield was higher under salt stress (Table S2).
A comparison of WSCs content, WSCs remobilization and the efficiency of remobilization showed that Bam had the highest values for these traits. WSCs remobilization strongly increased under salinity in Bam, but no significant difference was observed for the other genotypes. Additional data are given in Suppl. Table S2. These results indicated that Bam and Ghods are contrasting genotypes for these traits. Hence, they were selected for more detailed and precise analyses.
Physiological features of the tolerant genotype under salinity
Based on the results in experiment 2, there was little change in canopy temperature (CT) in Bam under salt stress (~0.5 °C), while it significantly increased in Ghods (~3 °C) in comparison with the well-watered plants. Bam had better water uptake under salt stress and its CT was about 2 °C lower than that of Ghods under salinity (Table 2).
Salt stress had significant effects on sodium and potassium uptake and their ratios. Ghods showed higher concentrations of sodium and higher Na+/K+ in its flag leaf than Bam under salt stress. This indicates that Bam, as a salt-tolerant cultivar, could control intake of sodium as a destructive ion into the main photosynthetic part of the shoot. In salt stressed plants, Na+/K+ increases mainly due to higher absorption rate of Na+, affecting several aspects of plant metabolism (Zhu 2002).
The results in experiment 2 revealed that remobilization and its efficiency based on the weight density, and grain yield were significantly affected by salt stress. A great yield loss was observed for Ghods (83 %) while it was almost half for Bam (47 %) (Table 2).
Stem reserve remobilization on the basis of weight density was induced in Bam under salinity and the efficiency of remobilization for Bam was significantly higher compared to Ghods. The maximum efficiency of remobilization was observed in the penultimate internode in both cultivars (Fig. 1), which is in concomitant to the previous studies showing the penultimate internode had the highest fructan content and remobilization efficiency in wheat and barley (Wardlaw and Willenbrink 1994; Blum 1998).
Salt stress-induced OA in the tolerant genotype
OA, as a leaf water status parameter, was measured at early anthesis by calculating the difference between the osmotic potential of the normal and salt-stressed tissues under saturated status. No significant differences in ΨFT were observed between cultivars under control treatment. While it was decreased under salt stress conditions for both cultivars and the decrease was greater in Bam compared to Ghods (Fig. 2). It appears that the osmolyte concentration was increased for Bam under salinity. The greater difference between ΨFTww and ΨFTss in Bam resulted in greater OA, which may play a significant role in its adaptation to stress conditions (Fig. 3).
Fructan content and remobilization under salinity in the tolerant vs sensitive genotype
Under salinity, the fructan content was increased in Bam while it was decreased in Ghods (Fig. 4). This increase probably occurred during vegetative growth under salt stress. In the well-watered treatment of Ghods, the drop in fructan concentration had been already started at least 10 days sooner than Bam. This indicates that Ghods depended on its fructan reserve during the reproductive stage (Fig. 4).
The post-anthesis changes in fructan content in the penultimate were compared between the genotypes to examine their capacity for fructan remobilization. Under salt stress, the maximum fructan content at anthesis was significantly higher in Bam (106 mg) compared to Ghods (44 mg). In addition, at the end of the grain filling period, the lowest fructan content was recorded for Bam (Fig. 4). Genotypic differences in the rate of the fructan degradation has been reported during grain filling period (Zhang et al. 2015).
Salinity had a significant effect on fructan remobilization and its efficiency. The mean comparison showed that Bam experienced greater fructan remobilization under stress compared to Ghods. Interestingly, Bam produced more fructan and remobilized it more efficiently during grain filling under salinity. Although Ghods produced abundant fructan under the well-watered condition, salinity restricted fructan production in this cultivar. Grain yield was correlated with remobilization on the basis of penultimate weight density (correlation coefficient: 0.96, P < 0.05) and fructan remobilization (correlation coefficient: 0.95, P < 0.05) under salt stress.
Expression pattern of fructan related genes under salt stress
The expression of five key genes involved in fructan biosynthesis and remobilization were analyzed at 10 days intervals in the penultimate. These genes were expressed differently at the transcriptional level both within intervals and between varieties in different treatments.
Major genes in fructan biosynthesis, 1-SST and 6-SFT, were differentially expressed under salt stress. The expression peak of these genes was observed at anthesis; then the expression decreased to nearly undetectable level at the middle of the grain filling period, around 20 day post-anthesis (DPA). A remarkable result was the up-regulation of 1-SST and 6-SFT in Bam and simultaneous down-regulation of them in Ghods under salt stress (Fig. 5).
The SUT1 transcript was detectable at anthesis in the penultimate internode and reached its maximum level around 10 DPA. Then it was declined to undetectable level at 40 DPA in the well-watered treatments but was still present at very low levels in the salt-stressed plants (Fig. 5). Although the trend of SUT1 expression in all treatments was similar and no significant change was observed between conditions in each cultivar, the expression levels of SUT1 was greater in Bam for both treatments compared to Ghods.
The expression of 1-FEH was up-regulated by salt stress in Bam, while it remained constant in Ghods. It was increased sharply in Bam at both the salt-stressed and well-watered conditions since 10 DPA, reached to the maximum around 20 DPA and then decreased (Fig. 5).
Under salt stress, the transcript level of IVR was raised sharply at 30 DPA in Bam, while it was slightly increased between 10 to 20 DPA and remained constant at 30 DPA in Ghods. It remained nearly unaffected during post-anthesis under the well-watered conditions in Bam and Ghods (Fig. 5).
Correlation of the studied traits under salinity
A significant correlation was observed between fructan content, weight density and the genes expressions in the penultimate under salt stress. The maximum fructan content and the highest expression of 6-SFT were observed at anthesis with a decreasing trend thereafter. A strong positive correlation was also observed between SUT1 expression, sugar content, weight and weight density under salinity (Table 3).
The fructan content in penultimate of Bam and Ghods was decreased at 10 to 20 DPA, as the expression of 1-FEH was increased. There was also a negative correlation between IVR expression and sucrose under salinity (Table 3).
Discussion
Genotypic variation in response to salt stress
Bam and Ghods cultivars provided useful comparisons because they appeared to respond differently to salt stress. Canopy temperature was controlled in Bam which might be partly due to efficient OA under salinity, while it significantly increased in Ghods which appeared to have weak OA mechanism. Considering more efficient OA in the tolerant genotype, Bam might result in more favorable leaf water content, which causes a more open stomata and sustained transpirational cooling under salinity. Better water uptake helps plants to continue CO2 influx towards the chloroplasts with greater photosynthesis that supplies the necessary photosynthates before destruction of the photosynthetic apparatus by the high concentration of sodium in salt-tolerant cultivars (Silva et al. 2007; Farouk 2011). The accumulation of excess in Na+ ions may decrease the stability of the photosystem II function (Watson et al. 2001). The salt-tolerant Bam cultivar avoided this harmful effect by maintaining lower leaf Na+ content and higher K+ vs Na+ by selective ion transport from soil to leaf. This could play a role in greater storage of carbohydrates than for salt-sensitive Ghods by longer photosynthesis. The inability of a salt-sensitive plant to prevent salt from reaching toxic levels in the transpiring leaves disrupts photosynthesis earlier and as a result produces lower stem reserves. The grain yield loss in Ghods, with its low capacity for remobilization, was more severe than for Bam with its high stem reserve remobilization at 12 dS m−1 salinity. It can imply that higher remobilization of the stem reserves helps the plant to fill the grains under stress conditions (Yang et al. 2004). It ultimately causes a better yield maintenance in salt-tolerant cultivars, although salt stress significantly decreased the number of spikelets per spike and total straw yield for both sensitive and tolerant cultivars (Maas et al. 1996; Poustini and Siosemardeh 2004).
Production and remobilization of fructan and their possible role in salt tolerance
Based on the results (in both the experiments 1 and 2), Bam, with great stem reserve remobilization compared to Ghods, had significantly higher grain yield under salinity. Moreover, the results in experiment 2 revealed that the fructan content in the penultimate was significantly increased in Bam under salinity, while it was decreased in Ghods. Salt-induced fructan synthesis has been previously shown in wheat seedlings (Kerepesi and Galiba 2000). Valluru and Van den Ende (2008) also reported that long-term stress increases soluble sugar concentrations and decreases starch. This could be a response to osmotic stress in a salt-tolerant cultivar (Livingston et al. 2009). In addition to the role of fructan as an osmolyte during OA (Livingston et al. 2009), it acts as a major carbohydrate reserve in about 15 % of flowering plant species (Valluru and Van den Ende 2008). Goggin and Setter (2004) found that fructan as a storage carbohydrate accounts for 70 % or more of grain dry matter under drought stress. According to their results, around anthesis, about 31 % of accumulated fructan in the stems of the rainfed plants was located in the penultimate internode.
The induced production of fructan (around anthesis or during vegetative growth) followed by its efficient remobilization (during grain filling) under salinity in Bam are two critical factors to the higher grain yield in this cultivar. A positive correlation between osmotic pressure, fructan content and fructan remobilization under salt stress implied that Bam utilized both mechanisms of OA via production of osmolytes like fructan and stem reserve remobilization. This diminished its yield dependency to current photosynthesis and prevented severe yield loss under salinity. There is some evidence that osmotic adjustment facilitates translocation of carbohydrates reserves during grain filling periods (Subbarao et al. 2000). It shows that fructans can play a dual role under salt stress for both OA with protective characteristics and as a main storage of polysaccharides in wheat stem to be used in grain filling. Suppression of fructan production in Ghods during salinity could be a result of its inferior ability for water uptake through OA, the accumulation of sodium in the flag leaf, and decreased capacity for photosynthesis and sucrose production. High fructan remobilization in Ghods in the well-watered treatment can imply the dependency of its grain filling on fructan reserves during the reproductive stage, whereas salt stress by restricting fructan production decreased the stem reserves and their remobilization. The other way round, the increased stem reserve and remobilization in Bam under salt stress, could to some extent compensate for the destructive effects of salinity on photosynthesis, and prevent severe yield loss in this cultivar (Fig. 6). According to Ma et al. (2014) dry matter reserved in the wheat stem contributed about 40 % to the grain yield under severe water stress and lost yields were partly compensated by dry matter remobilization.
A schematic salt-tolerant wheat genotype under salinity. Increased fructan production and remobilization under salinity can protect it from severe yield loss under the stress conditions. Photoshop was used to create the artwork. OA osmotic adjustment, RWC relative water content, WSCs water soluble carbohydrates
Correlation of fructan content and expression of the related genes under salinity
A significant correlation between fructan content and expression levels of rate-limiting genes in the biochemical pathways of fructan metabolism was observed. Based on the results, the transcript levels of the evaluated five key genes in fructan metabolism were different under salt stress in the two genotypes responded differently to salt stress in various genotypes. Up-regulation of 1-SST and 6-SFT along with fructan accumulation were observed in Bam under salinity, at anthesis, whereas down-regulation of 1-SST and 6-SFT in conjunction with fructan depletion were observed in Ghods. Several reports state that these genes are critical for fructan biosynthesis because they are soluble linear or branched polymers of fructose that produce graminan-type fructan and cooperate with 1-FFT gene in wheat (Van den Ende et al. 2003; Livingston et al. 2009; Huynh et al. 2012). Up-regulation of 1-SST and 6-SFT has been reported under drought stress and positively correlated with the total stem WSCs and fructan content (Xue et al. 2008). Also under cold stress, the levels of 1-SST and 6-SFT transcripts were increased in snow mold-resistant cultivars which accumulated more fructan than other cultivars (Kawakami and Yoshida 2002). Fructan accumulation in plants that normally do not produce fructan could also protect them from water stress (Cairns 2003). The fructan-producing transgenic tobacco lines showed high tolerance to drought, salinity and low temperature stresses (Pilon-Smits et al. 1995). Different combinations of 1-SST, 6-SFT and 1-FFT genes for fructan production in transgenic tobacco plants had a significant influence on tolerance to abiotic stresses. The combination of 1-SST and 6-SFT produced the highest fructan or soluble sugar content and the strongest salt tolerance (Bie et al. 2012). It can be assumed that there is a good potential for manipulation of these major genes in wheat so that they might raise the power of fructan production and remobilization during the grain filling period under salinity.
Salt stress up-regulated the expression of 1-FEH and IVR during the grain filling stage compared to the well-watered plants in Bam, whereas they remained constant in Ghods. There are several lines of evidence that remobilization of stored carbohydrates in wheat stems correlates with up-regulation of 1-FEH and depends on the hydrolysis of fructan by fructan exohydrolases which is an important contributor to grain filling under drought stress (Goggin and Setter 2004; Van den Ende et al. 2004; Xue et al. 2008; Joudi et al. 2012). Therefore, it is reasonable to assume that higher expression of 1-FEH during the fructan break down phase in Bam would be significant for maintaining the flow of carbon required for grain filling under salt stress (Fig. 6). According to Zhang et al. (2015) a cleaved amplified polymorphic marker of 1-FEH w3 has been introduced as a useful marker for the selection for high stem WSCs remobilization and high thousand grain weight in wheat breeding under terminal drought. The results showed that both aspects of fructan biosynthesis and fructan degradation can be induced under salt stress and contributed in remobilization efficiency.
Upper expression of SUT1 in Bam and its positive correlation with fructan content and stem weight during grain filling implied that a rapid sucrose export from the internodes to the grains has been happened. It was shown that SUT1 expression closely parallels grain growth.
Conclusion
Unavailability of enough carbohydrates resources for grain filling is the main limiting factor for yield maintenance under saline conditions. Preservation of current photosynthesis and/or stem reserves remobilization at the reproductive stage can be considered as the main solutions to this problem.
Under salt stress, stomatal hydraulic conductance, chlorophyll and leaf relative water content is decreased (Table S3) and subsequently canopy temperature is increased in salt-sensitive wheat cultivar. The photosynthetic apparatus and membrane structures are probably damaged due to sodium accumulation and increase in the sodium to potassium ratio. However, in a salt-tolerant genotype, a rise in osmolytes such as fructan (owing to up-regulation of 1-SST and 6-SFT) may lead to efficient OA which in turn result in maintenance of the water content and stomatal conductance, thus affording preservation of current photosynthesis and carbohydrates production to some extent. Such a plant could contain considerable stem reserves of carbohydrates. During grain filling, after long-term salinity (when the leaves already changed color to yellow), plants with high potential of stem reserve remobilization (through up-regulation of 1- FEH and IVR) can take advantage and use this capability to reduce the negative impact of salinity on grain yield (Fig. 6). Higher expression of SUT1 in salt-tolerant genotypes can also play an important role in higher sucrose transport and grain growth.
The current study confirmed that fructan remobilization might play an important role in grain filling under salt stress. Also, the close relation between fructan accumulation for OA and fructan remobilization, before and during the grain filling period, is proposed under salinity. Remobilization of fructans possibly over-produced during OA can save energy in plants under stress, although energy is needed for synthesis or transport of extra solutes during OA (Munns 2002). Based on the achieved results, genotypes with high potential for fructan production and remobilization under salt stress provide proper materials for salt tolerance breeding in wheat and so screening for these traits is suggested.
Most studies of salt tolerance have concentrated on the mechanisms that control the osmotic or ionic effects of salt stress. The present study examined the mechanism of sugar remobilization used by salt-tolerant genotypes to fill the grains and compared them to sensitive genotypes. It is suggested that the capability of fructan production and the remobilization would be effective contributors to salt-stress tolerance protecting the plants from severe yield loss under salinity.
Author contribution statement
MS, ZS and SG conceived and designed research. MS and ZS conducted experiments. BN contributed new reagents or analytical tools. MS and ZS analyzed data and wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- DPA:
-
Day post anthesis
- FEH:
-
Fructan exohydrolase
- IVR:
-
Vacuolar invertase
- OA:
-
Osmotic adjustment
- 6-SFT:
-
Sucrose: fructan 6-fructosyltransferase
- 1-SST:
-
Sucrose: sucrose 1-fructosyltransferase
- SUT:
-
Sucrose transporter
- WSCs:
-
Water soluble carbohydrates
References
Bazargani MM, Sarhadi E, Bushehri AAS, Matros A, Mock HP, Naghavi MR, Hajihoseini V, Mardi M, Hajirezaei M, Moradi F (2011) A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J Proteomics 74:1959–1973
Bie X, Wang K, She M, Du L, Zhang S, Li J, Gao X, Lin Z, Ye X (2012) Combinational transformation of three wheat genes encoding fructan biosynthesis enzymes confers increased fructan content and tolerance to abiotic stresses in tobacco. Plant Cell Rep 31:2229–2238
Blum A (1989) Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci 29:230–233
Blum A (1998) Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 100:77–83
Cairns AJ (2003) Fructan biosynthesis in transgenic plants. J Exp Bot 54:549–567
Ehdaie B, Alloush GA, Madore MA, Waines JG (2006a) Genotypic variation for stem reserves and mobilization in wheat: I. Postanthesis changes in internode dry matter. Crop Sci 46:735–746. doi:10.2135/cropsci2005.04-0033
Ehdaie B, Alloush GA, Madore MA, Waines JG (2006b) Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Sci 46:2093–2103
Farouk S (2011) Osmotic adjustment in wheat flag leaf in relation to flag leaf area and grain yield per plant. J Stress Physiol Biochem 7:117–138
Gebbing T, Schnyder S (1999) Pre-anthesis reserve utilization for protein and carbohydrate synthesis in grains of wheat. Plant Physiol 121:871–878
Godt DE, Roitsch T (1997) Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggest an important function in establishing and maintaining sink metabolism. Plant Physiol 115:273–282
Goggin DE, Setter TL (2004) Fructosyltransferase activity and fructan accumulation during development in wheat exposed to terminal drought. Funct Plant Biol 31:11–21
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499. doi:10.1146/annurev.arplant.51.1.463
Hincha DK, Popova AV, Cacela C (2006) Effects of sugars on the stability of lipid membranes during drying. In: Leitmannova Liu A (ed) Advances in planar lipid bilayers and liposomes, vol 3. Elsevier, Amsterdam, pp 189–217
Huynh BL, Mather DE, Schreiber AW, Toubia J, Baumann U, Shoaei Z, Stein N, Ariyadasa R, Stangoulis JC, Edwards J, Shirley N, Langridge P, Fleury D (2012) Clusters of genes encoding fructan biosynthesizing enzymes in wheat and barley. Plant Mol Biol 80:299–314
Joudi M, Ahmadi A, Mohamadi V, Abbasi A, Vergauwen R, Mohammadi H, Van den Ende W (2012) Comparison of fructan dynamics in two wheat cultivars with different capacities of accumulation and remobilization under drought stress. Physiol Plant 144:1–12. doi:10.1111/j.1399-3054.2011.01517.x
Kawakami A, Yoshida M (2002) Molecular characterization of sucrose:sucrose 1-fructosyltransferase and sucrose:fructan 6-fructosyltransferase associated with fructan accumulation in winter wheat during cold hardening. Biosci Biotech Biochem 66:2297–2305
Kerepesi I, Galiba G (2000) Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci 40:482–487
Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26:37–56
Livingston DP, Hincha DK, Heyer AG (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66:2007–2023
Ma J, Huang GB, Yang DL, Chai Q (2014) Dry matter remobilization and compensatory effects in various internodes of spring wheat under water stress. Crop Sci 54(1):331–339
Maas EV, Lesch SM, Francois LE, Grieve CM (1996) Contribution of individual culms to yield of salt-stressed wheat. Crop Sci 36:142–149
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nagaraj VJ, Altenbach D, Galati VL, Uscher M, Meyer AD, Boller T, Wiemken A (2004) Distinct regulation of sucrose: sucrose-1-fructosyltransferase (1-SST) and sucrose: fructan 6-fructosyltransferase (6-SFT), the key enzymes of fructan synthesis in barley leaves: 1-SST as the pacemaker. New Phytol 161:735–748
Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10:11. doi:10.1186/1471-2199-10-11
Peshev D, Vergauwen R, Moglia A, Hideg E, Van den Ende W (2013) Towards understanding vacuolar antioxidant mechanisms: a role for fructans? J Exp Bot 64:1025–1038
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Pilon-Smits EAH, Ebskamp MJM, Paul MJ, Jeuken MJW, Weis-beek PJ, Smeekens SCM (1995) Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol 107:125–130
Pollock CJ (1986) Fructans and the metabolism of sucrose in vascular plants. New Phytol 104:1–24
Pollock C, Cairns AJ (1991) Fructan metabolism in grasses and cereals. Annu Rev Plant Physiol Plant Mol Biol 42:77–101
Poustini K, Siosemardeh A (2004) Ion distribution in wheat cultivars in response to salinity stress. Field Crop Res 85:125–133
Rahman NA (2008) Determination of glucose and fructose from glucose isomerization process by high-performance liquid chromatography with UV detection. Mod Appl Sci 2:151–154
Roitsch T (1999) Source–sink regulation by sugar and stress. Curr Opin Plant Biol 2:198–206
Schnyder H (1993) The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling—a review. New Phytol 123:233–245
Sharbatkhari M, Galeshi S, Shobbar ZS, Nakhoda B, Shahbazi M (2013) Assessment of agro-physiological traits for salt tolerance in drought-tolerant wheat genotypes. Int J Plant Prod 7:437–454
Shiratake K (2007) Genetics of sucrose transporter in plants. Genes Genomes Genomics 1:73–80
Silva MA, Jifon JL, Da Silva JA, Sharma G (2007) Use of physiology parameters as fast tools to screen for drought tolerance in sugarcane. Braz J Plant Physiol 19:193–201
Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A (1995) Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA 92:11652–11656
Subbarao GV, Nam NH, Chauhan YS, Johanson C (2000) Osmotic adjustment, water relations and carbohydrate remobilization in pigeonpea under water deficits. J Plant Physiol 157:651–659
Trouverie J, Chateau-Joubert S, Thevenot C, Jacquemot MP, Prioul JL (2004) Regulation of vacuolar invertase by abscisic acid or glucose in leaves and roots from maize plantlets. Planta 219:894–905
Valluru R, Van den Ende W (2008) Plant fructans in stress environments: emerging concepts and future prospects. J Exp Bot 59:2905–2916. doi:10.1093/jxb/ern164
Van den Ende W, Clerens S, Vergauwen R, Van Riet L, Van Laere A, Yoshida M, Kawakami A (2003) Fructan 1-exohydrolases. Beta-(2,1)-trimmers during graminan biosynthesis in stems of wheat? Purification, characterization, mass mapping, and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 131:621–631
Van den Ende W, De Coninck B, Van Laere A (2004) Plant fructan exohydrolase: a role in signaling and defense? Trends Plant Sci 9:523–528
Van Laere A, Van den Ende W (2002) Inulin metabolism in dicots: chicory as a model system. Plant Cell Environ 25:803–815
Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Funct Plant Biol 21:255–271
Watson R, Pritchard J, Malone M (2001) Direct measurement of sodium and potassium in the transpiration stream of saltexcluding and non-excluding varieties of wheat. J Exp Bot 52:1873–1881
Xue GP, McIntyre CL, Jenkins CLD, Glassop D, Herwaarden AF, Shorter R (2008) Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat. Plant Physiol 146:441–454
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2004) Activities of fructan- and sucrose-metabolizing enzymes in wheat stems subjected to water stress during grain filling. Planta 220:331–343. doi:10.1007/s00425-004-1338-y
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Zhang J, Dell B, Conocono E, Waters I, Setter T, Appels R (2009) Water deficits in wheat: fructan exohydrolase (1-FEH) mRNA expression and relationship to soluble carbohydrate concentrations in two varieties. New Phytol 181:843–850
Zhang B, Li W, Chang X, Li R, Jing R (2014) Effects of favorable alleles for water-soluble carbohydrates at grain filling on grain weight under drought and heat stresses in wheat. PLoS One 9:1–12
Zhang J, Xu Y, Chen W, Dell B, Vergauwen R, Biddulph B, Khan N, Luo H, Appels R, Van den Ende W (2015) A wheat 1-FEH w3 variant underlies enzyme activity for stem WSC remobilization to grain under drought. New Phytol 205:293–305
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgments
This work was supported by Agricultural Biotechnology Research Institute of Iran (ABRII, Grant Number 7-05-05-90118). The authors are grateful to Dr. Sinem Bezircilioglu who kindly reviewed the manuscript in terms of English writing.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharbatkhari, M., Shobbar, ZS., Galeshi, S. et al. Wheat stem reserves and salinity tolerance: molecular dissection of fructan biosynthesis and remobilization to grains. Planta 244, 191–202 (2016). https://doi.org/10.1007/s00425-016-2497-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2497-3