Abstract
Within plant systems, two main forms of programmed cell death (PCD) exist: developmentally regulated and environmentally induced. The lace plant (Aponogeton madagascariensis) naturally undergoes developmentally regulated PCD to form perforations between longitudinal and transverse veins over its leaf surface. Developmental PCD in the lace plant has been well characterized; however, environmental PCD has never before been studied in this plant species. The results presented here portray heat shock (HS) treatment at 55°C for 20 min as a promising inducer of environmental PCD within lace plant protoplasts originally isolated from non-PCD areas of the plant. HS treatment produces cells displaying many characteristics of developmental PCD, including blebbing of the plasma membrane, increased number of hydrolytic vesicles and transvacuolar strands, nuclear condensation, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling positive nuclei, as well as increased Brownian motion within the vacuole. Results presented here for the first time provide evidence of chloroplasts in the vacuole of living protoplasts undergoing environmentally induced PCD. Findings suggest that the mitochondria play a critical role in the cell death process. Changes in mitochondrial dynamics were visualized in HS-treated cells, including loss of mitochondrial mobility, reduction in ΔΨm, as well as the proximal association with chloroplasts. The role of the mitochondrial permeability transition pore (PTP) was examined by pre-treatment with the PTP agonist cyclosporine A. Overall, HS is depicted as a reliable method to induce PCD within lace plant protoplasts, and proves to be a reliable technique to enable comparisons between environmentally induced and developmentally regulated PCD within one species of plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed cell death (PCD)
Programmed cell death is the regulated death of cells within an organism, and is ubiquitous in all eukaryotes (Lam and del Pozo 2000). PCD in plants can be broadly characterized as either environmentally induced or developmentally regulated (Gunawardena 2008). Environmentally induced PCD is an outcome of abiotic or biotic factors and includes examples such as heat shock (HS; McCabe et al. 1997; McCabe and Leaver 2000; Balk et al. 2003; Vacca et al. 2004, 2006; Scott and Logan 2008; Zhang et al. 2009), UV radiation (Danon and Gallois 1998; He et al. 2008), low oxygen treatment inducing the formation of lysigenous aerenchyma (Gunawardena et al. 2001), the use of compounds and chemical elements, including aluminum chloride (AlCl3) and cadmium (Cd; Yakimova et al. 2007, 2008), as well as pathogen infection (Mittler and Lam 1997). The initial four examples fit into the abiotic-induced category, while pathogen infection is caused by biotic factors. Within an experimental setting, environmentally induced PCD has been demonstrated in whole tissues as well as in protoplasts (single cells lacking the cell wall; McCabe and Leaver 2000; Reape and McCabe 2008). Protoplasts have been used as a versatile cell-based system, which maintain many of the same physiological properties of an intact plant cell, despite the removal of the cell wall, but with an increased level of manipulability (Pongchawee et al. 2006, 2007; Yoo et al. 2007; Scott and Logan 2008).
In contrast, developmentally regulated PCD occurs as part of normal development and presumably is a response to internal signals. Examples of developmentally regulated PCD include elimination of transitory organs and tissues (Filonova et al. 2008; Helmersson et al. 2008), xylem differentiation (Fakuda et al. 1998; Fakuda 2000), and leaf morphogenesis (Gunawardena et al. 2004, 2005) as is seen in the lace plant (Aponogeton madagascariensis) and Monstera.
Both forms of PCD display distinct morphological characteristics, including plasma membrane blebbing, increased formation of vesicles and transvacuolar strands, condensation of the cytoplasm and nucleus, changes in mitochondrial dynamics, cleavage of DNA at specific sites by endonucleases, as well as a positive terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) response (McCabe et al. 1997; Danon et al. 2000; McCabe and Leaver 2000; Balk et al. 2003; Gunawardena et al. 2004; Yao et al. 2004; Reisen et al. 2005; Conradt 2009).
Mitochondria and PCD
Mitochondria play a vital role in cellular metabolism in both plants and animals. Within animal systems, mitochondrial dynamics have been recognized to play an imperative role in the regulation of PCD, while this organelles role within plant PCD is still unclear (Jones 2000; Joza et al. 2001). In animal systems, mitochondria appear to undergo one of two distinct physiological changes leading to alterations in mitochondrial membrane potential (ΔΨm) and the release of internal membrane space (IMS) proteins. One strategy involves the formation of the permeability transition pore (PTP) by the coordination of the voltage-dependent anion channel (VDAC), AdNT and cyclophilin D (CyD), while the second strategy involves the Bcl family of proteins and utilizes only the VDAC (Green and Reed 1998). To date, evidence for the presence of PTP formation in plant PCD has been reported (Crompton 1999; Zamzami et al. 1995; Jacobson 1996; Balk et al. 1999; Jones 2000; Arpagaus et al. 2002; Diamond and McCabe 2007; Zhang et al. 2009); however, there is no evidence for the involvement of Bcl family proteins in plants (Balk et al. 1999; Balk and Leaver 2001). It is now known that cyclosporine A (CsA) can act in disrupting the PTP by inhibiting the binding of CyD to AdNT (Crompton 1999; Arpagaus et al. 2002; Lin et al. 2005; Diamond and McCabe 2007), leading to advances in PCD research focusing on the mitochondria.
Induction of cell death
The induction of PCD through the use of environmental factors such as HS has recently become a frequently used technique in plant systems; it is thought to alter cellular metabolism, leading to alterations in mitochondrial dynamics and inevitably complete cell death. Arabidopsis, tobacco BY-2 cells, and cucumber have all depicted alterations in cellular redox status, as well as disruption of mitochondrial dynamics following HS (Balk et al. 1999, 2003; Vacca et al. 2004, 2006; Yao et al. 2004; Scott and Logan 2008; Zhang et al. 2009). These changes in mitochondrial dynamics can be visualized when mitochondria are stained with a permeability sensitive dye such as chloromethyl-X-rosamine (CMXRos or Mitotracker Red), which is sequestered in the mitochondrial matrix as a function of the Nernst equation (Kroemer et al. 1997; Balk et al. 1999; Curtis and Wolpert 2002; Yao et al. 2004).
The lace plant
The lace plant [A. madagascariensis (Mirbel) H. Bruggen] is an aquatic monocot endemic to the river of Madagascar. Leaves of the plant are very thin, approximately four cell layers thick and are produced from a shoot apical meristem (SAM) at the apex of the spherical corm. The leaves of the lace plant form in a heteroblastic series; the first one to three leaves produced by the SAM are the juvenile leaves that do not form perforations, while the following leaves are adult leaves, which will form perforations. The lace plant is 1 of 40 species in the monogeneric family Aponogetonaceae, and is the only species which produces perforations in its leaves through the PCD process (Gunawardena et al. 2004, 2006, 2007). Proposed hypotheses for the purpose of these perforations are reviewed in Gunawardena and Dengler (2006).
Developmentally regulated PCD and the lace plant
In the lace plant, PCD begins between longitudinal and transverse veins in spaces known as areoles, and continues outward before stopping four to five cells (non-PCD) from the vascular tissue, creating a lattice-like pattern over the entire leaf surface (Gunawardena et al. 2004). The developmental morphology of the adult lace plant leaf has been divided into five stages as detailed in Gunawardena et al. (2004). Due to the predictability of perforation formation, A. madagascariensis makes an ideal model system for the study of developmentally regulated PCD in plants (Gunawardena et al. 2004, 2006, 2007; Gunawardena 2008; Wright et al. 2009; Elliott and Gunawardena 2010).
The present study aimed to investigate environmental PCD in the lace plant, A. madagascariensis, through HS at 55°C for 20 min. Cellular changes that occurred were examined using live cell imaging and characteristics compared with the developmentally counterpart within the whole leaf of the plant. An emphasis was placed on the role of the mitochondria, particularly the PTP’s role within the induced cell death system.
Materials and methods
Plant materials
Lace plants used for all experimental purposes were grown in axenic culture in magenta boxes as described by Gunawardena et al. (2006). Plants were maintained at 24°C with 12 h light/12 h dark cycles provided by daylight simulating fluorescent bulbs (Philips, Daylight Deluxe, F40T12/DX, Markham, Ontario, Canada) at approximately 125 μmol/m2 s. All experimental plant materials were propagated via subculture; all subculturing processes were carried out according to the protocol of Gunawardena et al. (2006). All chemicals were purchased from Sigma (St Louis, MO, USA), unless otherwise stated. All experiments were completed at least four times unless otherwise stated.
Confocal laser scanning microscopy
Confocal observations were performed using a Nikon Eclipse Ti confocal microscope (Nikon, Mississauga, Ontario, Canada) fitted with a digital camera (Nikon DS-Fi1), using EZ-C1 3.80 imaging software and Ti Control. Confocal microscope observations were performed using DIC optics with complimentary fluorescent images taken via a fluorescent fluorescein isothiocyanate (FITC; excitation 460–500 nm, emission 510–560 nm) or tetramethyl rhodamine iso-thiocyanate (TRITC; excitation 527–552 nm, emission 577–632 nm) laser. All composite plates were assembled using Adobe Photoshop Elements version 6.0.
Protoplast isolation
Perforation expansion leaves, from boxed plants between 1 and 2 months of age, were excised and digested in protoplast buffer containing 5 mM Mes, 0.6 M sorbitol, 2% (w/v) cellulase R10, and 0.5% pectolyase Y-23 for 4 h (Yakult Pharmaceutical, Tokyo, Japan). Enzyme incubation was followed by two washes in protoplast buffer, containing no enzymes, by centrifugation at 100g for 10 min each time. Protoplast samples were examined via confocal microscopy. Density and viability of protoplasts was determined using a hemocytometer (Bright-Line, Horsham, PA, USA) and fluorescein diacetate (FDA) or propidium iodide (PI) staining, respectively. Approximately 60.35 ± 1.08 × 105 protoplasts/g fresh weight, with a viability of 92.75 ± 2.25%, can be obtained from a single isolation. Due to the large variation in the size of lace plant protoplasts (25–100 μm), it is difficult to use a fine mesh for the separation of protoplasts and cell debris; therefore, it is common in protoplast isolations to have a small amount of cell debris present (Lord and Gunawardena 2010).
HS challenge
Originally, lace plant protoplasts were heated at 25, 55 or 75°C in order to determine the temperature that most effectively induced PCD. Given results from this preliminary work, freshly prepared protoplasts were then challenged by HS at 55°C for 20 min. HS was performed using a Nikon live cell culture chamber, equipped with a Bionomic controller (20/20 technology, BC-110) regulated by a heat exchanger (20/20 technology, HEC-400; Grand Rapids, MI, USA). Breathing quality air (5 psi) was pumped into a gas purge system (20/20 technology, P-502) for regulation; air was then pumped into a small humidity chamber, which was then subsequently pumped over the sample in the culture chamber to create a humid environment, preventing cells from desiccating. Preliminary experiments tracked HS-treated protoplasts over a 6 h period. Following this preliminary work, it was determined that allowing HS cells to recover at 24°C for 0, 30, 60, 90, or 120 min was sufficient and optimal. Control cells were isolated protoplasts which were not subjected to HS. Several cell samples were also heated at 75°C for 20 min in an effort to induce necrosis. This experiment was completed not only to enable the comparison of PCD cells with controls, but also to allow for comparison with the cells undergoing uncontrolled cell death. Prior to HS at 55°C for 20 min, several protoplast samples were pre-treated with 50 μM CsA for 30 min, at room temperature according to Lin et al. (2005, 2006). All operations and staining were carried out under dark conditions to minimize possible oxidative stress caused by light.
TUNEL
For LM-TUNEL assay, treated protoplasts were fixed with 4% paraformaldehyde for 1 h at room temperature. Protoplasts were then permeabilized with 10 μg/ml proteinase K at 4°C for 2 min. The free 3′-OH groups in the DNA were labeled by a fluorescein in situ cell death detection kit (Roche, Boehringer Mannheim, Germany) according to manufacturer’s instructions. TUNEL-stained samples were excited with the FITC cube (excitation 460–500 nm, emission 510–560 nm) on the confocal microscope.
DNA isolation and electrophoresis
Genomic DNA was isolated from HS-treated protoplasts isolated from an original 1 g perforation expansion leaf sample. Protoplasts isolated from this sample were divided into six 400 μl samples and frozen in liquid nitrogen at 0, 0.5, 1, 3, 5, and 24 h post-HS. Samples were ground by hand into a fine powder. Isolation of DNA was performed using a DNeasy plant mini kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer’s instructions. To observe DNA fragmentation, samples (0.5 μg/ml lane, final concentration) were run with a 100-bp ladder on a 1% ethidium bromide agarose gel at a constant 50 V.
Mitochondrial staining
Control, CsA + HS or HS-challenged protoplasts were incubated with 100 nM of the mitochondria-specific, membrane potential sensitive dye Mitotracker Red or CMXRos (Molecular Probes Eugene, OR, USA; dissolved in dimethylsulfoxide, DMSO), for 10 min at 24°C in the dark. CMXRos was excited with the TRITC cube (excitation 527–552 nm, emission 577–632 nm) using the confocal microscope.
Transmission electron microscopy (TEM)
Protoplast samples for TEM were fixed using a method optimized specifically for lace plant material. Fixation was initiated by adding cells to 2% glutaraldehyde (GA) in buffer (0.05 M sodium cacodylate, 0.4 M sorbitol, pH 6.5) for 1 h, followed by the addition (1:1, v/v) of 1% OsO4 in distilled water (dH2O). Fixed protoplasts were then washed twice in buffer (0.05 M sodium cacodylate, 0.4 M sorbitol, pH 6.5) for 20 min each and stored at 4°C over night. Cells were then embedded in 4% agarose (Type VII-A low gelling temperature; Sigma), cut into small pieces (approximately 0.5 cm × 0.5 cm) and dehydrated for 10 min each in 50, 70, 80, 90, and 95% ethanol and twice for 10 min each in 100% ethanol and propylene oxide. Embedded protoplasts were infiltrated in mixed propylene oxide and poly/bed (1:1) overnight, then infiltrated in fresh epoxy for 4 h, and transferred to fresh epoxy in capsules. Polymerization was performed in a 60°C oven for a total of 48 h. Semi-thin sections (2 μm thick) and ultra-thin sections (50 nm thick) were stained with uranyl acetate and lead citrate before observation with a Philips Tecnai 12 transmission electron microscope (TEM; Philips Electron Optics, Eindhoven, The Netherlands) operating at 80 kV and fitted with a Kodak (Rochester, NY, USA) Megaview II camera and software (AnalySIS, Soft Imaging System, Münster, Germany). All composite plates were assembled using Adobe Photoshop Elements version 6.0.
Statistical analysis
All data were assessed by a general linear model of variance (GLM Anova) and the means were compared using the Tukey test at 95% confidence intervals (P < 0.05). All statistical analyses were carried out using Minitab 15 Statistical Software-English (Minitab Inc., State College, PA, USA, 1972).
Results
The natural development of a lace plant leaf has been previously described with five developmental stages distinguished (Fig. 1). These stages include furled stage, “window” stage (Fig. 1a), perforation formation, perforation expansion (Fig. 1b) and mature (Fig. 1c). A method has been developed for sterile culture of plants (Fig. 1d; Gunawardena et al. 2004), and the transparent nature of the thin leaf facilitates live cell imaging. Several common characteristics of PCD have already been described throughout regular leaf morphogenesis in the lace plant, including plasma membrane blebbing, increased vesicle formation, condensation of the cytoplasm and nucleus, changes in mitochondrial dynamics as well as TUNEL positive nuclei. To date, preliminary results indicate indirect evidence for the involvement of caspase 1, and the up-regulation of ETR1 receptors in the PCD process within the lace plant (data not shown).
The lace plant (Aponogeton madagascariensis). a Window stage lace plant leaf, displaying transparent windows where PCD is occurring. b Perforation expansion lace plant leaf portraying the expansion of the perforated areole. c Mature lace plant leaf showing completed perforations between longitudinal and transverse veins. d Sterile lace plant in magenta box. Scale bars 100 μm (a–c), 1 cm (d)
Environmentally induced PCD characteristics
One hour following protoplast isolation, control protoplasts were still of high yield and approximately 97.75% viability (Fig. 2a, b) in contrast to cells allowed to rest for 1 h post-HS with approximately 31.25% viability (Fig. 2c, d). Viability of protoplasts was determined by FDA staining. Characteristics of PCD were not seen in control protoplasts. Control cells showed intact tonoplast and plasma membranes (Figs. 3a, b, 4a), healthy nuclei (Fig. 4b), healthy chloroplasts with intact stroma and starch granules (Figs. 3a, 4c), as well as healthy, and often dividing mitochondria (Fig. 4d).
Micrograph of lace plant protoplasts. Viable control protoplasts isolated from perforation expansion leaves. DIC image (a) and corresponding fluorescent image showing fluorescein diacetate (FDA) staining of living cells 1 h post-isolation (b). Cells exposed to HS challenge at 55°C for 20 min and allowed to rest for 1 h. DIC image (c) and corresponding fluorescent image showing FDA staining of living cells (d). Scale bars 50 μm
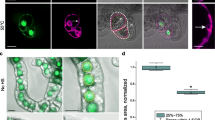
Micrographs of individual lace plant protoplasts 1 h post-isolation for controls, and 1 h post-HS for PCD and necrotic cells. Control protoplasts without signs of PCD, intact plasma membrane (black arrow, a), and healthy chloroplasts (white arrow, a), intact tonoplast membrane (black arrow, b). Protoplasts HS at 55°C for 20 min with common characteristics of PCD. Plasma membrane blebbing (black arrow, c), increased production of hydrolytic vesicles (black arrow, d), severe nuclear condensation (white arrow, d). Chloroplasts (white arrow, e) inside the vacuole, note intact tonoplast membrane (black arrow, e). Increased production of transvacuolar strands (black arrow, f). Protoplasts after HS treatment at 75°C for 20 min with common characteristics of necrosis. Swollen protoplast (black arrow, g), inevitably leading to complete cell lysis (black arrow, h). Scale bars 10 μm (c–f), 20 μm (a, b, g, h)
Transmission electron micrographs of control (a–d), and HS (e–h) lace plant protoplasts. Healthy protoplast displaying intact plasma membrane (red arrow) and tonoplast (black arrow, a). Healthy nucleus showing no signs of condensation or chromatin flocculation (b). Healthy chloroplast depicting intact inner and outer membrane, thylakoids, intact stroma (red arrow) and starch granules (black arrow, c). Healthy dividing mitochondria (black arrow) showing intact outer and inner membranes, and clearly non-degraded cristae; no mitochondrial swelling present (d). HS-treated protoplasts depicting plasma membrane blebbing (black arrow) and permeabilized tonoplast (red arrow, e). Magnified nucleus displaying condensation and chromatin flocculation (black arrow, f). Chloroplasts with intact inner and outer membranes, but degraded inner thylakoid membranes (black arrow) and decreases in starch granules (g). Slightly swollen and degraded mitochondria depicting degraded cristae and association with chloroplasts (h). Scale bars 2 μm (a, e, f), 1 μm (b, c, g), 0.5 μm (h), 200 nm (d)
HS has been used as a practical method to induce cell death in many species of plant, but has never before been used with the lace plant. Between 20 min and 2 h post-HS at 55°C, lace plant protoplasts displayed many key characteristics of PCD, including blebbing of the plasma membrane (Figs. 3c, 4e), increased vesicle formation (Fig. 3d), nuclear condensation (Figs. 3d, 4f), increased Brownian motion within the vacuole (see Online Resource 1), circular chloroplasts, with degraded inner membrane, stroma, starch granules, and thylakoids (Fig. 4g). In approximately one dozen protoplasts (out of 30 samples), chloroplasts were seen inside the central vacuole of the protoplast (Fig. 3e; see Online Resource 2). Also following HS, slightly swollen mitochondria appeared with degraded cristae (Fig. 4h); these mitochondria were often seen associated with compromised chloroplasts (Fig. 4h). An increased number of transvacuolar strands was observed in approximately 20% of HS-treated cells (Fig. 3f). Combined, these characteristics suggest HS as an excellent inducer for the study of environmentally induced cell death in lace plant protoplasts. In contrast, protoplasts which were heated at 75°C for 20 min to induce necrosis displayed characteristics of an uncontrolled version of cell death including cell swelling (Fig. 3g) and cell rupture (Fig. 3h).
Protoplast viability was tracked via PI staining at 30 min intervals over a 2 h period; control, CsA + HS and HS protoplast viability were compared (Fig. 5a). HS was found to significantly affect protoplast viability at 0, 30, 60, 90 and 120 min post-HS when compared to control cells (P < 0.05; Fig. 5b). CsA treatment was found to significantly affect protoplast viability at 0, 30, 60, 90, and 120 min post-HS when compared to control cells (P < 0.05; Fig. 5b). The percentage of dead protoplasts following HS rose exponentially and reached 97.75 ± 0.94% at 120 min post-HS (Fig. 5a, b). This value varied significantly from both CsA + HS protoplasts (81.75 ± 1.10%) and control protoplasts (5.75 ± 0.62%) at the same time interval (P < 0.05 for both; Fig. 5a, b). All cells which stained positive with PI for dead nuclei contained a shrunken, PCD appearance, and in very few cases did a protoplast appear to be swollen or have ruptured via necrosis (Fig. 5a).
Percentage of dead protoplasts following HS at 55°C for 20 min, compared to control and CsA + HS-treated protoplasts over a 2 h period as determined by PI staining. Control, CsA + HS and HS-treated protoplasts illustrating PI-stained nuclei at time 0 and 2 h with corresponding DIC images. Control protoplasts show less cell death symptoms then HS-treated protoplasts, while CsA + HS-treated protoplasts show an intermediate amount of cell death (a). Scale bars 70 μm. Cell death was tracked via PI staining at 30 min intervals for 2 h, followed by counting of PI-stained nuclei. Control protoplasts show a significantly lower amount of cell death then HS-treated protoplasts at all time intervals, while CsA + HS-treated protoplasts depict a statistically significant lower amount of cell death at 30, 60, 90, and 120 min compared to the HS treatment. Overall, CsA treatment decreased the amount of cell death seen in HS-treated protoplasts. Data represent mean ± standard error of four replicates (b)
Cessation of mitochondrial streaming
Following the determination of optimal dye loading concentrations and incubation time periods, protoplasts were incubated in 100 nM CMXRos for 10 min at 25°C, in the dark. Protoplasts stained via this method displayed intense mitochondrial staining, which could be easily observed within single protoplasts. Control protoplasts displayed active mitochondrial streaming (Fig. 6a; see Online Resource 3). Between approximately 20 min and 1 h post-HS, mitochondria depicted remarkable changes in motility; HS-treated protoplasts displayed cessation of mitochondrial streaming in approximately 65% of the isolates (Fig. 6b; Table 1; see Online Resource 3).
Quantification of changes in CMXRos-stained mitochondrial fluorescence intensity. Image of un-treated control (a) and HS-treated (b) protoplasts taken from video clip. The value of CMXRos fluorescence inside each of the three selected areas in each photograph was plotted over 400 s. Fluorescence intensities were recorded with EZ-C1 3.80 imaging software and normalized with excel software. Scale bars 25 μm
Also in this interval of time, mitochondria within the HS cells appeared to form small aggregates, and occasionally appeared to be proximally associated with the chloroplasts (Fig. 4h). This aggregate formation was found in approximately 30% of the HS-treated protoplasts, but not seen in control cells (Fig. 4c, d). Protoplasts pre-treated with CsA still showed cessation of mitochondrial streaming between approximately 20 min and 1 h post-HS (Table 1).
Further analysis of changes in mitochondria motility was performed with CMXRos-stained protoplasts by quantifying the variation in mitochondrial fluorescence in arbitrarily chosen areas indicated by ellipses (Fig. 6a, b). Variation in fluorescence intensity within these ellipses was used as a measure of mitochondrial streaming. Oscillation in the control graph (Fig. 6a) indicates rapid mitochondrial streaming within the cell, whereas little oscillation in the HS-treated graph (Fig. 6b) indicates little to no mitochondrial streaming. More details of mitochondrial motility can be found online in the Online Resource 3.
Decrease in mitochondrial ΔΨm
Control, CsA + HS and HS-treated protoplasts stained with CMXRos were also used to make inferences regarding mitochondrial ΔΨm during environmentally induced cell death. Regardless of which pathway was used by mitochondria to induce cell death, the increased release of IMS proteins during the HS challenge is indicative for a reduction in mitochondrial ΔΨm, and therefore can be visualized via changes in mitochondria fluorescence. Control protoplasts labeled with CMXRos showed vivid mitochondrial staining at time 0, 60, and 120 min post-isolation (Fig. 7; Table 1). In contrast, protoplasts pre-treated with CsA followed by HS showed a slight decrease in mitochondrial fluorescence at 0, 60, and 120 min post-HS when compared to controls (Fig. 7; Table 1). Protoplasts treated with just a HS challenge showed rapid decreases in CMXRos staining leading to almost no viable mitochondria 2 h post-HS (Fig. 7; Table 1). CsA + HS-treated protoplasts displayed an intermediate decrease in mitochondrial ΔΨm between control- and HS-treated cells. It should also be noted that within HS challenged protoplast samples, the largest decrease in mitochondrial fluorescence occurred between 1 and 2 h post-HS (Fig. 7; Table 1). The results for each treatment were observed in at least 65% of the sample protoplasts.
Visualization of representative protoplasts mitochondrial membrane depolarization during control, CsA + HS, and HS treatment. Control protoplasts at time 0, 60, and 120 min show intense CMXRos mitochondrial staining. CsA + HS-treated protoplasts at the same time intervals show slightly decreased CMXRos staining, indicating a slight loss of mitochondrial ΔΨm. HS-treated protoplasts illustrate mitochondrial staining at time 0, but rapid decreases in CMXRos staining following 60 and 120 min. This loss of CMXRos staining in HS-treated cells depicts a rapid decrease in mitochondrial ΔΨm as compared to both control and CsA + HS-treated cells. Note the most rapid loss of mitochondrial ΔΨm can be seen between 60 and 120 min in HS challenged cells. Scale bars 25 μm
TUNEL, DNA isolation and electrophoresis
PCD confirmation was supported by the presence of 3′-OH nick ends detected via LM florescent TUNEL (Fig. 8). At 2 h post-isolation, no TUNEL positive nuclei were present in control cells (Fig. 8a–c). Approximately 20% of CsA + HS-treated cells, which were allowed to rest for 2 h post-HS, had TUNEL positive nuclei (Fig. 8d–f). Approximately 55% of HS-treated cells, which were allowed to rest for 2 h post-HS treatment, showed TUNEL positive nuclei (Fig. 8g–l). HS protoplasts, when allowed to rest for 18 h post-HS, still displayed approximately 55% TUNEL positive nuclei (data not shown). No DNA laddering was visualized via electrophoresis at any interval post-HS (data not shown).
LM-TUNEL positive protoplasts. DIC, fluorescent and overlay images. a–c Control protoplasts were isolated, allowed to rest for 2 h, fixed, and labeled with fluorescent TUNEL. No TUNEL positive nuclei are present in control protoplasts. d–f CsA + HS-treated protoplasts were pre-treated with CsA, followed by heating at 55°C for 20 min, allowed to rest for 2 h, fixed, and labeled with fluorescent TUNEL. Approximately 20% of CsA + HS-treated cells depicted TUNEL positive nuclei. g–l HS-treated protoplasts were heated at 55°C for 20 min and allowed to rest for 2 h, fixed, and labeled with fluorescent TUNEL. Approximately 55% of protoplasts depicted TUNEL positive nuclei. These results suggest that DNA fragmentation does not occur in control cells, occurs at an intermediate level in CsA + HS cells, and occurs in slightly more than half HS-treated cells. Scale bars 25 μm (a–c, g–l), 50 μm (d–f)
Discussion
Results presented here indicate that HS treatment at 55°C for 20 min is an excellent inducer of cell death in lace plant protoplasts, and caused an increase in cell death compared to control cells at 1 h post-HS, as per FDA and PI staining. The decline in living cells was anticipated given the severity of the HS challenge as well as drastic changes in cellular morphology visualized in HS-treated cells. These changes in cellular morphology were representative of those normally seen in cells which have undergone PCD, including the overall shrinkage of cells versus the swelling and rupture associated with necrosis (McCabe et al. 1997; McCabe and Leaver 2000).
Organelles involved in PCD
Chloroplast degradation in the lace plant-induced cell death system mimicked many of the same characteristics seen in the developmentally regulated counterpart. Changes in chloroplast shape, size, matrix morphology and the presence of starch granules were all noted in this induced death system, and have been reported previously in the developmentally regulated system (Wright et al. 2009). Wright et al. (2009) also made inferences regarding chloroplast fate, but due the thickness of whole lace plant leaves, and the static nature of TEM, were not able to determine if chloroplasts were entering the vacuole to be degraded. To date, findings of chloroplasts entering the vacuole have been reported in Arabidopsis thaliana (Wada et al. 2009), French bean (Minamikawa et al. 2001) and wheat (Wittenbach et al. 1982), all during developmental PCD leaf senescence. Live cell imaging evidence presented here for the first time depicts chloroplasts within the vacuole of a protoplast undergoing induced PCD (Online resource 2). Unlike the findings of Wright et al. (2009), dumbbell-shaped dividing chloroplasts were only seen in control cells, and not in early stages of cell death. This lack of dividing chloroplasts may be a result of the strong stress following HS, unlike the possibly more gradual effect of developmentally regulated PCD signals.
An increased number of transvacuolar strands was also noted in both the induced and developmentally regulated cell death systems in the lace plant (Wright et al. 2009). An increase in transvacuolar strands was also shown during induced cell death by osmotic stress in tobacco suspension cultures (Reisen et al. 2005). Increases in transvacuolar strands could aid the movement of organelles such as chloroplasts and mitochondria to the edge of the nucleus or to inside the vacuole in lace plant cells. As was anticipated, an increase in hydrolytic vesicle formation was also seen within both death systems. The increase in these vesicles would presumably aid in the degradation of dead or dying organelles as PCD progresses.
Changes in nuclear shape and size were noted within both induced and developmentally regulated death systems. Nuclear condensation is a common characteristic of PCD and is possibly driven by the condensation of chromatin (Ryerson and Heath 1996; McCabe et al. 1997). TUNEL positive nuclei were seen in HS-challenged cells and increased exponentially following HS. The presence of TUNEL positive nuclei was also noted in the developmentally regulated system (Gunawardena et al. 2004). DNA laddering was not present in the induced cell death system at any interval following HS. However, DNA laddering was seen following HS-induced death in several species, including carrot (McCabe et al. 1997) and Arabidopsis (McCabe and Leaver 2000). The lack of DNA laddering has been noted previously within several developmental PCD examples including leaf morphogenesis in the whole lace plant leaves (Gunawardena et al. 2004), and treachery element differentiation in Zinnia (Fakuda et al. 1998; Fakuda 2000).
The mitochondria, an organelle commonly thought to be involved in plant PCD
HS challenge has been demonstrated to cause PCD in a variety of plant species, including Arabidopsis (McCabe and Leaver 2000; Balk et al. 2003; Yao et al. 2004; Scott and Logan 2008; Zhang et al. 2009), BY-2 tobacco cells (Vacca et al. 2004, 2006) and cucumber (Balk et al. 1999). Yao et al. (2004), Scott and Logan (2008) and Zhang et al. (2009) reported changes in mitochondria, such as the formation of the PTP, the release of IMS proteins, and the MPT following the death induction signal, but preceding morphological cell changes. Within the lace plant-induced cell death system, it is evident that PCD is occurring, and given the drastic changes in mitochondrial dynamics, it is also evident that mitochondria are playing a role in the cell death process; however, the order of the death events is just beginning to be elucidated.
Cessation of mitochondrial streaming appears to be one of the earliest occurrences in the lace plant-induced cell death system, between approximately 20 min and 1 h post-HS. This cessation of streaming was also demonstrated during HS-induced cell death by Zhang et al. (2009) in A. thaliana protoplasts, as well as Vacca et al. (2004, 2006) in tobacco BY-2 cells. This impairment of mitochondrial metabolism and movement following HS challenge is thought to be highly correlated with the induction of PCD (Vacca et al. 2004, 2006) and is suggested to indicate an acute change in cellular redox status within the cell (Zhang et al. 2009). Within this time period, mitochondria also appear to form aggregates, and in some cases form associations with chloroplasts. This aggregate formation has been noted by both Yao et al. (2004) and Scott and Logan (2008) in Arabidopsis protoplasts and could be an attempt to reserve energy stores within the dying cell, or to offer the last remaining energy from the organelle to chloroplasts for further death-associated processes.
Following cessation of streaming, between 1 and 2 h post-HS, mitochondria begin to lose their ΔΨm, as noted by substantial decreases in CMXRos staining. This decrease in ΔΨm was noted previously in Arabidopsis protoplasts by Yao et al. (2004) 2–3 h post-HS, and by Scott and Logan (2008) 1–4 h post-HS. Preliminary work completed on whole lace plant leaves undergoing developmental PCD depicts similar patterns in mitochondrial dynamics as noted above. Mitochondria initially show vivid mitochondrial staining and streaming, followed by the formation of large aggregates of mitochondria, which move rapidly in the cytoplasm. This rapid movement is followed by the loss of streaming and finally the loss of mitochondrial straining (data not shown). Overall, at the 2 h mark within lace plant protoplasts, it is realistic to assume that the mitochondria are no longer viable. This decrease in viable mitochondria after HS follows drastic morphological changes which have occurred within the cells, including the blebbing of the plasma membrane, increases in hydrolytic vesicles, the increase of TUNEL positive nuclei, as well as the overall shrinkage of the protoplast. In general, plant mitochondria have been suggested to play a pivotal role in the integration of environmental and developmental signals that trigger cell death and must be further studied in order elucidate the role of the organelle in other non-model plant species (Balk and Leaver 2001; Jones 2000).
Implications of CsA on induced cell death
CsA appeared to have a strong affect on the induction and continuance of environmental cell death in the lace plant. Pre-treatment of lace plant protoplasts with 50 μM CsA for 30 min at room temperature caused a significant decrease in the amount of dead cells at 30, 60, 90, and 120 min post-HS as compared to control- and HS-treated cells. This effect of CsA is relatively rapid compared to results presented by Scott and Logan (2008), in which CsA pre-treatment only significantly decreased Arabidopsis cell death at 24 h post-HS. The variation in CsA effect could be due to lower concentrations and incubation times utilized by Scott and Logan (2008). As expected, CsA pre-treatment also decreased the percentage of TUNEL positive nuclei within cell samples at 2 h post-HS. The finding that CsA partially inhibits cell death, characterizing the cells as intermediate between control and HS-treated cells, has been noted previously in Arabidopsis (Yao et al. 2004), tobacco (Lin et al. 2005, 2006) and sycamore (Contran et al. 2007).
Pre-treatment of protoplasts with CsA prior to HS also had an effect on mitochondrial ΔΨm. CsA partially delayed the decrease in mitochondrial ΔΨm, causing CMXRos staining to be visible for longer following HS. This delayed decrease in ΔΨm was also noted by Lin et al. (2005, 2006) in tobacco protoplasts, following pre-treatment with CsA. This delayed decrease in ΔΨm may be directly correlated with the later onset of cell death and DNA fragmentation as noted in the previous paragraph; this supports the role of mitochondria as a key player in the induced cell death process.
Unexpectedly, pre-treatment with CsA did not have an effect on the cessation of mitochondrial streaming as compared to HS samples. Protoplasts pre-treated with CsA still showed cessation of mitochondrial streaming. This result suggests that the cessation of streaming may not be correlated with the PTP and hence CsA has no effect on this organelle characteristic.
Overall, CsA appears to have an effect on the induction and maintenance of induced PCD within the lace plant. Given the effect of this PTP inhibitor, it can be suggested that induced cell death within the lace plant is at least partially governed by the association between the VDAC, AdNT, and the matrix localized protein CyD, all which form the PTP within the mitochondria (Diamond and McCabe 2007).
Conclusions
In plants, the exact signal transduction pathway which causes PCD is not yet fully understood. The results presented here portray HS at 55°C for 20 min in lace plants as a promising inducer of PCD within plant protoplasts, which according to our observations can be most accurately examined between 20 min and 2 h post-HS. HS treatment produced cells which contain many characteristic of PCD. PCD was confirmed through the use of fluorescent TUNEL, in which 3′-OH nick ends were visualized in HS-treated protoplasts beginning 1 h post-HS. TUNEL positive nuclei were not present in control samples. Results presented here demonstrate, to the best of our knowledge, the first example of chloroplasts being brought into the vacuole of protoplasts undergoing environmentally induced PCD. The results also indicate that the mitochondria, particularly the PTP, may play a role in the induced cell death process. Thus, HS treatment proves to be a reliable technique to enable the comparison of environmentally induced and developmentally regulated PCD within one species of plant.
Abbreviations
- CMXRos:
-
Chloromethyl-X-rosamine
- CyD:
-
Cyclophilin D
- CsA:
-
Cyclosporin A
- FDA:
-
Fluorescein diacetate
- FITC:
-
Fluorescent fluorescein isothiocyanate
- GA:
-
Glutaraldehyde
- HS:
-
Heat shock
- ΔΨm :
-
Membrane potential
- PTP:
-
Permeability transition pore
- PCD:
-
Programmed cell death
- PI:
-
Propidium iodide
- SAM:
-
Shoot apical meristem
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- TRITC:
-
Tetramethyl rhodamine iso-thiocyanate
- TEM:
-
Transmission electron microscopy
- VDAC:
-
Voltage-dependent anion channel
References
Arpagaus S, Rawyler A, Braendle R (2002) Occurrence and characteristics of the mitochondrial permeability transition in plants. J Biol Chem 277:1780–1787
Balk J, Leaver CJ (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13:1803–1818
Balk J, Leaver CJ, McCabe PF (1999) Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett 463:151–154
Balk J, Chew SK, Leaver CJ, McCabe PF (2003) The intermembrane space of plant mitochondria contains a DNase activity that may be involved in programmed cell death. Plant J 34:573–583
Conradt B (2009) Genetic control of programmed cell death during animal development. Annu Rev Genet 43:493–523
Contran N, Cerana R, Crosti P, Malerba M (2007) Cyclosporin A inhibits programmed cell death and cytochrome c release induced by fusicoccin in sycamore cells. Protoplasma 231:193–199
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249
Curtis MJ, Wolpert TJ (2002) The oat mitochondrial permeability transition and its implication in victorin binding and induced cell death. Plant J 29:295–312
Danon A, Gallois P (1998) UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Lett 437:131–136
Danon A, Delorme V, Mailhac N, Gallois P (2000) Plant programmed cell death: a common way to die. Plant Physiol Biochem 38:647–655
Diamond M, McCabe PF (2007) The mitochondrion and plant programmed cell death. In: Logan DC (ed) Annual plant reviews; plant mitochondria. Blackwell, Oxford, pp 308–329
Elliott A, Gunawardena AHLAN (2010) Calcium inhibition halts developmental programmed cell death in the lace plant, Aponogeton madagascariensis? Botany 88:206–210
Fakuda H (2000) Programmed cell death of tracheary elements as a paradigm in plants. Plant Mol Biol 44:245–253
Fakuda H, Watanabe Y, Kuriyama H, Aoyagi S, Sugiyama M, Yamamoto R, Demura T, Minami A (1998) Programming of cell death during xylogenesis. J Plant Res 111:253–256
Filonova LH, Suárez MF, Bozhkov PV (2008) Detection of programmed cell death in plant embryos. Methods Mol Biol 427:173–179
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Gunawardena AHLAN (2008) Programmed cell death and tissue remodeling in plants. J Exp Bot 59(3):445–451
Gunawardena AHLAN, Dengler NG (2006) Alternative modes of leaf dissection in monocotyledons. Bot J Linn Soc 150:25–44
Gunawardena AHLAN, Navachandrabala C, Kane M, Dengler NG (2006) Lace plant: a novel system for studying developmental programmed cell death. In: Teixeira da Silva JA (ed) Floriculture, ornamental and plant biotechnology: advances and topical issues, vol 1. Global Science Books, Middlesex, UK, pp 157–162
Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001) Characterisation of programmed cell death during aerenchyma formation induced by hypoxia and in roots of maize (Zea mays L.). Planta 212:205–214
Gunawardena AHLAN, Greenwood JS, Dengler NG (2004) Programmed cell death remodels lace plant leaf shape during development. Plant Cell 16:60–73
Gunawardena AHLAN, Sault K, Donnelly P, Greenwood JS, Dengler NG (2005) Programmed cell death and leaf morphogenesis in Monstera oblique. Planta 221:607–618
Gunawardena AHLAN, Greenwood JS, Dengler NG (2007) Cell wall degradation and modification during programmed cell death in lace plant, Aponogeton madagascariensis (Aponogetonaceae). Am J Bot 94:1116–1128
He R, Drury GE, Rotari VI, Gordon A, Willer M, Farzaneh T, Woltering EJ, Gallois P (2008) Metacaspase-8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis. J Biol Chem 283:774–783
Helmersson A, von Arnold S, Bozhkov P (2008) The level of free intracellular zinc mediates programmed cell death/cell survival decisions in plant embryos. Plant Physiol 147:1158–1167
Jacobson MD (1996) Reactive oxygen species and programmed cell death. Trends Biochem Sci 21:83–86
Jones A (2000) Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci 5:225–230
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CYJ, Sasaki T, Elia AJ, Cheng HYM, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zúñiga-Pflücker JC, Kroemer G, Penninger JM (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549–554
Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Rev Immunol Today 18:44–51
Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44:417–428
Lin J, Wang Y, Wang G (2005) Salt stress-induced programmed cell death via Ca2+-mediated mitochondrial permeability transition in tobacco protoplasts. Plant Growth Regul 45:243–250
Lin J, Wang Y, Wang G (2006) Salt stressed-induced programmed cell death in tobacco protoplasts is mediated by reactive oxygen species and mitochondrial permeability transition pore status. J Plant Physiol 163:731–739
Lord CEN, Gunawardena AHLAN (2010) Isolation of leaf protoplasts from the submerged aquatic monocot Aponogeton madagascariensis. Am J Plant Sci Biotechnol (in press)
McCabe PF, Leaver CJ (2000) Programmed cell death in cell cultures. Plant Mol Biol 44:359–368
McCabe PF, Levine A, Meijer PJ, Tapon NA, Pennell RI (1997) A programmed cell death pathway activated in carrot cells cultures at low density. Plant J 12:267–280
Minamikawa T, Toyooka K, Okamoto T, Hara-Nishimura I, Nishimura M (2001) Degradation of ribulose-bisphosphate carboxylase by vacuolar enzymes of senescing French bean leaves: immunocytochemical and ultrastructural observations. Protoplasma 218:144–153
Mittler R, Lam E (1997) Characterization activities and DNA fragmentation induced upon hypersensitive response cell death and mechanical stress. Plant Mol Biol 34:209–221
Pongchawee K, Na-Nakorn U, Lamseejan S, Poompuang S, Phansiri S (2006) Factors affecting protoplast isolation and culture of Anubias nana Engler. Int J Bot 2:193–200
Pongchawee K, Na-Nakorn U, Lamseejan S, Poompuang S, Phansiri S (2007) Protoplast isolation and culture of aquatic plant Cryptocoryne wendtii De Wit. Nat Sci 41:300–310
Reape T, McCabe PF (2008) Apoptotic-like programmed cell death in plants. New Phytol 180:13–26
Reisen D, Marty F, Leborgne-Castel N (2005) New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamic during osmotic stress. BMC Plant Biol 5:1–13
Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8:393–402
Scott I, Logan DC (2008) Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol 177:90–101
Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L (2004) Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol 134:1100–1112
Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E (2006) Cytochrome c is released in reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiol 14:208–219
Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, Makino A (2009) Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol 149:885–893
Wittenbach VA, Lin W, Hebert RR (1982) Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol 69:98–102
Wright H, Van Doorn WG, Gunawardena AHLAN (2009) In vivo study of developmentally programmed cell death using the lace plant (Aponogeton madagascariensis; Aponogetonaceae) leaf model system. Am J Bot 96:865–876
Yakimova ET, Kapchina-Toteva VM, Woltering EJ (2007) Signal transduction events in aluminum-induced cell death in tomato suspension cells. J Plant Physiol 164:702–708
Yakimova ET, Woltering EJ, Kapchina-Toteva Vm, Harren FJM, Cristescu SM (2008) Cadmium toxicity in cultured tomato cells—role of ethylene, proteases and oxidative stress in cell death signaling. Cell Biol Int 32:1521–1529
Yao N, Eisfelder BJ, Marvin J, Greenberg JT (2004) The mitochondrion—an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40:596–610
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssire JL, Petit PX, Kroemer G (1995) Reduction in mitochondrial potential constitutes an early irreversible step of programmed cell lymphocyte deal in vivo. J Exp Med 181:1661–1672
Zhang L, Li Y, Xing D, Gao C (2009) Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J Exp Bot 60:2073–2091
Acknowledgments
The authors thank Dr. M. E. Kane (University of Florida, USA) for providing the original sterile lace plant cultures, Jaime Wertman (Dalhousie University, Canada) for critical review of this article, Bruno J. Roy (Dalhousie University, Canada) for assistance in formatting supplementary videos, Dr. Nancy Dengler (University of Toronto, Canada) and Dr. Wouter G. van Doorn (Wageningen University, The Netherlands) for help throughout the manuscript, and Dr. Jennifer Sheen (Mass General Hospital, Molecular Biology, Boston, MA, USA) for help regarding protoplast isolation. The authors also greatly acknowledge the Canadian Foundation for Innovation (CFI) for the Leaders Opportunity Fund, the Natural Sciences and Engineering Research Council (NSERC) for discovery and equipment grants for A.G., and Dalhousie University for partial doctoral funding for C.L.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found online at http://dx.doi.org/10.1007/s00425-012-1825-5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2010_1304_MOESM1_ESM.mov
Online Resource 1 Increased brownian motion of small unknown objects in the vacuole of a protoplast HS at 55°C for 20 min. Scale bar = 15μm (MOV 21,505 kb)
425_2010_1304_MOESM2_ESM.mov
Online Resource 2 Protoplasts HS at 55°C for 20 min and allowed to rest for 1 h at room temperature. Note chloroplasts actively entering the vacuole of a cell, followed by slight brownian motion within the vacuole. Chloroplasts are presumably brought into the vacuole to be degraded. Chloroplast material floating freely in cell culture in cellular debris left from enzymatic isolation. Scale bar = 10μm (MOV 11,164 kb)
425_2010_1304_MOESM3_ESM.mov
Online Resource 3a Control protoplast depicting CMXRos stained mitochondria (red) and chlorophyll autofluorescence (green). Note actively streaming mitochondria in control protoplast. Also note the abundance of chloroplasts within the cell. Scale bar = 10μm (MOV 1,318 kb)
425_2010_1304_MOESM4_ESM.mov
Online Resource 3b Protoplast HS at 55°C for 20 min followed by a 30 min rest period at room temperature. CMXRos stained mitochondria (red) and chlorophyll autofluorescence (green). Note there is no actively streaming mitochondria within the HS challenged cell, when compared to the control sample (Online resource 3a). Also note the decrease in chloroplast abundance within the cell. Scale bar = 10μm (MOV 1,136 kb)
Rights and permissions
About this article
Cite this article
Lord, C.E.N., Gunawardena, A.H.L.A.N. Environmentally induced programmed cell death in leaf protoplasts of Aponogeton madagascariensis . Planta 233, 407–421 (2011). https://doi.org/10.1007/s00425-010-1304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1304-9