Abstract
In this report, the distributions of calreticulin (CRT) and its transcripts in Haemanthus pollen, pollen tubes, and somatic cells of the hollow pistil were studied. Immunoblot analysis of protein extracts from mature anthers, dry and germinated pollen, growing pollen tubes, and unpollinated/pollinated pistils revealed a strong expression of CRT. Both in vitro and in situ studies confirmed the presence of CRT mRNA and protein in pollen/pollen tubes and somatic cells of the pistil transmitting tract. The co-localization of these molecules in ER of these cells suggests that the rough ER is a site of CRT translation. In the pistil, accumulation of the protein in pollen tubes, transmitting tract epidermis (tte), and micropylar cells of the ovule (mc) was correlated with the increased level of exchangeable calcium. Therefore, CRT as a Ca2+-binding/buffering protein, may be involved in mechanism of regulation calcium homeostasis in these cells. The functional role of the protein in pollen–pistil interactions, apart from its postulated function in cellular Ca2+ homeostasis, is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calreticulin is an unconventional, highly conserved Ca2+-binding/buffering lectin-like chaperone containing a KDEL/HDEL retention signal for ER lumen localization (see review by Michalak et al. 2009). Although CRT has been suggested to work as a multifunctional and ubiquitous protein involved in multiple intra- and extracellular processes, research on CRT has mainly focused on its role in regulating intracellular Ca2+ homeostasis, signaling, and chaperone activity involved in quality control within the secretory pathway. In all cells where it has been investigated, CRT has been found in the ER. However, it should be noted that CRT has also been detected outside the ER lumen, including cytoplasm, nucleus, and the cell surface/extracellular matrix (see review by Michalak et al. 2009). Recent studies have identified a novel CRT isoform in mammals (Persson et al. 2002) and two or more genes encoding CRT isoforms have been found in plants (Persson et al. 2003), suggesting functional diversity and possible variations in expression patterns among CRTs.
Calreticulin is highly abundant in most plant cell types. The protein shares the same molecular structure identified for its animal homologue and seems to have similar functional properties (see review by Mariani et al. 2003). One of the most important roles of CRT is calcium-storing function in the ER of plant cells (Persson et al. 2001; Wyatt et al. 2002). Plant CRT is often glycosylated (Coughlan et al. 1997; Navazio et al. 2002; Persson et al. 2003) and phosphorylated by protein kinase CK2 (Baldan et al. 1996). Besides its primary location in ER (Denecke et al. 1995; Napier et al. 1995; Opas et al. 1996; Lenartowska et al. 2002; Nardi et al. 2006), plant CRT has been found in the Golgi apparatus and vesicles (Borisjuk et al. 1998; Navazio et al. 2002; Nardi et al. 2006), nucleus and nuclear envelope (Denecke et al. 1995; Napier et al. 1995; Lenartowska et al. 2002; Jia et al. 2008), in the cytoplasm (Lenartowska et al. 2002; Navazio et al. 2002, Jia et al. 2008), on the spindle apparatus of dividing cells (Denecke et al. 1995), and associated with patches on the plasma membrane and with plasmodesmata (Baluška et al. 2001; Laporte et al. 2003; Bayer et al. 2004; Chen et al. 2005). In rice endosperm, CRT accumulates in ER-derived prolamine protein bodies and glutelin protein storage vacuoles (Torres et al. 2001) and in protein bodies in maize callus cells (Šamaj et al. 2008). CRT has never been found in vacuoles, although this cell compartment, as well as the cell wall, is generally regarded to be the main sites of Ca2+ storage in plants.
Based on the patterns of CRT gene expression and protein localization, there are several proposed functions for plant CRT. The best documented are for roles in cell proliferation (Dresselhaus et al. 1996), signal transduction (Droillard et al. 1997; Li et al. 2003; Komatsu et al. 2009), regeneration (Li and Komatsu 2000; Jin et al. 2005), gravistimulation (Heilmann et al. 2001), and stress/pathogen attack response (Denecke et al. 1995; Jaubert et al. 2002; Sharma et al. 2004; Chen et al. 2005; Jia et al. 2008). CRT has also been suggested to be involved in cell-to-cell communication via plasmodesmata (Baluška et al. 2001; Bayer et al. 2004). However, there have been very few systematic analyses of plant CRT function and thus much remains to be understood about the expression, physiological role, and subcellular distribution of CRT isoforms in plants.
It has long been recognized that variations of intracellular Ca2+ levels are involved in pollen tube growth and guidance (see reviews by Holdaway-Clarke and Hepler 2003; Kroeger et al. 2008). Consistent with its role as a Ca2+-binding protein, there are some indications that CRT may also be involved in reproductive events in plants. An elevated level of CRT gene expression has been correlated with fertilization (Dresselhaus et al. 1996; Nelson et al. 1997; Williams et al. 1997) and embryo development (Chen et al. 1994; Borisjuk et al. 1998). The protein has also been found in different flower organs including the anther, ovary, and seeds (Denecke et al. 1995; Hassan et al. 1995; Coughlan et al. 1997; Nelson et al. 1997; Navazio et al. 2002; Hsieh and Huang 2005; Nardi et al. 2006). Moreover, CRT is present in pollen and pollen tubes growing in vitro (Navazio et al. 1998; Nardi et al. 1998; Nardi et al. 2006). Our previous study on Petunia revealed that CRT is expressed in both pollen tubes growing in situ and in the transmitting cells of the closed style (Lenartowska et al. 2001; 2002). An important question is whether this CRT expression pattern is universal in the cells involved in pollen–pistil interactions or if it depends on the anatomical structure of the pistil transmitting tract. Thus, we have examined the localizations of CRT mRNA and protein in germinating pollen/growing pollen tubes in vitro and in situ in the hollow pistil of Heamanthus.
Materials and methods
Plant material
Anthers, pollen, and pistils of Haemanthus albiflos L. (commercial cultivars grown at room temperature in the Institute of Genaral and Molecular Biology, Nicolaus Copernicus University, Toruń, Poland) were used for study. Pollen was collected after dehiscence and used either as fresh material or dehydrated and stored at 4°C. For immunoblot analysis, closed anthers (dissected from flowers before and after anthesis) and pistils (unpollinated and pollinated at anthesis) were frozen in liquid nitrogen and stored at −80°C. For light/electron microscopy studies, samples of unpollinated and pollinated pistils were fixed, dehydrated in ethanol, and embedded as previously described (Lenartowska et al. 2002).
In vitro pollen germination/pollen tube growth
Pollen was germinated in liquid culture media as follows: 15 mM Mes, 1.6 mM BO3H, 1 mM KCl, 0.1 mM CaCl2, 7% sucrose, pH 5.5. Cultures were grown in culture dishes on a rotating platform at room temperature for at least 2 h, and germination rate and pollen tube length were assessed by light microscopy. In vitro cultivated pollen/pollen tubes were collected by centrifugation for 1 min at 200g, and pelleted cells were immediately frozen in liquid nitrogen for immunoblotting or fixed for FISH/immunolocalization.
Western blot analysis
A polyclonal antibody against maize CRT (CRT PAb) was used (Napier et al. 1995), and the specificity of the primary antibody was verified using maize seedlings as a positive control. The samples (mature anthers before/after anthesis, dehydrated pollen, in vitro cultivated pollen tubes, and unpollinated/pollinated pistils at different time after pollination) were homogenized in liquid nitrogen and soluble proteins were extracted with 50 mM Tris–HCl (pH 7.5), 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 5 mM NaF, 10 μg/ml aprotinine, and 10 μg/ml leupeptine. The homogenates were centrifuged at 16,000g for 30 min at 4°C and the protein concentration of the supernatants was determined (Bradford 1976). Equal amounts of proteins (30 μg per lane) were separated by electrophoresis on a 12.0% SDS–PAGE gel and molecular weights were determined with standard molecular marker (Fermentas). Proteins were subsequently transferred to a PVDF Hybond-P membrane (Amersham Pharmacia Biotech) and blotted with the CRT PAb (1:2000 dilution). Protein bands were detected by ECL (Amersham Pharmacia Biotech), according to the manufacturer’s guidelines.
Confocal/fluorescence microscopy
In vitro cultivated pollen/pollen tubes as well as unpollinated and pollinated samples of dissected styles and ovules were fixed with freshly prepared 4% formaldehyde in phosphate-buffered saline (PBS), pH 7.2, overnight at 4°C. Samples were washed in PBS and 0.1 M citrate buffer (55 mM citric acid, 125 mM sodium citrate, pH 4.8), digested in a mixture of 1% cellulase R10 (Serva), 27 U/ml pectinase (Sigma), and 0.01% citrate buffer, pH 4.8, for 45 min at 37°C. Then cells were permeabilized with 0.1% Triton X-100 (Sigma) in PBS and placed on microscope slides covered with Biobond (BioCell). For sectioning, tissue specimens (styles and ovules) were washed in PBS, dehydrated in ethanol, embedded in butyl methyl methacrylate (BMM, Fluka), sectioned with a diamond knife into semi-thin sections (Leica Ultracut), and transferred onto microscope slides covered with Biobond (BioCell).
A three-step method was developed to simultaneously label DNA, CRT protein, and CRT mRNA in in vitro-cultivated cells and sections. In the first step, CRT mRNA was localized by fluorescent in situ hybridization (FISH) using an antisense digoxygenin (DIG)-UTP-labeled RNA probe generated by in vitro transcription using T7 polymerase following the manufacturer’s protocol (Roche). A maize CRT 1.6 kb cDNA clone (Napier et al. 1995) was used as the template to transcribe the probe. Hybridization was carried out in 50% formamide overnight at 42°C. Signals were detected using primary mouse anti-DIG and secondary goat-anti-mouse IgG-Alexa Fluor 488 antibodies (Roche and Molecular Probes, respectively). In the second step, localization of CRT protein was determined. Non-specific protein binding sites were blocked with 3% bovine serum albumin (BSA) in PBS, pH 7.2. After blocking, cells and sections were treated with CRT PAb followed by incubation with goat anti-rabbit IgG-Alexa Fluor 546 (Molecular Probes), both diluted 1:100 in PBS with 1% BSA. Controls were performed without the probe and primary antibody. In the final step, DNA was stained with 4′,6-diamidino-2-phenylindole in 4 μg/ml (DAPI, Fluka) concentration. Images of in vitro cultivated cells were acquired using the software package EZ 2000 Viewer connected to a confocal Nikon microscope PCM 2000-Eclipse TE 300. Semi-thin sections were analyzed using a Nikon Eclipse 80i fluorescence microscope and images were collected using a Nikon digital camera DS-5Mc and Lucia General software (Laboratory Imaging).
Transmission electron microscopy
For immunolocalization of CRT and callose on ultrathin sections of pollinated pistils, an immunogold double labeling technique was developed. The chemical fixation, embedding, and sectioning were performed as previously described (Lenartowska et al. 2002). After blocking with 5% BSA (Sigma) in PBS, pH 7.2, sections were treated with two kinds of primary antibodies: CRT PAb and monoclonal anti-(1 → 3)-β-glucan antibody (anti-Cal MAb, Biosupplies), diluted 1:20 and 1:50, respectively, in PBS with 1% BSA. Antibody binding was detected with the following secondary antibodies (BioCell): 20 nm diameter gold-conjugated goat anti-rabbit (for CRT) and 10 nm diameter gold-conjugated goat anti-mouse (for callose), both diluted 1:100. In the control, incubations with the primary antibodies were omitted. The sections were stained with 2.5% uranyl acetate and examined using a JEOL 1010 transmission electron microscope at 80 kV. Localization of loosely bound Ca2+ (exchangeable calcium) in pollinated pistils was determined cytochemically using potassium antimonite precipitation (Bednarska et al. 2005).
Results
Calreticulin expression
We first wished to determine if CRT is expressed during Haemanthus pollen maturation and germination, and pollen tube growth in vitro and in situ. A maize anti-CRT antibody (Napier et al. 1995) was expected to cross-react with Haemanthus CRT. To confirm the specificity of the antibody in Haemanthus, we first compared protein samples from the two species by Western-blot. While the antibody recognized a single 55 kDa band in extracts made from maize seedlings (used as a positive control), three bands were observed in Haemanthus tissue samples: a doublet at 55 kDa and a single band at 45 kDa (Fig. 1, compare lanes 1 and 2–9). The 45-kDa band may be a shorter isoform of CRT, as plants have been shown to contain two or more CRT isoforms with different molecular weights corresponding to differences in N-linked glycosylation (Chen et al. 1994; Nelson et al. 1997; Navazio et al. 1998; Persson et al. 2003; Hsieh and Huang 2005). Alternatively, the smaller band could be a degradation product. Identical bands were observed in all Haemanthus samples investigated: mature anthers dissected before and after flower anthesis (Fig. 1, lanes 2 and 3, respectively), dry pollen and in vitro growing pollen tubes (Fig. 1, lanes 4 and 5, respectively), unpollinated (Fig. 1 lane 6), and pollinated pistils at different times after pollination (Fig. 1, lanes 7, 8 and 9). Comparable high levels of CRT were observed in all Haemanthus samples.
Western blotting of total protein extracts using CRT PAb from maize (lane 1) and Haemanthus (lanes 2–9). Lanes: 1, maize seedlings; 2, closed anther before anthesis; 3, closed anther after anthesis; 4, dry pollen; 5, in vitro growing pollen tubes; 6, unpollinated pistil; 7–9, pollinated pistils (6, 24, and 48 h after pollination, when pollen tubes grow in the stigma, style and ovary, respectively)
Expression and localization of CRT in germinated pollen and growing pollen tubes in vitro
To begin to characterize the role CRT may play in pollen germination/pollen tube growth, we wished to determine the sub-cellular localization of CRT transcripts and protein in rehydrated and germinated pollen, and in growing pollen tubes of Haemanthus. Samples were processed for FISH and immunolabeling and visualized by confocal microscopy. In pollen hydrated in culture medium, diffuse labeling was observed for both transcripts (Fig. 2a, green) and protein (Fig. 2d, red). The strongest signals detected were associated with the aperture regions (Fig. 2a and d, arrows). The preferential accumulation of CRT transcripts and protein at apertures was also observed in germinating pollen (Fig. 2b, e, f, respectively). In germinating pollen, CRT mRNA (green) was detected predominantly along the edge of the cell (Fig. 2b), whereas CRT protein (red) was more uniformly distributed (Fig. 2e, f). Enrichment of CRT protein was not observed at an extra-apical zone of the tip-growing cell (Fig. 2f, arrow). In germinating pollen, CRT mRNA was also detected in vegetative nuclei (Fig. 2b) and at a second (inactive) aperture region (Fig. 2b, arrow).
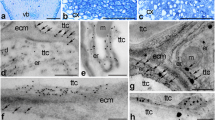
Localization of CRT mRNA (green) and protein (red) in germinated pollen and pollen tubes growing in vitro (a–j) and in situ (k–n) by fluorescence microscopy. a and d, pollen re-hydrated in culture medium, arrows show the apertures; b, c, e, f, pollen germinating in culture medium, arrows in b and c show inactive aperture or extra-apical zone of the tube, respectively; h–j, double localization of CRT and its transcript in pollen tubes growing in vitro, arrow in h shows hybridization signal in and around vegetative nuclei, arrows in i show CRT preferential localization in cytoplasmic periphery and in some patches (arrowheads); j, merge; k–n, double labeling of CRT mRNA and protein in unpollinated (k) and pollinated (l–n) pistils, arrowheads show CRT mRNA localization around tte cells nuclei; c and g, control sections. c style or micropylar canal, gn generative nuclei, mc micropylar cells, pg pollen grain, pt pollen tube, vn vegetative nuclei. Bar 10 μm
Double labeling experiments using both CRT antisense probe and CRT PAb clearly showed co-localization of CRT protein (red) and its transcripts (green) in elongated pollen tubes growing in vitro (Fig. 2h–j). Both CRT mRNA and protein were found to be highly abundant in the shank of the tube, whereas weaker labeling of the apical zone was observed. By contrast, the area of the pollen grain that became empty of cytoplasm at this stage became almost completely devoid of CRT mRNA and protein. In pollen tubes, CRT transcripts were fairly uniformly distributed through the cell from the base to the sub-apical zone of the tube. A strong hybridization signal was seen in the region of the vegetative nucleus (Fig. 2h, arrow), but very little signal was detected in the region of the generative nucleus (Fig. 2h, arrowhead). CRT protein was enriched at the periphery of the tube (Fig. 2i, arrows), and several patches within the basal regions of the pollen tube revealed relatively high levels of CRT protein (Fig. 2i, arrowheads). In in vitro cultivated cells, the positions of generative and vegetative nuclei were marked by DAPI staining (Fig. 2j, inset). Control in which the probe and CRT PAb were omitted resulted in a complete lack of labeling (Fig. 2c). The observed mRNA and protein localization is consistent with CRT playing a role in pollen germination and pollen tube growth.
Expression and localization of CRT in situ
To determine the localization pattern of CRT mRNA and protein during pollen - pistil interactions, we sectioned pistils before and after pollination. Cross-sections of unpollinated (Fig. 2k) and pollinated (Fig. 2l–n) pistils showed preferential localizations of CRT mRNA (green) and protein (red) in the tte cells (Fig. 2k–m). In those cells, the mRNA and protein were detected in the cytoplasm, and the transcripts were also found in close proximity to the tte cell nuclei (Fig. 2k and m, arrowheads). After pollination, a particularly dense co-localization of CRT protein and its transcripts was observed in pollen tubes (Fig. 2l, m, orange-yellow fluorescence). The pollen tube tip showed uniform fluorescence (Fig. 2l), whereas in the vacuolated tube region, mRNA and protein were preferentially localized in the peripheral cytoplasm and/or the cell wall of the tube (Fig. 2m). In contrast to the styles, the micropyle region in the ovule after pistil pollination contained a high abundance of CRT mRNA and protein (Fig. 2n). Both CRT transcripts (green) and protein (red) accumulated in the cells surrounding the micropylar canal; however, CRT protein was distributed mainly in the peripheral regions of these cells (Fig. 2n). After pollination, a strong hybridization signal and immunolabeling were also found in pollen tubes that were in close proximity to the micropyle (Fig. 2n). Chemical fixation probably removed the exudate that is secreted into the stylar canal of the pollinated pistil, so we were not able to detect any signals from it. The positions of cells nuclei were marked by DAPI staining on the sections. Control in which the probe and CRT PAb were omitted resulted in a complete lack of labeling (Fig. 2g).
Immunogold CRT and callose localization in pollinated pistils
Light microscopy resolution does not allow one to state exactly the sub-cellular compartments labeled with CRT PAb. To obtain higher-resolution localization of CRT, we performed immuno-electron microscopy on in situ germinating pollen and growing pollen tubes. CRT protein was distributed rather uniformly in the cytoplasm of early-rehydrated pollen grains (Fig. 3a). In germinated pollen, the highest level of labeling was limited to the aperture region and peripheral cytoplasm (Fig. 3b, c). In rehydrated as well as germinated pollen the cytoplasmic regions labeled with CRT PAb could mainly correspond to ER (Fig. 3a, arrows), Golgi stacks, and vesicles frequently occurring in the cytoplasm of the male gametophyte, what has been shown by ultrastructural studies in many different species. No staining was observed in vacuoles and in any layer of the pollen sporoderm. In growing pollen tubes, CRT protein labeling was strongest towards the peripheral cytoplasm, adjacent to the plasma membrane (Fig. 3e, f) where long ER cisternae were present (Fig. 3e). Some gold traces were also found in the inner cell wall of the pollen tube (Fig. 3f). Double labeling experiments using both CRT PAb and anti-Cal MAb clearly showed that CRT co-localized tightly with callose at the pollen tube inner cell wall (Fig. 3g, black and white arrowheads, respectively). By contrast, callose was not co-localized with CRT on secretory vesicles present in the tube cytoplasm (Fig. 3g, black arrowheads). No CRT labeling was observed in vacuoles or the fibrillar outer cell wall of the tube (Fig. 3f, g). In the stylar somatic tte cells CRT was mainly localized in the Golgi stacks (Fig. 4a, arrows) and in the ER (Fig. 4b, arrows). Double staining clearly demonstrated that CRT also co-localized tightly with callose at the plasmodesmata connecting tte cells (Fig. 5a, b). Because the finding of CRT in the cell wall was somewhat unexpected (see “Discussion”), we conducted several controls to confirm the specificity of the immunogold reaction. No background was observed when control sections were incubated with only secondary gold-labeled antibody (Fig. 3d). Furthermore, binding of the CRT PAb was almost completely inhibited when the sections were pretreated with proteinase K (Lenartowska et al. 2002). The specificity of used CRT PAb to CRT was verified by Napier et al. (1995). Therefore, the inner cell wall staining reflects the presence of CRT in this region.
Immunogold localization of CRT and callose in germinating pollen and growing pollen tubes in situ. a early rehydrated pollen, arrows show the possible ER position; b, c germinating pollen; d control; e, f growing pollen tubes; g double localization of CRT (20 nm gold, black arrowheads) and callose (10 nm gold, white arrowheads) in growing pollen tube. a amyloplast, c cytoplasm, cw cell wall, er endoplasmic reticulum, ex exine, icw inner cell wall of the tube, in intine, ocw outer cell wall of the tube, v vacuole. Bar 200 nm
Exchangeable calcium is observed in regions where CRT was found
Loosely bound Ca2+ (exchangeable calcium) represents a structurally stable pool that can associate with a variety of Ca2+-binding proteins, and transfer calcium into other forms as needed (Wick and Hepler 1982; Zhao et al. 2002). Because CRT is a Ca2+-binding/buffering protein, we hypothesized that exchangeable calcium would be in the same cellular regions in which we saw CRT localization. To test this idea, ultrathin sections through the style and ovule of pollinated pistils were fixed with 2% antimonate to precipitate loosely bound Ca2+. A large mass of Ca2+ precipitates was observed occupying the pollen tubes growing on the pistil, as well as stylar tte cells and the micropyle region of the ovule (Fig. 6). In the sub-tip zone of the pollen tube, which is rich in mitochondria and Golgi stacks, precipitates were localized mainly at the border between plasma membrane and cell wall (Fig. 6a, arrows), and also in dictyosomes (Fig. 6b, arrows). In the vacuolated region of the tube, precipitates most strongly labeled peripheral cytoplasm (Fig. 6c, arrows) and the cell wall: both its inner (Fig. 6c, white arrowheads) and outer (Fig. 6c, black arrowheads) layers. Some precipitates were also found in the extracellular matrix of the stylar transmitting tract and at the surface of the tte cells surrounding the stylar canal (Fig. 6c). Numerous precipitates were also localized in the ER, which was enriched at the peripheral cytoplasm of these cells (Fig. 6d, arrows). The precipitates at the micropyle region of the ovule were mainly localized in the cytoplasm and in the extended walls of the cells surrounding the micropylar canal (Fig. 6e, f). Consistent with CRT being a Ca2+-binding protein, high levels of loosely bound Ca2+ were often observed in the same regions of the pollen tubes and pistil transmitting tract cells where CRT was found.
Subcellular loosely bound Ca2+ localization in pollinated pistil. a–d cross-sections of pollinated style; e, f longitudinal sections of ovule micropylar region. a, b sub-tip region of the tube, arrows in a show Ca2+ precipitates at the border line between plasma membrane and cell wall, arrows in b show Golgi stacks; c, vacuolated zone of the tube, Ca2+ precipitates are present in peripheral cytoplasm (arrows), inner and outer cell wall (white and black arrowheads, respectively); d, Ca2+ precipitates accumulated in the ER of tte cells (arrows). c cytoplasm, cw cell wall, ecm extracellular matrix, er endoplasmic reticulum, g Golgi stacks, m mitochondrium, mc micropylar cell, pt pollen tube, tte transmitting tract epidermis. Bars 2 μm (e), 1 μm (a, c, f), 200 nm (b and d)
Discussion
Our results show that CRT is highly and constitutively expressed in mature anthers and in the cells involved in pollen–pistil interactions, such as pollen/pollen tubes and somatic cells of the pistil transmitting tract. These findings support the idea that CRT, which is responsible for Ca2+ homeostasis, may be regulated as a housekeeping gene during pollen maturation, pollen germination, and pollen tube growth. Thus, it is surprising that previous studies in tobacco revealed a stronger expression of CRT protein in developing and mature anthers than in dry pollen or germinating pollen and pollen tubes growing in vitro (Nardi et al. 2006). The reason for this difference should be explored in the future. Nevertheless, early embryo development in the Haemanthus fertilized ovaries may be accompanied by an elevated level of CRT expression as earlier studies have revealed (Chen et al. 1994; Nelson et al. 1997; Borisjuk et al. 1998).
Two major biochemical mechanisms driving pollen germination and pollen tube elongation were documented by recent studies: a tip-focused Ca2+ gradient within the pollen tube cytoplasm and the contribution of actin filaments to the elongation process (see reviews by Holdaway-Clarke and Hepler 2003; Cheung and Wu 2008). Upon pollen rehydration and germination, intracellular Ca2+ level at the germinal aperture increases to a higher level than in other regions, and a tip-focused Ca2+ gradient is then established and sustained while a pollen tube grows (see review by Holdaway-Clarke and Hepler 2003). This stable Ca2+ gradient is required for the proper organization and dynamics of the actin cytoskeleton that differs between the shank and apical region of the tube (Li et al. 2001; Lovy-Wheeler et al. 2005; Lenartowska and Michalska 2008). The actin cytoskeleton is essential for polarized pollen tube growth (Cárdenas et al. 2005, 2008) and several actin-binding proteins (ABPs) that affect actin assembly and dynamics have been identified in pollen/pollen tubes (see reviews by Ren and Xiang 2007; Cai and Cresti 2008). Although many of them are Ca2+-regulated and some Ca2+-binding signaling molecules have been found in pollen and pollen tubes, how the tip-focused Ca2+ gradient may be sensed and mediated during tube tip growth is still unknown. While the sites and mechanism of Ca2+ mobile storage in growing pollen tubes have not been identified, two likely candidates are (1) internal stores (membrane compartments within the cytoplasm, e.g., ER, Golgi, and vesicles) and (2) external stores, that is, the cell wall. Our confocal and immuno-electron microscopy data clearly show that both of these speculated stores are enriched in CRT. Furthermore, our data show a correlation between high level of CRT expression and presence of exchangeable calcium in Haemanthus pollen tubes. In the pollen tubes growing in situ CRT and exchangeable calcium co-localized at the regions where ER and Golgi stacks were present: sub-apex and shank of the tube. This pool of calcium thereby could represent Ca2+ bound by CRT (and other Ca2+-binding proteins) that becomes an important source of easily releasable calcium when and where it is needed (Zhao et al. 2002, Michalak et al. 2009). We therefore suggest that the Ca2+-binding/buffering activity of CRT is involved in modulation of local Ca2+ concentrations at the aperture of germinated pollen and during pollen tube growth, which seem to be critical for polar tip-growth of the tube.
Double localization of CRT mRNA and protein on sectioned pistils revealed that CRT is specifically expressed in the stylar tte cells which lines the hollow style in Haemanthus pistil and in the cells of the ovule micropylar region. Both cell types also revealed a relatively high level of exchangeable calcium accumulated in their cytoplasm and cell wall. Thus, CRT may be also involved in regulation Ca2+ homeostasis in these cells, as well as in growing pollen tube. In tte cells, CRT was mainly localized in ER and Golgi stacks, and co-localized tightly with callose at the plasmodesmata. Work in other plants has also demonstrated CRT accumulation within plasmodesmata, suggesting that CRT may regulate the architecture of these structures via its Ca2+-buffering capacity and thus be involved in cell-to-cell communication (Baluška et al. 2001; Laporte et al. 2003; Bayer et al. 2004).
In the present work, CRT localization was observed in the Haemanthus pollen tube inner cell wall. Similar results were found in Petunia (Lenartowska et al. 2002). However, studies on in vitro cultivated tobacco pollen tubes revealed relatively intense label of CRT that was seen adjacent to the pollen tube cell wall but did not show a significant label in the cell wall itself (Nardi et al. 2006). These differences are probably due to the different conditions of pollen tube growth. There is a series of signaling events in pollen–pistil interactions starting at the stigma and ending at the ovary that guide the pollen tube to reach the micropyle which do not function in vitro. Other studies have demonstrated that a pollen-specific protein LP28 and two transmitting tissue-specific proteins, 120 kDa glycoprotein and PELPIII, were also located in the callosic cell wall of developing pollen and pollen tubes growing in situ (Lind et al. 1996; Mogami et al. 2002; Graaf et al. 2003). Therefore, it is possible that CRT found in callosic cell wall may be translocated from ER/Golgi into it and play a role in external Ca2+ storage in pollen tubes growing in situ, while deposition of the protein in that compartment is not required in vitro. On the other hand, our data revealing the typical localization of CRT in Golgi stacks and vesicles would be involved in the secretion of cell wall precursors during the tip-growth of the tube.
An important question is when and where CRT mRNA and protein are synthesized. Several studies in Hyacinthus, Nicotiana, and Liriodendron suggest that mature pollen, although transcriptionally silent, contains the pool of long-lived RNA which is probably synthesized during pollen development and stored until the time of pollen germination and tube growth (Navazio et al. 1998; Nardi et al. 2006; Zienkiewicz et al. 2008). Our results demonstrating the presence of CRT gene transcripts and protein in rehydrated and germinated pollen and growing pollen tubes support this idea. On the other hand, our data showing hybridization signals in vegetative nuclei of germinated pollen and growing pollen tubes suggest that CRT mRNA synthesis could be re-initiated following germination. This is consistent with data indicating that de novo RNA synthesis is probably occurring during pollen germination and tube elongation (Fernando et al. 2001; Zienkiewicz et al. 2008). We have also revealed a co-localization of CRT mRNA and protein in Haemanthus germinated pollen and growing pollen tubes in vitro, and preferential CRT localization in the ER of the pollen tubes growing in the pistil. Furthermore, our data confirmed the presence of CRT transcripts on the ER surface in Petunia (Lenartowska et al. 2001). Thus, we suggest that the rough ER is the site of CRT translation.
Taken together, we conclude that CRT in mature anthers, germinating pollen, growing pollen tubes, and somatic cells of the pistil transmitting tract could regulate development and biological functions of the cells engaged in pollen–pistil interactions by modulating Ca2+ homeostasis involving both internal and external Ca2+ stores. In addition, our previous (Lenartowska et al. 2002) and present studies show similar patterns of CRT localization in pollen tubes growing in anatomically different types of pistil styles (closed in Petunia and hollow in Haemanthus) and suggest that this CRT distribution is universal during pollen–pistil interactions.
Abbreviations
- BSA:
-
Bovine serum albumin
- BMM:
-
Butyl methyl methacrylate
- MAb:
-
Monoclonal antibody
- PAb:
-
Polyclonal antibody
- CRT:
-
Calreticulin
- mRNA:
-
Messenger RNA
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- FISH:
-
Fluorescent in situ hybridization
- Mes:
-
Sigma BioUltra Buffer
References
Baldan B, Navazio L, Friso A, Mariani P, Meggio F (1996) Plant calreticulin is specifically and efficiently phosphorylated by protein kinase CK2. Biochem Biophys Res Commun 221:498–502
Baluška F, Cvrčková F, Kendrick-Jones, Volkmann D (2001) Sink plasmodesmata as gateways for phloem unloading. Myosin VIII and calreticulin as molecular determinants of sink strength? Plant Physiol 126:39–46
Bayer E, Thomas CL, Maule AJ (2004) Plasmodesmata in Arabidopsis thaliana suspension cells. Protoplasma 223:93–102
Bednarska E, Lenartowska M, Niekraś L (2005) Localization of pectins and Ca2+ ions in unpollinated and pollinated wet (Petunia hybrida Hort.) and dry (Haemanthus albiflos L.) stigmas. Folia Histochem Cytobiol 43:249–259
Borisjuk N, Sitailo L, Adler K, Malysheva L, Tewes A, Borisjuk L, Manteuffel R (1998) Calreticulin expression in plant cells: developmental regulation, tissue specificity and intracellular distribution. Planta 206:504–514
Bradford MM (1976) A rapid and sensitive method of the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cai G, Cresti M (2008) Organelle motility in the pollen tube: a lale of 20 years. J Exp Bot 26:1–15
Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK (2005) Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil Cyto 61:112–127
Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146:1611–1621
Chen F, Hayes PM, Mulrooney DM, Pan A (1994) Identification and characterization of cDNA clones encoding plant calreticulinin barley. Plant Cell 6:835–843
Chen MH, Tian GW, Gafni Y, Citovsky V (2005) Effects of calreticulin on viral cell-to-cell movement. Plant Physiol 138:1866–1876
Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59:547–572
Coughlan SJ, Hastings C, Winfrey R Jr (1997) Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol Biol 34:897–911
Denecke J, Carlsson LE, Vidal S, Höglund AS, Ek B, van Zeijl MJ, Sinjorgo KM, Palva ET (1995) The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell 7:391–406
Dresselhaus T, Hagel C, Lorz H, Kranz E (1996) Isolation of a full length cDNA encoding calreticulin from a PCR library of in vitro zygotes of maize. Plant Mol Biol 31:23–34
Droillard MJ, Guclu J, Le Caer JP, Mathieu Y, Guern J, Lauriere C (1997) Identification of calreticulin-like protein as one of phosphoproteins modulated in response to oligogalacturonides in tobacco cells. Planta 202:341–348
Fernando DD, Owens JN, Yu X, Ekramoddullah AKM (2001) RNA and protein synthesis during in vitro pollen germination and tube elongation in Pinus monticola and other conifers. Sex Plant Reprod 13:259–264
Graaf BHJ, Knuiman BA, Derksen J, Mariani C (2003) Characterization and localization of the transmitting tract tissue-specific PELP III proteins of Nicotiana tabacum. J Exp Bot 54:55–63
Hassan AM, Wesson C, Trumble WR (1995) Calreticulin is the major Ca2+-storage proteinin the endoplasmic reticulum of the pea plant (Pisum sativum). Biochem Biophys Res Commun 211:54–59
Heilmann I, Shin J, Huang J, Perera IY, Davies E (2001) Transient dissociation of polyribosomes and concurrent recruitment of calreticulin and calmodulin transcripts in gravistimulated maize pulvini. Plant Physiol 127:1193–1203
Holdaway-Clarke T, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytologist 159:539–563
Hsieh K, Huang AH (2005) Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J 43:889–899
Jaubert S, Ledger TN, Laffaire JB, Piotte C, Abad P, Rosso MN (2002) Direct identification of stylet secreted proteins from root-knot nematodes by a proteomic approach. Mol Biochem Parasitol 121:205–211
Jia XY, Xu CY, Jing RL, Li RZ, Mao XG, Wang JP, Chang XP (2008) Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. J Exp Bot 59:739–751
Jin ZL, Hong JK, Yang KA, Koo JC, Choi YJ, Chung WS, Yun DJ, Lee SY, Cho MJ, Lim CO (2005) Over-expression of Chinese cabbage calreticulin 1, BrCRT1, enhances shoot and root regeneration, but retards plant growth in transgenic tobacco. Transgenic Res 14:619–626
Komatsu S, Jan A, Koga Y (2009) Characterization of a histidine- and alanine-rich protein showing interaction with calreticulin in rice. Amino Acids 36:137–146
Kroeger JH, Geitmann A, Grant M (2008) Model for calcium dependent oscillatory growth in pollen tubes. J Theor Biol 253:363–374
Laporte C, Vetter G, Loudes AM, Robinson DG, Hillmer S, Stussi-Garaud C, Ritzenthaler C (2003) Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY-2 cells. Plant Cell 15:2058–2075
Lenartowska M, Michalska A (2008) Actin filament organization and polarity in pollen tubes revealed by myosin II subfragment 1 decoration. Planta 228:891–896
Lenartowska M, Lenartowski R, Bednarska E (2001) Localization of the calreticulin gene mRNA in unpollinated and pollinated styles of Petunia hybrida Hort. J Appl Genet 42:15–20
Lenartowska M, Karaś K, Marshall J, Napier R, Bednarska E (2002) Immunocytochemical evidence of calreticulin-like protein in pollen tubes and styles of Petunia hybrida Hort. Protoplasma 219:23–30
Li Z, Komatsu S (2000) Molecular cloning and characterization of calreticulin, a calcium-binding protein involved in the regeneration of rice cultured suspension cells. Eur J Biochem 267:737–745
Li Y, Zee SY, Liu YM, Huang BQ, Yen LF (2001) Circular F-actin bundless and G-actin gradient in pollen and pollen tubes of Lilium davidii. Planta 213:722–730
Li Z, Onodera H, Ugaki M, Tanaka H, Komatsu S (2003) Characterization of calreticulin as a phosphoprotein interacting with could-induced protein kinase in rice. Bioll Pharm Bull 26:256–261
Lind JL, Bönig I, Clarke AE, Anderson MA (1996) A style-specific 120 kDa glycoprotein enters pollen tubes of Nicotiana alata in vivo. Sex Plant Reprod 9:75–86
Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK (2005) Enhanced fixation reveals the apical cortical fringe of actin filaments as consistent feature of the pollen tube. Planta 221:95–104
Mariani P, Navazio L, Zuppini A (2003) Calreticulin and the endoplasmatic reticulum in plant cell biology. In: Michalak M, Eggleton P (eds) Calreticulin, 2nd edn. Landes Bioscence, Georgetown, pp 94–101
Michalak M, Groenedyk J, Szabo E, Gold LI, Opas M (2009) Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 417:651–666
Mogami N, Shiota H, Tanaka I (2002) LP28, a lily pollen-specific LEA-like protein, is located in the callosic cell wall during male gametogenesis. Sex Plant Reprod 15:57–63
Napier RM, Trueman S, Henderson J, Boyce JM, Hawes C, Fricker MD, Venis MA (1995) Purification, sequencing and functions of calreticulin from maize. J Exp Bot 46:1603–1613
Nardi MC, Giacomelli E, Dainese P, Fitchette-Laine AC, Faye L, Baldan B, Navazio L, Mariani P (1998) Ginkgo biloba L. expresses calreticulin, the major calcium-binding reticuloplasmin in eukaryotic cells. Botanica Acta 111:66–70
Nardi CN, Feron R, Navazio L, Mariani P, Pierson E, Wolters-Arts M, Knuiman B, Mariani C, Derksen J (2006) Expression and localization of calreticulin in tobacco anthers and pollen tubes. Planta 223:1263–1271
Navazio L, Sponga L, Dainese P, Fitchette-Laine AC, Faye L, Baldan B, Mariani P (1998) The calcium binding protein calreticulin in pollen of Liriodendron tulipifera L. Plant Sci 131:35–42
Navazio L, Miuzzo M, Royle L, Baldan B, Varotto S, Merry AH, Harvey DJ, Dwek RA, Rudd PM, Mariani P (2002) Monitoring endoplasmic reticulum-to-Golgi traffic of a plant calreticulin by protein glycosylation analysis. Biochemistry 41:14141–14149
Nelson DE, Glaunsinger B, Bohnert HJ (1997) Abundant accumulation of the calcium-binding molecular chaperone calreticulin in specific floral tissues of Arabidopsis thaliana. Plant Physiol 114:29–37
Opas M, Tharin S, Milner RE, Michalak M (1996) Identification and localization of calreticulin in plant cells. Protoplasma 191:164–171
Persson S, Wyatt SE, Love J, Thompson WF, Robertson D, Boss WF (2001) The Ca2+ status of the endoplasmic reticulum is alerted by induction of calreticulin expression in transgenic plants. Plant Physiol 126:1092–1104
Persson S, Rosenquist M, Sommarin M (2002) Identification of a novel calreticulin isoform (Crt2) in human and mouse. Gene 297:151–158
Persson S, Rosenquist M, Svensson K, Galvão R, Boss WF, Sommarin M (2003) Phylogenetic analyses and expression studies reveal two distinct groups of calreticulin isoforms in higher plants. Plant Physiol 133:1385–1396
Ren H, Xiang Y (2007) The function of actin-binding protein in pollen tube growth. Protoplasma 230:171–182
Šamaj J, Salaj J, Obert B, Baluška F, Menzel D, Volkmann D (2008) Calreticulin mRNA and protein are localized to protein bodies in storage maize callus cells. Plant Cell Rep 27:231–239
Sharma A, Isogai M, Yamamoto T, Sakaguchi K, Hashimoto J, Komatsu S (2004) A novel interaction between calreticulin and ubiquitin-like nuclear protein in rice. Plant Cell Physiol 45:684–692
Torres E, Gonzalez-Melendi P, Stöger E, Shaw P, Twyman RM, Nicholson L, Vaquero C, Fischer R, Christou P, Perrin Y (2001) Native and artificial reticuloplasmins co-accumulate in distinct domains of the endoplasmic reticulum and in post-endoplasmic reticulum compartments. Plant Physiol 127:1212–1223
Wick SM, Hepler PK (1982) Selective localization of intracellular calcium with potassium antimonite. J Hisiochem Cytochem 30:1190–1204
Williams CM, Zhang G, Michalak M, Cass DJ (1997) Calcium induced protein phosphorylation and changes in levels of calmodulin and calreticulin in maize sperm cells. Sex Plant Reprod 10:83–88
Wyatt SE, Tsou PL, Robertson D (2002) Expression of the high capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res 11:1–10
Zhao J, Yu FL, Liang SP, Zhou C, Yang HY (2002) Changes of calcium distribution in egg cells, zygotes and two-called proembryos of rice (Oryza sativa L.). Sex Plant Reprod 14:331–337
Zienkiewicz K, Zienkiewicz A, Rodriguez-Garcia MI, Smoliński DJ, Świdziński M, Bednarska E (2008) Transcriptional activity and distribution of splicing machinery elements during Hyacinthus orientalis pollen tube growth. Protoplasma 233:129–139
Acknowledgments
The authors thank J.M. Boyce (Oxford University, Oxford, UK) for CRT cDNA plasmid and R.M. Napier (University of Warwick, Wellesbourne, UK) for CRT antibody. We thank O. Narbutt and M. Świdziński for their excellent technical assistance, and D.J. Frank (Washington University in St. Louis, US) and M. Opas (University of Toronto, Toronto, Canada) for critical reading of the manuscript. This project was supported by the Ministry of Science and Higher Education, grants 3 P04C 051 23 and N303 023 32/1034.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to the memory of our friend Bogdan Wróbel.
Rights and permissions
About this article
Cite this article
Lenartowska, M., Lenartowski, R., Smoliński, D.J. et al. Calreticulin expression and localization in plant cells during pollen–pistil interactions. Planta 231, 67–77 (2009). https://doi.org/10.1007/s00425-009-1024-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-1024-1