Abstract
Spatially defined growth of cells in culture is a useful model for studies ranging from the characterization of cellular motility to the analysis of network behaviour in structurally defined ensembles of excitable cells. Current methodological approaches for obtaining patterned growth include sophisticated modifications of surface chemistry, stamping techniques and microfluidics. The implementation of most of these techniques requires the availability of highly specialized apparatus and some of the methods are specific for certain cell types and/or substrate materials. The goal of the present study was to develop a cell-patterning technique that can be implemented by any laboratory working with cell culture and that is highly adaptable in terms of cell types and substrate materials. The method is based on a photolithographic process that permits the patterned deposition of attachment factors of choice on surfaces previously coated with agar with a spatial resolution (maximal deviation from a straight line) of ±3 µm. Because agar efficiently prevents cell adhesion, patterned growth obtained with this technique displays virtually no off-pattern cell attachment. The method permitted the patterning of cardiomyocytes, fibroblasts and HeLa cells on either glass substrates or polymer-coated materials with a spatial resolution of a few micrometers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, patterned growth of cells in culture has received increasing attention in the context of studying cellular interactions in networks of excitable cells of both cardiac and neuronal origin. For patterned growth of neurons, techniques available include lift-off procedures involving silicon masks [3], spatially defined coating of substrates with attachment factors using stamping techniques [2, 18] or microfluidics [4, 10] and modifications of the surface chemistry of the substrates [5, 6]. For cultured cardiomyocytes, the principles of patterned growth were pioneered in the early 70s by Lieberman and colleagues who developed methods for growing linear cell structures or spheroidal cell aggregates from dissociated embryonic chick heart cells [14]. The reproducible construction of defined two-dimensional growth patterns of cardiomyocytes in primary culture became feasible with the introduction of photolithographic techniques [16]. That method was based on coating glass cover-slips with a photosensitive resist which prevented the attachment of cardiomyocytes. The photoresist-coated glass was exposed to a mask carrying the desired growth patterns and, following development of the substrates, freshly isolated cardiomyocytes adhered preferentially to the re-exposed glass surface. Whereas this method worked well for cardiomyocytes in primary culture [9, 15], it was not suited for other cell types like fibroblasts and HeLa cells because these cells tended to adhere to the photoresist as well. Moreover, the method was occasionally hampered by batch-to-batch variations in the quality of the photoresist that resulted in a substantial degree of off-pattern adhesion. In addition, the technique worked only with glass surfaces. To overcome these problems, we have developed a new method using photolithography for the patterned deposition of attachment factors. Because adhesion-promoting substances can be chosen according to the specific requirements of a given cell type in culture, this new technique permits the patterned growth of a variety of different cell types with a high degree of reproducibility and virtually no off-pattern adhesion of cells over extended periods of time in culture. Moreover, besides patterning of glass substrates, the technique is suitable for coating of polymer surfaces, thus permitting, for example, the construction of geometrically defined cell patterns on polymer-insulated arrays of extracellular electrodes. Because the technique needs little specialized instrumentation, it can be readily implemented in any laboratory working with cell culture.
Materials and methods
Cell culture
Cardiomyocytes
Primary cultures of neonatal rat ventricular cardiomyocytes were obtained using established procedures. [16] In brief, hearts from between four and six neonatal rats (Wistar, 1–2 days old) were excised, the ventricles minced with scissors and the resulting small tissue pieces dissociated in Hank's balanced salt solution (HBSS without Ca2+ and Mg2+; Gibco, Basel, Switzerland) containing trypsin (0.1%, Boehringer, Rotkreuz, Switzerland) and pancreatin (60 µg/ml, Sigma, Buchs, Switzerland). The dispersed cells were, after centrifugation, resuspended in medium M199 (Gibco). The medium was supplemented with penicillin (20 U/ml; Fakola, Basel, Switzerland), streptomycin (20 µg/ml; Fakola), vitamin B12 (20 µg/ml; Sigma), 100 µM bromodeoxyuridine (Sigma) and 10% neonatal calf serum (Boehringer). The cell suspension was preplated in large culture flasks to reduce the fibroblast content and the myocytes remaining in suspension were seeded at a density of 1.9×103 cells/mm2 on patterned substrates (see below). The cultures were kept in an incubator at 35 °C in a humidified atmosphere containing 1.2% CO2. Medium exchanges were performed on the 1st day after seeding and every other day thereafter with supplemented medium M199 containing a reduced concentration of serum (5%).
Fibroblasts
Fibroblast cultures of cardiac origin were derived from cells obtained during the preplating step of the cardiomyocyte culturing procedures (see above). Because fibroblasts attach more rapidly to the plastic culture-ware than cardiomyocytes [1], most of the cells adhering to the culture flasks after 2 h preplating were fibroblasts. These cells were kept in the incubator under conditions identical to those of cardiomyocytes. After 2–3 days, the fibroblasts formed uniform monolayers at which time they were split by incubating the monolayers for 10 min with the same dissociation solution used for disintegration of cardiac tissue. The resulting cell suspension was triturated using a large-bore pipette to ensure complete dissociation of the cells. Thereafter, the suspension was centrifuged and the resulting cell pellet resuspended in M199 containing 10% neonatal calf serum, penicillin, streptomycin and vitamin B12. After counting the dissociated cells in a haemocytometer, they were seeded at a density of ~1×102/mm2 in culture flasks and the medium exchanged after 1 h. This early wash step ensured that the number of contaminating cardiomyocytes was substantially decreased and resulted in virtually pure fibroblast cultures after 2–3 splittings (splitting intervals 4–6 days). Thereafter, the fibroblasts were seeded at a density of 300 cells/mm2 on substrates patterned with collagen.
HeLa cells
Wild-type HeLa cells (kindly provided by Prof. R. Weingart, Dept. of Physiology, University of Bern, Switzerland) were grown in culture flasks following the protocol used for fibroblasts. After harvesting, HeLa cells were seeded at a density of 300 cells/mm2 on substrates patterned with collagen.
Preparation of patterned substrates
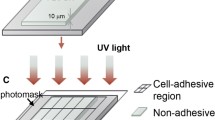
The various steps involved in the fabrication of glass substrates supporting patterned growth of cultured cells are summarized in Fig. 1.
Steps involved in the fabrication of culture substrates supporting patterned growth. After cleaning, the substrates were coated sequentially with layers of agar and photoresist before being exposed to a photographic mask carrying the desired growth pattern. The pattern was then spray-developed, coated with attachment factors and the remaining photoresist stripped from the substrate, yielding a spatially defined deposition of attachment factors according to which the cells adhered in culture. For details see Materials and methods
Step 1: cleaning
Prior to coating, the glass substrates (round cover-slips, diameter: 22 mm, thickness: 0.13–0.16 mm, Assistent, Haska, Bern, Switzerland) were thoroughly cleaned as follows: after removal of dirt and organic material by rinsing with acetone, the cover-slips were immersed in stirred H2SO4 (95%) for 10 min, rinsed in distilled water, immersed in stirred NaOH (1 M) for 10 min and rinsed in distilled water. The cover-slips were then stored in distilled water until further use.
Step 2: agar coating
The glass substrates were mounted on a custom-made spinning device permitting up to 10,000 rpm. Spin coating was performed at 5,000 rpm by applying 1 ml hot agar solution with a syringe to the glass surface. The agar solution consisted of 0.6% (w/w) agar (agar-agar, microbiology grade, Merck, Germany) in distilled water heated to 90 °C. After coating, the substrates were stored in a desiccator until further use.
Step 3: photoresist coating
Steps 3–5 were performed under yellow light illumination, to which the photoresist was not sensitive. The agar-coated substrates were removed individually from the desiccator, cleaned with compressed air to ensure absence of dust particles and placed on the spinning device. A drop of photoresist (KTFR-resist, Amtec, Amden, Switzerland) was placed in the middle of the glass. After a few seconds of spinning at ~200 rpm to ensure uniform spreading of the drop over the entire glass surface, rotational speed was increased to 7,000 rpm for 10 s. This resulted in a thin and uniform layer of photoresist. After coating, substrates were dried for 2 h at 80 °C in an oven before being stored under light-tight conditions until further use.
Step 4: exposure
The desired patterns were transferred to the photoresist coated glasses by UV exposure using masks that had been fabricated using procedures described previously [16]. Briefly, patterns of interest were designed on computers at ~10× magnification, printed at high resolution and reduced photographically to match the size of the substrates (Kodalith Ortho Film 6556 type 3, Kodak). The substrates were brought into close contact with the film mask and illuminated for 2 min in a UV light box (AV-Berna, Berna, Bern, Switzerland) of the type normally used for the fabrication of printed circuit boards.
Step 5: development
To develop the patterns, the exposed substrates were placed on the spinning device, spun at 7,000 rpm and the developer (xylol) applied evenly for 12 s using an air-brush (Badger 150-5, Badger Airbrush, Franklin Park, Ill., USA).
Step 6: coating with attachment factors
The developed substrates were immersed for 1 min in a solution consisting of 0.2 mg/ml collagen type IV (Sigma/Fluka) dissolved in a 12% solution of glacial acid in HBSS and then washed thoroughly with distilled water before being immersed in multi-well plates containing distilled water and placed in an incubator for ~1 h.
Step 7: stripping of photoresist
Incubation of the substrates in distilled water for ~1 h at 35 °C caused the photoresist to soften to a degree that either induced its spontaneous detachment from the substrate or at least reduced adhesion to such an extent that it could be easily removed mechanically with a fine forceps. After this step, the substrates were washed with Hank's balanced salt solution (Gibco) and placed in a UV illuminator for sterilization. The sterilized substrates were either used immediately in culture or stored in a desiccated jar for later use.
Step 8: cell seeding and washing
The substrates, now consisting of an agar coat covered by a patterned layer of attachment factors, were incubated with freshly dissociated cells for 20–24 h and then were washed twice with growth medium. This wash step removed all unattached cells, i.e. the cells on the agar regions not coated by attachment factors.
Multi-electrode arrays
For the assessment of impulse propagation characteristics in cardiac tissue over extended periods of time, cardiomyocytes were cultured on substrates containing multiple extracellular electrodes (multielectrode arrays, MEAs). The substrates consisted of glass plates (21×21 mm, 0.7 mm thick) carrying up to 72 transparent ITO (indium-tin oxide) recording/stimulation electrodes [13]. The leads of the electrodes were insulated with a polymer layer (SU-8, 5 µm thick). To obtain patterned cell growth over the MEAs, they were treated in an identical manner to pure glass substrates (as above) with the exception of the cleaning step, which was performed as follows: (i) rinsing with ethanol (100%), (ii) rinsing with ethanol (70%), (iii) rinsing with doubly distilled water and (iv) drying with compressed air. This cleaning procedure was necessary to avoid damage to the insulating polymer layer.
Immunofluorescence
To characterize the patterned deposition of attachment factors, collagen IV-coated substrates were subjected to indirect immunofluorescence by using primary antibodies directed against collagen type IV (mouse monoclonal antibody, Zymed, Mächler, Switzerland) and a secondary fluorescent antibody (Alexa 488 goat anti-mouse IgG, Molecular Probes, Leiden, The Netherlands). The preparations were imaged on an inverted microscope equipped for epifluorescence (Axiovert 35 M, Zeiss, Zürich, Switzerland) using a slow-scan camera (UltraPix, AstroCam, Gloor Instruments, Uster, Switzerland).
Results and Discussion
Characterization of the culture substrates
To assess the spatial resolution of the patterned deposition of attachment factors, substrates having undergone the coating procedure with collagen type IV were stained using indirect immunofluorescence. As shown by the mask in Fig. 2A, the patterned region measured 10×10 mm and consisted of "isthmus" structures, i.e. regions where two prospective cell sheets of variable dimensions were connected to each other by a narrow cell strand. The magnified detail of the mask illustrates that the isthmus was formed by two mutually apposed T-shaped structures. As shown by the immunofluorescence images in Fig. 2B, these structures were reproduced faithfully by the coating process (dark: agar-coated regions; bright: collagen-coated regions) with the exception of slight irregularities along the straight borders and rounding of corners. When measured along linear borders over extended distances, border non-uniformities were characterized by random deviations from a straight line of maximally ±3 µm. These deviations from the original pattern are due to imperfections of the mask and of the exposure process. Whereas these spatial irregularities were barely relevant for the multiple-cell-wide structures shown in this study, they are likely to become a limiting factor when trying to produce single-cell-wide strands. In these cases, the use of etched chromium masks instead of film masks and of an illuminator with a collimated light beam instead of a diffuse UV light source is likely to increase the spatial resolution to an extent which permits the production of such narrow structures.
Patterned collagen deposition. A Example of a photolithography mask used for the generation of "isthmus" patterns. The entire mask is shown on the left (diameter of the circle: 20 mm), the right-hand illustration shows a single isthmus formed by two T-shaped structures facing each other at higher magnification (black: agar-coated region, white: collagen-coated region). B Series of immunofluorescent images showing the spatially defined deposition of collagen IV (bright areas) in this type of pattern at increasing magnification
Patterned growth of cardiomyocytes in primary culture
Representative examples illustrating patterned growth of cardiomyocytes in primary culture are shown in Fig. 3. Figure 3A is an overview of a simple structure consisting of a monolayer region with emerging linear cell strands (strand width: 80 µm; entire length of individual strands: 10 mm). Clearly, regions coated with agar completely prevented cell attachment, thus resulting in highly defined two-dimensional growth patterns with virtually no off-pattern adhesion. Figure 3B shows an enlargement of the region where a cardiomyocyte cell strand contacted the monolayer region. Individual cells, some with cross-striations, can be easily identified. The geometry of this particular preparation deviated from the original mask inasmuch as that the lateral borders of the cell strand did not contact the monolayer at an acute angle but formed a funnel. The degree of funnel formation was dependent on the degree of contractile activity of the cultures: whereas preparations showing no or minimal contractile activity displayed only minimal formation of funnel-like structures, preparations which were active over prolonged periods of time showed funnels similar to that illustrated in Fig. 3B. This observation can be explained by assuming that prolonged contractile activity dislodges the cells in the corners from their original site of attachment and induces them to form a bridge over the agar-coated regions. The formation of funnels could be counteracted to some extent by designing right-angled regions with T-shaped structures as shown in Fig. 2. There, the pattern already provided space for cell dislodgment (the wings of the Ts) thus resulting in connections between strands and monolayer sheets that were closer to right-angled (data not shown). The remaining two panels of Fig. 3 illustrate more complex cell patterns. Figure 3C shows part of a sheet of cells forming a regular right angled network. Such a network can be looked upon as a model of an anisotropic sheet of cardiac tissue with infrequent lateral connections. Because the frequency and extent of lateral electrical coupling can be systematically varied, such a cell network might serve e.g. as a reproducible experimental preparation for the assessment of the effects of cardiac fibrosis on impulse propagation characteristics [19]. Figure 3D shows a model structure designed to investigate the feasibility and characteristics of anatomically fixed micro-reentrant excitation. The entire structure measured 1×1 mm. It consisted of a 30-µm-wide cell strand (~two cells across) forming a loop. On top, the strand entered an abrupt tissue expansion, which is known to give rise to unidirectional conduction blocks [15]. On the right, the strand released periodically open-ended strands of identical widths. These strands slow conduction by acting as repetitive current sinks along the main strand [12]. Following excitation of the preparation by extracellular stimulation of the free cell strand on the left, the combination of unidirectional conduction block and slow conduction is expected to give rise to counter-clockwise micro-reentrant excitation. This particular pattern marks the limit of the spatial resolution of the patterning technique presented. Although smaller structures can be obtained, the yield of continuous structures began to decline when the minimal width of strands fell below 20 µm. Because this is mostly due to the above-mentioned irregularities of the patterned deposition of attachment factors in the range of a few micrometres, this limitation might possibly be overcome by using etched chromium masks and collimated light sources.
Patterned growth of cardiomyocytes. A Phase-contrast image of part of a monolayer culture with 80-µm-wide and 10-mm-long linear cell strands. B Magnified view of a single junction between cell monolayer and cell strand. The shape of the cellular junction deviates slightly from the original mask by forming a narrow funnel instead of being rectangular. C Detail of a lattice pattern (overall dimensions 10×10 mm). D Overview of a pattern suitable for investigating the characteristics of anatomically fixed micro-reentrant circuits (overall dimensions of the rectangular pattern: 1×1 mm)
Patterned growth of cardiomyocytes on MEAs
Whereas the examples shown above illustrate the feasibility of the method when used in conjunction with glass substrates, patterned growth might sometimes be desirable on materials other than glass. This is e.g. the case when trying to apply the technique of patterned growth on arrays of extracellular electrodes that are coated with an electrically insulating layer of a polymer. As illustrated by Fig. 4, the method was equally effective for achieving patterned growth on this type of substrate. The only difference in the fabrication process of the substrates concerned the cleaning, which required the use of solvents that did not harm the insulating polymer layer. Moreover, instead of a random exposure of the substrates with the masks, the spatial matching of growth patterns to the specific layout of extracellular electrodes required the masks to be aligned to a given electrode pattern by using a microscope prior to exposure.
Patterned growth of cardiomyocytes on arrays of extracellular electrodes. Phase-contrast image of a linear cell strand with branches at defined intervals. The preparation was cultured on an array of transparent extracellular electrodes (located in the central regions of the junctions, black arrows). The white arrows denote the right edge of the lead of the central electrode. The multiple bright or dark particles present over the entire preparation (either in or out of focus) correspond to free floating cellular debris
Patterned growth of fibroblasts and HeLa cells
Examples of patterned growth of fibroblasts of cardiac origin and HeLa cells are shown in Figs. 5 and 6, respectively. For both cell types, the patterning mask used corresponded to that depicted in Fig. 2. The overview of a patterned growth fibroblast culture shown in Fig. 5A illustrates that regions coated with agar again completely suppressed off-pattern cell attachment. From the higher magnification images in Fig. 5B and C it is evident that fibroblasts, similar to cardiomyocytes, tended to span cornered structures. In the case of fibroblasts, this behaviour might be explained by the high degree of motility of this cell type [11, 17], resulting in cells crawling along the edges of the preparation and, upon reaching corners, spanning across the agar-coated regions. Alternatively, proliferation of fibroblasts in culture [7, 8] might induce apposition of new cells in the cornered regions which then could stretch in both directions along the originally adherent cells. That indeed migratory capacity might be important for this spanning process is suggested by the finding that HeLa cells, which do not migrate, did not show such behaviour (Fig. 6B and C) but adhered closely to the original isthmus structure of the deposited attachment factors. The results with both fibroblasts and HeLa cells illustrate that the technique of patterned deposition of attachment factors can be used for producing geometrically defined 2-dimensional tissue sheets with cell types different from cardiomyocytes.
Patterned growth of fibroblasts of cardiac origin. A The overview of a part of a monolayer culture with periodic 80-µm-wide and 10-mm-long linear cell strands illustrates that, also for the case of fibroblasts, there is no off-pattern cell attachment. B, C Details of the junction of an isthmus structure (same pattern as that shown in Fig. 2): even though the patterned deposition of collagen formed a "double-T", there was still a modest degree of funnel formation by the fibroblasts (for further explanation see text)
Patterned growth of HeLa cells. The type of mask used was identical to that shown in Fig. 2. A An overview showing the complete absence of cell attachment in regions coated with agar. B, C Higher magnification, phase-contrast images showing that HeLa cells complied faithfully with the original pattern and revealed the "double-T" structure of the original mask
In conclusion, patterned growth of cells in culture is a valuable experimental model for studying cellular network behaviour of excitable cells. In such studies, it is essential that growth patterns can be adapted continuously to given experimental questions. The method presented is suited for such an adaptive process because it takes no more than a day to design and implement new growth patterns. Moreover and in contrast to other methods, the technique makes little use of complex technologies, thus permitting its implementation in any laboratory working with cell culture. As shown above, the method is suited for patterning the growth of different types of adherent cells on different types of substrates with no off-pattern cell attachment and it should be straightforward to adapt the technique to cell types and substrate materials different from those investigated in this study.
References
Blondel B, Roijen I, Cheneval JP (1971) Heart cells in culture: a simple method for increasing the proportion of myoblasts. Experientia 27:356–358
Branch DW, Corey JM, Weyhenmeyer JA, Brewer GJ, Wheeler BC (1998) Microstamp patterns of biomolecules for high-resolution neuronal networks. Med Biol Eng Comput 36:135–141
Chien CG, Pine J (1991) An apparatus for recording synaptic potentials from neuronal cultures using voltage-sensitive fluorescent dyes. J Neurosci Methods 38:93–105
Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, Ingber DE, Whitesides GM (2000) Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc Natl Acad Sci USA 14:2408–2413
Corey JM, Wheeler BC, Brewer GJ (1996) Micrometer resolution silane-based patterning of hippocampal neurons: critical variables in photoresist and laser ablation processes for substrate fabrication. IEEE Trans Biomed Eng 43:944–955
Detrait E, Lhoest JB, Knoops B, Bertrand P, van den Bosch de Aguilar P (1998) Orientation of cell adhesion and growth on patterned heterogeneous polystyrene surface. J Neurosci Methods 84:193–204
Dostal DE, Booz GW, Baker KM (1996) Angiotensin II signalling pathways in cardiac fibroblasts: conventional versus novel mechanisms in mediating cardiac growth and function. Mol Cell Biochem 157:15–21
Eghbali M (1992) Cardiac fibroblasts: function, regulation of gene expression, and phenotypic modulation. Basic Res Cardiol 87:183–189
Fast VG, Kléber AG (1995) Cardiac tissue geometry as a determinant of unidirectional conduction block: assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc Res 29:697–707
Folch A, Toner M (1998) Cellular micropatterns on biocompatible materials. Biotechnol Prog 14:388–392
Kolega J, Shure MS, Chen WT, Young ND (1982) Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci 54:23–34
Kucera JP, Kléber AG, Rohr S (1998) Slow conduction in cardiac tissue. II. Effects of branching tissue geometry. Circ Res 83:795–805
Kucera JP, Heuschkel MO, Renaud P, Rohr S (2000) Power-law behavior of beat rate variability in monolayer cultures of neonatal rat ventricular myocytes. Circ Res 86:1140–1145
Lieberman M, Horres CR, Shigeto N, Ebihara L, Aiton JF, Johnson EA (1981) Cardiac muscle with controlled geometry. Application to electrophysiological and ion transport studies. In: Nelson PG, Lieberman M (eds) Excitable cells in tissue culture. Plenum, New York, pp 379–408
Rohr S, Salzberg BM (1994) Characterization of impulse propagation at the microscopic level across geometrically defined expansions of excitable tissue. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures. J Gen Physiol 104:287–309
Rohr S, Schölly DM, Kleber AG (1991) Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res 68:114–130
Sheetz MP, Felsenfeld DP, Galbraith CG (1998) Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol 8:51–54
Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE (1994) Engineering cell shape and function. Science 264:696–698
Spach MS, Dolber PC (1986) Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res 58:356–371
Acknowledgements
This study was supported by the Swiss National Science Foundation (Grant Nr. 31-64914.01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohr, S., Flückiger-Labrada, R. & Kucera, J.P. Photolithographically defined deposition of attachment factors as a versatile method for patterning the growth of different cell types in culture. Pflugers Arch - Eur J Physiol 446, 125–132 (2003). https://doi.org/10.1007/s00424-002-1000-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-1000-0