Abstract
Purpose
This study aimed to investigate the clinical efficacy of lower-extremity ultrasonography screening with early intervention for deep venous thrombosis (DVT) on the incidence of venous thromboembolism (VTE) after minimally invasive surgery (MIS) for gastric cancer (GC).
Methods
Between January 2012 and December 2019, 1070 patients were diagnosed with both clinical and pathological stage I–III GC and underwent MIS at our institution. Routine ultrasonographic screening for DVT in lower extremities is performed before MIS. Patients diagnosed with DVT were preoperatively administered anticoagulant therapy. Enoxaparin was routinely administrated after surgery irrespective of the presence of DVT. The incidence of postoperative symptomatic VTE was examined retrospectively.
Results
A total of 74 (6.9%) patients were preoperatively diagnosed with DVT. Multivariate analyses revealed that age > 70 years (p = 0.015), female sex (p < 0.001), and positive serum D-dimer test (p < 0.001) were significant and independent risk factors for preoperative DVT. The incidence of symptomatic postoperative VTE was 1 (0.09%); symptomatic VTE developed in one patient among patients without DVT, whereas no patient with DVT developed VTE.
Conclusions
Preoperative DVT screening using lower-extremity ultrasonography followed by preoperative anticoagulant therapy should be considered as a useful strategy to safely perform MIS for GC without increasing the incidence of VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with cancer are at 4–sevenfold increased risk for venous thromboembolism (VTE), which comprises deep venous thrombosis (DVT) and pulmonary thromboembolism (PTE) [1, 2]. PTE occurs when a thrombus traveling through the veins lodges in pulmonary arteries, leading to obstruction [3], and is an infrequent but highly lethal complication of major surgery [4]. Approximately 80% of PTEs result from the DVT of lower extremities [5]; therefore, DVT prophylaxis is important for the prevention of lethal VTE. Interestingly, the risk of severe PTE is relatively lower in Japan than in Western countries. The reported postoperative PTE incidence after major abdominal surgery is 0.14% according to a recent study of the Japanese National Clinical Database [6]. However, a prospective study from Japan previously reported that the rate of postoperative DVT was 23.7% [7], a rate comparable to those reported in studies from Europe and North America [8]. Therefore, risk of postoperative lethal VTE might be comparable between Japanese patients and those in Western countries.
Minimally invasive surgery (MIS) has been rapidly increasing with recent technological advances. Especially in gastric cancer (GC), laparoscopic gastrectomy has gained widespread use as a minimally invasive and safe curative procedure [9,10,11,12,13,14]. In our institution, we have recently demonstrated the comparability of short- and long-term outcomes between laparoscopic gastrectomy and open gastrectomy [15, 16], and MIS is the first-choice standard radical procedure for GC [17]. However, increased intraabdominal pressure from pneumoperitoneum can reduce peak venous flow velocity, thereby promoting thrombus formation especially in lower extremities [18]. In fact, we treated a patient with GC who developed fatal PTE after MIS in 2010. Since then, we have routinely administered postoperative anticoagulant therapy with enoxaparin. In addition, we have launched preoperative ultrasonographic screening for DVT in lower extremities in 2011 based on the hypothesis that the correct diagnosis of DVT in lower extremities before surgery and subsequent initiation of anticoagulant therapy should prevent the incidence of fatal postoperative VTE. In the present study, we investigated the clinical efficacy of preoperative ultrasonographic screening with early intervention for DVT to reduce the incidence of severe postoperative VTE after MIS for GC.
Methods
Patients
Between January 1, 2012, and December 31, 2019, 1285 consecutive patients were referred to our division with operable primary GC. In the present study, 1070 patients diagnosed with clinical and pathological stage I–III GC were enrolled after the exclusion of patients with clinical or pathological stage IV GC (n = 104), remnant GC (n = 36), open gastrectomy (n = 21), double cancer (n = 15), palliative or limited lymphadenectomy due to insufficient physical function (n = 24), lack of ultrasonographic evaluation (n = 14), and protocol deviation by using fondaparinux as a postoperative anticoagulation (n = 1). The cohort comprised 317 and 753 patients who underwent robotic and laparoscopic surgery, respectively. No patient had a history of previous VTE within the year prior to study enrollment.
Cancer staging was performed based on the findings of contrast-enhanced computed tomography (CT), gastrography, endoscopy, and endoscopic ultrasonography before the beginning of any treatment and, when applicable, after the completion of chemotherapy, as previously described [19, 20]. The cancer stage was determined according to the 15th edition of the Japanese Classification of Gastric Carcinoma [21]. The extent of systematic lymph node dissection was determined on the basis of the Japanese Gastric Cancer Treatment Guidelines 2018 [21]. Details on indications for radical gastrectomy, assessment of physical function, selection of operators, operative procedures, perioperative management in radical gastrectomy, extent of gastric resection and lymph node dissection, type of anastomosis, diagnosis and treatment for pancreatic fistula, postoperative chemotherapy, and oncologic follow-up have been previously reported [15,16,17, 19, 20, 22,23,24,25,26].
Diagnosis of DVT
During the study period, the ultrasonographic examination of lower extremities and serum D-dimer measurements were routinely performed to detect DVT prior to surgery. DVT was classified into two types: proximal DVT involving the popliteal vein and above and distal DVT involving the region distal to the popliteal vein. All ultrasonographic procedures were performed in the ultrasound unit by medical ultrasonographers and supervised by ultrasonographers certified by the Japan Society of Ultrasonics in Medicine. Neither contrast venography nor CT venography of lower extremities was performed in the present study.
Perioperative management for thromboembolism prophylaxis
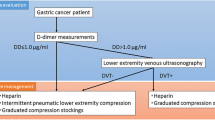
Patients diagnosed with DVT by ultrasonographic screening before surgery were administered preoperative anticoagulant therapy according to the recommendations for VTE prophylaxis from a clinical team comprising board-certified cardiovascular surgeons and anesthesiologists at our institution (Fig. 1). From 2012 to 2015, 12 000 U/day unfractionated heparin was intravenously administrated for a minimum of 3 days and discontinued 6 h before surgery. Starting in 2016, direct oral anticoagulants (DOACs), including edoxaban, apixaban, and rivaroxaban, were administered orally for a minimum of 7 days, and bridging therapy with 10 000–15 000 U/day unfractionated heparin was administered for 2 days before surgery, which was discontinued 6 h before surgery (Fig. 1). For patients with DVT in the femoral or iliac vein and fragile DVT, a retrievable inferior vena cava filter was placed before surgery, and these patients did not receive anticoagulant therapy. Patients without DVT did not receive preoperative anticoagulant therapy. Perioperative management for VTE was uniformly performed irrespective of the presence of DVT, according to the Japanese Guideline for Prevention of Venous Thromboembolism [27]. Specifically, elastic stockings (ES) and intermittent pneumatic compression (IPC) were used routinely from the initiation of general anesthesia until the morning of postoperative day (POD) 1 in all patients after obtaining their informed consent, regardless of the presence or absence of DVTs, according to the in-hospital recommendations of the clinical team for preventing postoperative VTE. During surgery, patient was placed in a 12° head-up position with the pneumoperitoneum at 10 mmHg. Intraoperative infusion volume was controlled by the anesthesiologist considering the patient’s age and underlying diseases. After surgery, all patients were administered oxygen (2 L/min) until an oxygen saturation level (SpO2) of ≥ 95% was achieved in room air, and SpO2 was monitored continuously until the morning of POD1 and at least three times per day afterwards. On POD1, patients began walking and drinking. Blood tests were performed at POD1, 2, 3, 5, and 7, and X-rays were taken at POD1, 3, and 4. Meals were initiated from POD3. Furthermore, as prophylactic anticoagulant therapy, enoxaparin (2000 IU) was subcutaneously administered twice daily for 7 days, starting at 24 h after surgery until POD7, irrespective of the DVT status. However, the final decision regarding the administration was determined based on the intraoperative findings and the patients’ condition on POD1. We did not perform enoxaparin therapy for patients with massive intraoperative bleeding, clinically suspicious of postoperative intraperitoneal bleeding or pancreatic fistula, and comorbidity with renal dysfunction. Contrast-enhanced CT, which was not routinely performed, was used in patients with DVT-suggestive symptoms, including dyspnea and chest pain, and in those with a decrease in SpO2 to 90% or lower. After surgery, DOAC administration for DVT prophylaxis was discontinued. The withdrawal protocol of antithrombotic agents for patients who received antithrombotic therapy because of other diseases is shown in Fig. 1. Aspirin and clopidogrel were withdrawn from 7 to 14 days before surgery, respectively, without heparin bridging therapy and restarted after removing the drain. Warfarin and DOACs were withdrawn from 4 to 2 days before surgery, respectively, and bridging therapy with 10 000–15 000 U/day heparin was administered for 2 days before surgery and was discontinued 6 h before surgery. From the morning of POD2, 10 000–15 000 U/day of unfractionated heparin was administered. Warfarin and DOACs were resumed after drain removal and heparin was discontinued (Fig. 1).
Flowchart of perioperative management for antithrombotic therapy. DVT, deep venous thrombosis; DOAC, direct oral anticoagulants. For patients who received heparin bridging therapy, heparin administration was discontinued 6 h before surgery. For patients who received preoperative antiplatelet drugs, including aspirin and clopidogrel, heparin bridging therapy was not administered. For patients who received antithrombotic therapy because of other diseases, the antithrombotic agents were resumed after initiating a drain
Measurements
The primary endpoint was the incidence of postoperative symptomatic VTE. The clinicopathological characteristics and short-term surgical outcomes including operative time; estimated blood loss; morbidity rate within 30 days after operation; rate of intraabdominal infectious complications, including leakage, postoperative pancreatic fistula, and intraabdominal abscess; mortality rate within 30 days after operation; and length of postoperative hospital stay were assessed as secondary endpoints. All postoperative complications that were grade IIIa or above based on the Clavien–Dindo classification were recorded [28] and classified in accordance with the Japan Clinical Oncology Group Postoperative Complication Criteria according to Clavien–Dindo ver. 2.0 [29]. Total operative time was defined as the time from the start of abdominal incision until the end of complete wound closure. Blood loss was estimated by weighing suctioned blood and blood-absorbed gauze pieces. Postoperative major bleeding was defined as follows, as previously described [30]: retroperitoneal, intracranial, intraocular, adrenal, endocardial, spinal, or surgical site bleeding requiring surgical intervention; clinically overt bleeding with ≥ 2 g/dL decrease in hemoglobin; or the need for transfusion of ≥ 800 mL red blood cells within 48 h from the suspicion of bleeding based on symptoms.
Statistical analysis
All analyses were performed using IBM SPSS Statistics 26 (IBM Corporation, Armonk, NY, USA). Between-group comparisons were examined by the χ2 or Mann–Whitney U test. Univariate χ2 test and multivariate logistic regression analyses were performed to determine factors associated with an increased risk of preoperative DVT in lower extremities. Data were expressed as medians with ranges or odds ratios (ORs) with 95% confidence intervals (CIs), unless otherwise noted. A two-tailed p value of < 0.05 was considered to indicate statistical significance.
Results
Prevalence of DVT
In the present study, 74 (6.9%) of the 1070 patients comprising the cohort were preoperatively diagnosed with DVT, and all patients with DVT were asymptomatic. The anatomical distribution of DVT is summarized in Table 1. A total of 92 thrombi were detected. Further, 9 patients had bilateral DVT, and 12 patients had multi-site DVT. In addition, 2 patients had only proximal DVT, whereas 65 patients had only distal DVT. The most common DVT site was soleal vein, followed by peroneal, and common femoral veins (Table 1).
Characteristics of patients with DVT
The patient characteristics are summarized in Table 2. Age, sex, American Society of Anesthesiologists grade, cStage, cT and cN status, tumor size, presence of cardiovascular disease, and positive serum D-dimer test (≥ 1.0 μg/mL) were significantly different between the patients with and without DVT. In addition, the serum D-dimer levels were significantly higher in patients with DVT than in those without DVT. In contrast, 24 patients (32.4%) had negative D-dimer assay results (< 1.0 μg/mL). However, there were no significant differences in the characteristics between the D-dimer-positive and D-dimer-negative patients among those with DVT. A total of 135 patients received antithrombotic therapy, including anticoagulant therapy and antiplatelet therapy due to other diseases before ultrasonographic examination; however, there were no significant differences in the patient characteristics between these two subgroups.
Risk factors of DVT
We next performed univariate and multivariate analyses to identify the risk factors for DVT (Table 3). The univariate analysis showed that age > 70 years, female sex, American Society of Anesthesiologists grade 2 or higher, cStage ≥ II, tumor size ≥ 30 mm, presence of cardiovascular disease, and positive serum D-dimer test were risk factors for DVT. Furthermore, the multivariate analysis identified age > 70 years (OR, 2.431; 95% CI, 1.185–4.986; p = 0.015), female sex (OR, 2.973; 95% CI, 1.700–5.201; p < 0.001), and positive serum D-dimer test (OR, 5.367; 95% CI, 2.918–9.872; p < 0.001) as independent risk factors for preoperative DVT in patients scheduled for GC surgery.
Preoperative anticoagulant therapy and perioperative management
The details of perioperative management for DVT are summarized in Fig. 2. Among the 74 patients diagnosed with DVT, 69 (93.2%) received any anticoagulant therapy, including intravenous unfractionated heparin administration in 40 patients and DOACs for ≥ 7 days in 29 patients (edoxaban in 19, apixaban in 6, and rivaroxaban in 4 patients, without obvious criteria regarding DOAC selection). There were no major bleeding events nor clinically relevant non-major bleeding events during treatment before surgery. In contrast, three patients with distal DVT were not administered any antithrombotic agents before surgery due to high risk of massive tumor bleeding. In two patients with proximal DVT, a retrievable filter was placed in inferior vena cava before surgery without preoperative anticoagulant therapy. All 74 patients received ES, and 61 (82.4%) patients received IPC.
Starting on POD1, 62 patients received enoxaparin therapy, whereas 12 did not. The 996 patients without DVTs received intraoperative ES and IPC. In this group, 175 patients did not receive postoperative anticoagulant therapy, whereas the remaining 821 patients received any postoperative anticoagulant therapy (enoxaparin therapy in 804, unfractionated heparin therapy in 17), as shown in Fig. 2. A total of 187 patients did not receive postoperative enoxaparin therapy based on the surgeon’s decision considering the intraoperative findings and the patient’s condition on POD1. Each reason is detailed as follows: intraoperative blood loss of ≥ 100 mL (45/187), drain amylase levels of ≥ 1,000 U/L on POD1 (66/187), high levels of inflammatory markers (WBC ≥ 15,000/μL or CRP ≥ 10 mg/dL; 26/187), partially bloody drain fluid on visual inspection (10/187), serum estimated glomerular filtration rate level of ≤ 45 mL/min/1.73 on POD1 (4/187), and unknown reasons (36/187).
Surgical and short-term outcomes
The surgical and short-term outcomes are summarized in Table 4. There were no significant differences in type of resection, type of approach, extent of lymphadenectomy, operative time, amount of bleeding, postoperative complications, rate of reoperation, mortality, and the length of hospital stay after surgery between patients with and without DVT.
The causes of mortality within 30 days after surgery were anastomotic leakage in one patient and pancreatic fistula leading to rapture of splenic artery pseudoaneurysm in one patient. There was no mortality due to postoperative VTE. The details of the postoperative complications are summarized in Table 5. No patients experienced symptomatic DVT in the DVT ( +) and DVT ( −) groups. Symptomatic VTE occurred in only one patient without DVT, whereas no patients with DVT developed symptomatic VTE. The only patient who developed PTE in the absence of DVT was an 80-year-old male diagnosed with cStage III (cT4N + M0) GC who underwent laparoscopic total gastrectomy. Contrast-enhanced CT on POD3 due to dyspnea and hypoxemia with a decreased SpO2 of 90% revealed PTE in the right peripheral pulmonary artery. This patient, who did not receive postoperative enoxaparin due to high levels of inflammatory markers on POD1 (WBC: 15,600/μL, CRP: 9.5 mg/dL), recovered following treatment with intubation and anticoagulant therapy. On the other hand, during the study period, 270 (25.2%) patients underwent unplanned contrast-enhanced CT to rule out postoperative complications, and asymptomatic PTE in the distal branch of the right pulmonary artery was identified in only one patient without DVT. This patient had chronic occlusive pulmonary disease, and PTE was detected by chance when contrast-enhanced CT was performed on POD3 to rule out pneumonia and abdominal infection. This patient also did not receive anticoagulant therapy due to massive intraoperative blood loss (total blood loss, 394 mL). This patient recovered after warfarin therapy for 3 months. Finally, there were no significant differences in the rates of other complications between the patients with and without DVT. Major bleeding occurred in eight patients, including five with CD grade ≥ IIIa and three with CD grade II. The summary of these eight patients is presented in Table 6. Three patients with CD grade II showed stable vital signs and no obvious signs of extravasation or intraperitoneal hematoma on contrast-enhanced CT. Thus, their conditions improved after blood transfusion. In the 883 patients who received enoxaparin therapy, a major bleeding event occurred in 3 patients (0.3%). In contrast, a major bleeding event occurred in 5 (2.7%) of the 187 patients who did not receive enoxaparin therapy (Fig. 2). A CD grade IIIb bowel obstruction occurred in one patient due to the torsion of the jejunum, and reoperation was performed on POD7.
Discussion
In the present study, including 1070 patients who underwent preoperative lower-extremity ultrasonographic DVT screening before planned MIS for GC, postoperative symptomatic VTE occurred in only one patient after MIS for GC. Notably, none of the 74 (6.9%) patients diagnosed with preoperative DVT in the lower extremities, who were considered a potentially high-risk subpopulation for postoperative VTE, showed progression to postoperative lethal VTE owing to our perioperative prophylactic management. In addition, the surgical outcomes were not significantly worse in the patients with DVT than those without DVT. Therefore, our findings suggest that our approach, including the accurate diagnosis of DVT in lower extremities by preoperative ultrasonographic screening and the subsequent initiation of anticoagulant therapy, can prevent the incidence of fatal postoperative VTE.
Our finding that preoperative lower-extremity ultrasonography led to the detection of DVT in 6.9% of the patients is in close agreement with the findings of a study by Tanizawa et al., who reported a DVT detection rate of 7.5% using the same approach [31]. In contrast, the DVT detection rate was 1.3% in a study by Wada et al. using lower-extremity ultrasonography only in patients with positive serum D-dimer test [32]. These two previous studies have indicated that female sex, age > 80 years, Eastern Cooperative Oncology Group performance status ≥ 1, presence of central venous catheter, and preoperative chemotherapy are risk factors for DVT [31, 32]. In addition, Lee et al. reported that the 2-year cumulative incidence of VTE in patients with stage IV GC was 24.4% [33], indicating a high risk for DVT. Furthermore, in contrast to previously published studies [31,32,33], we excluded patients with stage IV GC in the current study, focusing instead on patients with resectable GC, and identified female sex, age > 70 years, and positive serum D-dimer test as important risk factors for DVT. Therefore, our findings lend further support for female sex and older age as important risk factors for DVT in patients with resectable GC. Because women after menopause may be predisposed toward developing DVT, further investigation regarding an association between menopause and occurrence of DVTs is desired.
None of the 74 patients diagnosed with DVT before surgery developed postoperative symptomatic VTE or experienced worse surgical outcomes at least partly because of preoperative anticoagulant therapy. In addition, only 1 patient developed postoperative PTE among a total of 996 patients who were not diagnosed with DVT before surgery. We consider that this greatly successful outcome was not only because of preoperative anticoagulant therapy but also because of postoperative therapy with the low-molecular-weight heparin enoxaparin, as previous studies demonstrated its efficacy in preventing VTE [34,35,36]. It is needless to say that early walking is important to prevent DVT/VTE, as suggested in the recent reports focusing on ERAS protocols [37, 38]. In contrast, the use of intraoperative IPC in patients with DVT is controversial. Although we believe that it might contribute to the prevention of VTE, some reports have suggested that compression might dislodge the clots, consequently causing VTE [39, 40]. In our study, no patients with DVTs showed progression to postoperative VTEs. Hence, the use of intraoperative IPC appears to be, at the least, not an absolute contraindication in patients with DVTs, when combined with active perioperative use of anticoagulant therapy.
In contrast to that in Western countries [4], the incidence of postoperative PTE has been reported to be very low in Japan; a recent study based on the Japanese National Clinical Database between 2011 and 2013 has reported that the frequency of postoperative PTE after gastrectomy, including total gastrectomy, was very low at 0.11% (175/159 478) [6], comparable to the results of the present study (0.09%) and the study by Tanizawa et al. (0.18%) [31]. This difference may result from the differences in coagulation function owing to racially divergent genetic backgrounds between the Western and Asian populations, including the Japanese population [36, 41]. Therefore, aggressive management to prevent VTE may not be required for Japanese patients. However, the risk of VTE in Japanese patients preoperatively diagnosed with DVTs has not been clarified. In addition, few studies have focused on the incidence of postoperative VTE after MIS for GC, although several have suggested that the incidence of VTE is comparable between MIS and open surgery for colorectal cancer [42]. Therefore, we believe that complete management for preventing postoperative VTE, including preoperative screening for DVT and subsequent early intervention, has great value in terms of prophylaxis for lethal complications, which has been highlighted in this study.
As another important finding, 24 patients (32.4%) had DVT despite a negative serum D-dimer assay result in the present study. Unfortunately, we could not determine the significant differences in the characteristics of the D-dimer-positive and D-dimer-negative patients with DVT. Although the D-dimer assay is a safe and reliable tool to determine the presence of thrombi with high sensitivity (97%–100%) and high negative predictive value, especially in the acute phase [43, 44], its reactivity for chronic or old thrombi is not clear. Therefore, the ultrasonographic examination in the present study might have detected chronic DVT unresponsive to the D-dimer assay. However, the medical ultrasonographers at our institution cannot accurately distinguish between patients with acute and chronic DVTs [45], which is a major limitation of this study. Perioperative anticoagulant therapy was performed for all DVT-positive patients diagnosed via ultrasonography, regardless of the acute or chronic status of the thrombi, according to in-hospital recommendations to prevent thrombus growth and minimize the risk for postoperative VTE. Additional studies to further develop a novel methodology for identifying only chronic thrombi are desired.
The present study has several other limitations that should be acknowledged. First, this was a single-center, retrospective and nonrandomized study. Therefore, the influence of several sources of patient bias could not be excluded. In addition, this study enrolled only Japanese patients. Therefore, we should consider how racially divergent genetic backgrounds affect coagulation function [36, 41]. Second, although preoperative ultrasonography was routinely performed to detect DVT, postoperative ultrasonographic surveillance for DVT was not routinely used. Therefore, the consequences of DVT after surgery were not evaluated in the present study. Previous studies have reported that VTE may sometimes develop beyond the first 30 days after surgery, although the majority of postoperative VTEs occur within 30 days after surgery [46, 47]; therefore, long-term follow-up using ultrasonography is warranted. Third, the VTE diagnosis was primarily dependent on patient symptoms in the present study and not on scheduled surveillance. In fact, contrast-enhanced CT scans were performed specifically to rule out postoperative complications in only 25% of the patients and were not used routinely to detect VTE. As a result, only one patient with asymptomatic VTE was diagnosed by chance, and contrast-enhanced CT was performed in only one patient with symptomatic VTE based on the presence of dyspnea and hypoxemia. Therefore, the present study may underestimate the postoperative VTE incidence. Prospective studies of postoperative VTE evaluation using scheduled contrast-enhanced CT scans are warranted to resolve this issue. Fourth, although central venous catheter-related VTE is a well-known risk for postoperative VTE [48], the presence of central venous catheter was not thoroughly investigated in the current study; thus, its relationship with VTE incidence should be elucidated.
In conclusion, preoperative DVT screening using lower-extremity ultrasonography followed by perioperative anticoagulant therapy should be considered as a useful strategy to safely perform MIS in patients with GC without increasing the VTE incidence.
Data availability
All data are presented in this manuscript.
Code availability
Not applicable.
References
Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC (2013) Epidemiology of cancer-associated venous thrombosis. Blood 122(10):1712–1723. https://doi.org/10.1182/blood-2013-04-460121
Li A, Garcia DA, Lyman GH, Carrier M (2019) Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res 173:158–163. https://doi.org/10.1016/j.thromres.2018.02.144
Crawford F, Andras A, Welch K, Sheares K, Keeling D (2016) Chappell FM (2016) D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev 8:Cd010864. https://doi.org/10.1002/14651858.CD010864.pub2
Rogers SO Jr, Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, Khuri SF (2007) Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 204(6):1211–1221. https://doi.org/10.1016/j.jamcollsurg.2007.02.072
Tapson VF (2004) Acute pulmonary embolism. Cardiol Clin 22(3):353-365,v. https://doi.org/10.1016/j.ccl.2004.04.002
Hata T, Ikeda M, Miyata H, Nomura M, Gotoh M, Sakon M, Yamamoto K, Wakabayashi G, Seto Y, Mori M, Doki Y (2019) Frequency and risk factors for venous thromboembolism after gastroenterological surgery based on the Japanese National Clinical Database (516 217 cases). Ann Gastroenterol Surg 3(5):534–543. https://doi.org/10.1002/ags3.12275
Sakon M, Maehara Y, Yoshikawa H, Akaza H (2006) Incidence of venous thromboembolism following major abdominal surgery: a multi-center, prospective epidemiological study in Japan. J Thromb Haemost 4(3):581–586. https://doi.org/10.1111/j.1538-7836.2006.01786.x
Clagett GP, Anderson FA Jr, Geerts W, Heit JA, Knudson M, Lieberman JR, Merli GJ, Wheeler HB (1998) Prevention of venous thromboembolism. Chest 114(5 Suppl):531s–560s. https://doi.org/10.1378/chest.114.5_supplement.531s
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 263(1):28–35. https://doi.org/10.1097/sla.0000000000001346
Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W, Ryu SW, Jin SH, Oh SJ, Ryu KW, Kim MC, Ahn HS, Park YK, Kim YH, Hwang SH, Kim JW, Cho GS (2019) A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer 22(1):214–222. https://doi.org/10.1007/s10120-018-0864-4
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 5(4):506–513. https://doi.org/10.1001/jamaoncol.2018.6727
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M (2017) Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 20(4):699–708. https://doi.org/10.1007/s10120-016-0646-9
Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, Koeda K, Nunobe S, Yoshikawa T, Terashima M, Sasako M (2020) Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 5(2):142–151. https://doi.org/10.1016/s2468-1253(19)30332-2
Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K, Takiguchi N, Kaji M, Okitsu H, Yoshikawa T, Terashima M (2019) Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 22(5):999–1008. https://doi.org/10.1007/s10120-019-00929-9
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I (2013) Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 27(1):286–294. https://doi.org/10.1007/s00464-012-2442-x
Nakauchi M, Suda K, Kadoya S, Inaba K, Ishida Y, Uyama I (2016) Technical aspects and short- and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective study. Surg Endosc 30(10):4632–4639. https://doi.org/10.1007/s00464-015-4726-4
Uyama I, Suda K, Satoh S (2013) Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer 13(1):19–25. https://doi.org/10.5230/jgc.2013.13.1.19
Nguyen NT, Cronan M, Braley S, Rivers R, Wolfe BM (2003) Duplex ultrasound assessment of femoral venous flow during laparoscopic and open gastric bypass. Surg Endosc 17(2):285–290. https://doi.org/10.1007/s00464-002-8812-z
Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I (2015) Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 29(3):673–685. https://doi.org/10.1007/s00464-014-3718-0
Shibasaki S, Suda K, Nakauchi M, Nakamura K, Kikuchi K, Inaba K, Uyama I (2020) Non-robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra-abdominal infectious complications: a single-center, retrospective and propensity score-matched analysis. World J Gastroenterol 26(11):1172–1184. https://doi.org/10.3748/wjg.v26.i11.1172
(2021) Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 24(1):1–21. https://doi.org/10.1007/s10120-020-01042-y
Shibasaki S, Suda K, Nakauchi M, Nakamura T, Kadoya S, Kikuchi K, Inaba K, Uyama I (2018) Outermost layer-oriented medial approach for infrapyloric nodal dissection in laparoscopic distal gastrectomy. Surg Endosc 32(4):2137–2148. https://doi.org/10.1007/s00464-018-6111-6
Nakamura K, Suda K, Suzuki A, Nakauchi M, Shibasaki S, Kikuchi K, Nakamura T, Kadoya S, Inaba K, Uyama I (2018) Intracorporeal isosceles right triangle-shaped anastomosis in totally laparoscopic distal gastrectomy. Surg Laparosc Endosc Percutan Tech 28(3):193–201. https://doi.org/10.1097/sle.0000000000000535
Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S (2012) Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 36(2):331–337. https://doi.org/10.1007/s00268-011-1352-8
Shibasaki S, Suda K, Nakauchi M, Kikuchi K, Kadoya S, Ishida Y, Inaba K, Uyama I (2017) Robotic valvuloplastic esophagogastrostomy using double flap technique following proximal gastrectomy: technical aspects and short-term outcomes. Surg Endosc 31(10):4283–4297. https://doi.org/10.1007/s00464-017-5489-x
Shibasaki S, Suda K, Nakauchi M, Nakamura K, Tanaka T, Kikuchi K, Inaba K, Uyama I (2020) Impact of the Endoscopic Surgical Skill Qualification System on the safety of laparoscopic gastrectomy for gastric cancer. Surg Endosc. https://doi.org/10.1007/s00464-020-08102-5
(2011) Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J 75(5):1258–1281. https://doi.org/10.1253/circj.cj-88-0010
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, Sasako M (2016) Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 46(6):668–685. https://doi.org/10.1007/s00595-015-1236-x
Hata T, Yasui M, Ikeda M, Miyake M, Ide Y, Okuyama M, Ikenaga M, Kitani K, Morita S, Matsuda C, Mizushima T, Yamamoto H, Murata K, Sekimoto M, Nezu R, Mori M, Doki Y (2019) Efficacy and safety of anticoagulant prophylaxis for prevention of postoperative venous thromboembolism in Japanese patients undergoing laparoscopic colorectal cancer surgery. Ann Gastroenterol Surg 3(5):568–575. https://doi.org/10.1002/ags3.12279
Tanizawa Y, Bando E, Kawamura T, Tokunaga M, Makuuchi R, Iida K, Nanri K, Yoneyama M, Terashima M (2017) Prevalence of deep venous thrombosis detected by ultrasonography before surgery in patients with gastric cancer: a retrospective study of 1140 consecutive patients. Gastric Cancer 20(5):878–886. https://doi.org/10.1007/s10120-016-0677-2
Wada T, Fujiwara H, Morita S, Fukagawa T, Katai H (2017) Incidence of and risk factors for preoperative deep venous thrombosis in patients undergoing gastric cancer surgery. Gastric Cancer 20(5):872–877. https://doi.org/10.1007/s10120-017-0690-0
Lee KW, Bang SM, Kim S, Lee HJ, Shin DY, Koh Y, Lee YG, Cha Y, Kim YJ, Kim JH, Park DJ, Kim HH, Oh D, Lee JS (2010) The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J Thromb Haemost 8(3):540–547. https://doi.org/10.1111/j.1538-7836.2009.03731.x
Dranitsaris G, Jelincic V, Choe Y (2012) Meta-regression analysis to indirectly compare prophylaxis with dalteparin or enoxaparin in patients at high risk for venous thromboembolic events. Clin Appl Thromb Hemost 18(3):233–242. https://doi.org/10.1177/1076029611426869
Sakon M, Kobayashi T, Shimazui T (2010) Efficacy and safety of enoxaparin in Japanese patients undergoing curative abdominal or pelvic cancer surgery: results from a multicenter, randomized, open-label study. Thromb Res 125(3):e65-70. https://doi.org/10.1016/j.thromres.2009.09.009
Jung YJ, Seo HS, Park CH, Jeon HM, Kim JI, Yim HW, Song KY (2018) Venous thromboembolism incidence and prophylaxis use after gastrectomy among Korean patients with gastric adenocarcinoma: the PROTECTOR randomized clinical trial. JAMA Surg 153(10):939–946. https://doi.org/10.1001/jamasurg.2018.2081
Bell BR, Bastien PE, Douketis JD (2015) Prevention of venous thromboembolism in the Enhanced Recovery After Surgery (ERAS) setting: an evidence-based review. Can J Anaesth 62(2):194–202. https://doi.org/10.1007/s12630-014-0262-2
Talec P, Gaujoux S, Samama CM (2016) Early ambulation and prevention of post-operative thrombo-embolic risk. J Visc Surg 153(6s):S11-s14. https://doi.org/10.1016/j.jviscsurg.2016.09.002 (1037-1052)
Rabe E, Partsch H, Morrison N, Meissner MH, Mosti G, Lattimer CR, Carpentier PH, Gaillard S, Jünger M, Urbanek T, Hafner J, Patel M, Wu S, Caprini J, Lurie F, Hirsch T (2020) Risks and contraindications of medical compression treatment - a critical reappraisal An international consensus statement. Phlebology 35(7):447–460. https://doi.org/10.1177/0268355520909066
Badireddy M, Mudipalli VR (2020) Deep Venous Thrombosis Prophylaxis StatPearls. StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., Treasure Island
Lee CH, Lin LJ, Cheng CL, Kao Yang YH, Chen JY, Tsai LM (2010) Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost 8(7):1515–1523. https://doi.org/10.1111/j.1538-7836.2010.03873.x
Cui G, Wang X, Yao W, Li H (2013) Incidence of postoperative venous thromboembolism after laparoscopic versus open colorectal cancer surgery: a meta-analysis. Surg Laparosc Endosc Percutan Tech 23(2):128–134. https://doi.org/10.1097/SLE.0b013e3182827cef
van der Graaf F, van den Borne H, van der Kolk M, de Wild PJ, Janssen GW, van Uum SH (2000) Exclusion of deep venous thrombosis with D-dimer testing–comparison of 13 D-dimer methods in 99 outpatients suspected of deep venous thrombosis using venography as reference standard. Thromb Haemost 83(2):191–198
Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M, Pauker SG, Makdissi R, Guyatt GH (2012) Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e351S-e418S. https://doi.org/10.1378/chest.11-2299
Mumoli N, Mastroiacovo D, Giorgi-Pierfranceschi M, Pesavento R, Mochi M, Cei M, Pomero F, Mazzone A, Vitale J, Ageno W, Dentali F (2018) Ultrasound elastography is useful to distinguish acute and chronic deep vein thrombosis. J Thromb Haemost 16(12):2482–2491. https://doi.org/10.1111/jth.14297
Matsuoka Y, Morimatsu H (2019) Incidence rates of postoperative pulmonary embolisms in symptomatic and asymptomatic patients, detected by diagnostic images - a single-center retrospective study. Circ J 83(2):432–440. https://doi.org/10.1253/circj.CJ-18-0729
Hope WW, Demeter BL, Newcomb WL, Schmelzer TM, Schiffern LM, Heniford BT, Sing RF (2007) Postoperative pulmonary embolism: timing, diagnosis, treatment, and outcomes. Am J Surg 194(6):814–818. https://doi.org/10.1016/j.amjsurg.2007.08.014 (discussion 818-819)
Verso M, Agnelli G (2003) Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 21(19):3665–3675. https://doi.org/10.1200/jco.2003.08.008
Acknowledgements
The authors would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Author information
Authors and Affiliations
Contributions
All the authors have fully met the ICMJE authorship criteria as follows. Study conception and design, Kazumitsu Suzuki, Susumu Shibasaki, Ichiro Uyama, and Koichi Suda; acquisition of the data, Kazumitsu Suzuki, Kenichi Nakamura, Tsuyoshi Tanaka, and Kenji Kikuchi; analysis and interpretation of the data, Kazumitsu Suzuki, Susumu Shibasaki, Koichi Suda, Masaya Nakauchi, Shingo Akimoto, and Kazuki Inaba; drafting of the manuscript, Kazumitsu Suzuki, Susumu Shibasaki, and Koichi Suda; critical revision of the manuscript, Susumu Shibasaki, Ichiro Uyama, and Koichi Suda. All authors read and approved the final manuscript. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The present study was approved by the Institutional Review Board of Fujita Health University (HM18-409).
Consent to participate
Informed consent for this study was obtained through an opt-out method.
Consent for publication
Not applicable.
Conflict of interest
Kazumitsu Suzuki, Susumu Shibasaki, Masaya Nakauchi, Kenichi Nakamura, Shingo Akimoto, Tsuyoshi Tanaka, Kenji Kikuchi, Kazuki Inaba, Koichi Suda, and Ichiro Uyama have no commercial association with or financial involvement that might pose a conflict of interest in connection with the submitted article. Ichiro Uyama has received lecture fees from Intuitive Surgical, Inc., outside of the submitted work. Koichi Suda, Tsuyoshi Tanaka, and Kenji Kikuchi have been funded by Medicaroid, Inc. in relation to the Collaborative Laboratory for Research and Development in Advanced Surgical Technology, Fujita Health University. Koichi Suda has also received advisory fees from Medicaroid, Inc., outside of the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suzuki, K., Shibasaki, S., Nakauchi, M. et al. Impact of routine preoperative sonographic screening with early intervention for deep venous thrombosis in lower extremities on preventing postoperative venous thromboembolism in patients with gastric cancer scheduled for minimally invasive surgery. Langenbecks Arch Surg 407, 597–608 (2022). https://doi.org/10.1007/s00423-021-02315-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02315-5