Abstract
Background
We hypothesized that the Endoscopic Surgical Skill Qualification System (ESSQS) can shorten operative time, resulting in a decrease in postoperative morbidity. Here, we aimed to clarify whether ESSQS-qualified surgeons could decrease the incidence of complications.
Methods
Between January 2009 and June 2019, 1042 patients diagnosed with both clinical and pathological Stage ≤ III gastric cancer and undergoing LG were enrolled. In all LG procedures involving ESSQS-qualified surgeons, these served as the operator or the instructive assistant. The short-term outcomes were retrospectively compared between the ESSQS-qualified and the non-ESSQS-qualified surgeons using a propensity-score matched analysis.
Results
After propensity-score matching, 321 patients were included in each group. No significant differences were observed in morbidity rate, and length of hospitalization following surgery, although the non-ESSQS-qualified surgeon group had a significantly longer total operative time (Non-ESSQS-qualified group, 368 [170–779] min vs. ESSQS-qualified group, 316 [147–772] min; p < 0.001), and larger estimated blood loss (Non-ESSQS-qualified group, 28 [0–702] mL vs. ESSQS-qualified group 25, [0–1069] mL; p = 0.042). Multivariate analysis revealed that operative time ≥ 360 min (OR 1.818 [1.069–3.094], p = 0.027) was identified as the only significant independent risk factor determining morbidity.
Conclusions
The incidence of postoperative morbidity did not differ between patients operated by the qualified and nonqualified surgeons, as long as ESSQS-qualified surgeons provide intraoperative instructions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, laparoscopic gastrectomy (LG) has gained popularity, especially for early gastric cancer (GC), being minimally invasive and non-inferior to open gastrectomy (OG) with regard to both short- and long-term outcomes [1,2,3,4,5,6]. However, the disadvantages of LG compared with OG may include prolongation of operative time and a longer learning curve [7, 8]. Furthermore, several recent studies using the nationwide web-based database in Japan revealed that LG resulted in higher postoperative local complications compared with OG [9,10,11]. These findings suggest that LG is a technically demanding procedure, and there may be a considerable technical gap between expert and nonexpert surgeons.
With a view to developing a tool for reliable and reproducible evaluation of the surgical techniques of surgeons, the Endoscopic Surgical Skill Qualification System (ESSQS) was launched in 2004 by the Japanese Society for Endoscopic Surgery (JSES) [12], and it has contributed to the improvement and standardization of LG [12, 13]. With the maturation of this system, ESSQS-qualified surgeons are regarded as highly skilled surgeons. However, it remains unknown how the difference in surgical skill might influence on operative time. In addition, it remains unclear whether prolongation of the operative time by trainee operators increases the incidence of complications.
Since we demonstrated the comparability of laparoscopic D2 gastrectomy and open D2 gastrectomy with regard to short- and long-term outcomes [14, 15], minimally invasive surgery has been the standard radical procedure for GC at our institute [16]. In addition, recent studies have demonstrated that prolongation of operative time is an independent risk factor for postoperative morbidity [17, 18]. Hence, we hypothesized that ESSQS-qualified surgeons, regarded as skillful surgeons, may be able to shorten the operative time, leading to a decrease of postoperative morbidity. The aim of this study was to clarify whether qualified surgeons could decrease the incidence of complications.
Materials and methods
Patients
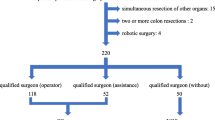
Between January 2009 and June 2019, 1716 consecutive patients were referred to our division with primary GC, for which surgical treatment was applicable. Informed consent was obtained from all patients. In the present study, 1,042 patients with both clinical and pathological Stage ≤ III GC were enrolled, whereas the remaining 674 patients were excluded because of clinical or pathological Stage IV GC (n = 166), remnant GC (n = 53), double cancer (n = 20), OG (n = 25), robotic gastrectomy (n = 359), or palliative or limited lymphadenectomy (n = 51) due to insufficient physical function. The patient selection process is summarized in Fig. 1. Clinical tumor staging was determined according to the 15th edition of the Japanese Classification of Gastric Carcinoma [19]. Cancer staging was performed based on the findings of contrast-enhanced computed tomography, gastrography, endoscopy, and endosonography before the beginning of any treatment and when applicable, after the completion of chemotherapy, as previously described [18, 20]. The extent of systematic lymph node (LN) dissection was performed based on the Japanese Gastric Cancer Treatment Guidelines 2018 [21]. Detailed indications for radical gastrectomy, assessment of physical function, operative procedures, perioperative management in radical gastrectomy, extent of gastric resection and LN dissection, type of anastomosis, diagnosis and treatment for pancreatic fistula, and postoperative chemotherapy in addition to oncologic follow-up have previously been reported [14,15,16,17,18, 20, 22,23,24]. This study was approved by the institutional review board of the Fujita Health University.
Surgical operator selection
In all LG procedures, the ESSQS-qualified surgeons were involved as either the operator or the instructive assistant. When a non-ESSQS-qualified surgeon performed LG, an ESSQS-qualified surgeon, as an assistant surgeon, made his/her best effort to help the operating surgeons safely complete LG. In our institute, the surgeons’ skill level and experience vary greatly with regard to OG, LG, and other laparoscopic surgeries, and not all are ESSQS-qualified. Although a uniform or systematic educational program was not established at the initiation of the study, the criteria for selection of surgical operator were determined according to our basic policy, including the following: First step was to learn the surgical procedures by benefiting from the experience of the first assistant or the scope operator in the first year. Second step was to perform laparoscopic distal gastrectomy (LDG) with D1+ dissection in the second year. Third step was to perform LDG with D2 dissection or laparoscopic total gastrectomy (LTG) with D1+ dissection in the third year. Fourth step was to perform LTG with D2 dissection procedures in the fourth year and onwards. The expert gastric surgeon (I.U.), who had performed more than 1500 LG procedures, finally identified the operating surgeon and the assistant surgeons for each patient, supervising all LG procedures. To monitor the operative quality, we had video conferences once a week for every operation using edited surgical videos. Furthermore, surgeons had the opportunity to review full surgical videos for any selected operation once a week.
Measurements
All patients were observed for 30 days following surgery. The clinicopathological characteristics and short-term surgical outcomes, including operative time, surgeon console time, estimated blood loss, the number of dissected lymph nodes, total morbidity rate, mortality rate, and length of postoperative hospitalization were evaluated as secondary endpoints. All postoperative complications were classified in accordance with the Japan Clinical Oncology Group Postoperative Complication Criteria [25]. The primary endpoint of this single-center retrospective analysis was morbidity (Clavien–Dindo [CD] Grade ≥ IIIa) [26]. Total operative time was defined as the time from the beginning of the abdominal incision until the end of complete wound closure. Blood loss was estimated by weighing suctioned blood and gauze pieces with absorbed blood. Intra-abdominal infectious complications included anastomotic leakage, pancreatic fistula, and intraperitoneal abscess. An expert surgeon was defined as a surgeon with an experience of 100 or more LG with D2 dissection. Except three expert surgeons (I.U., S.K., and S.S.), the number of experienced LG cases by each operating surgeon was counted from his/her initial case at our institution, irrespective of the prior LG experience before he/she joined our institution.
Propensity-score matched analysis
Propensity-score matched (PSM) analysis was used to limit confounders and overcome possible patient selection bias. Propensity scores for all patients were calculated using a logistic regression model based on the following variables: age, gender, body mass index (BMI), American Society of Anesthesiologist (ASA) grade, presence of neoadjuvant chemotherapy, history of laparotomy, cT, cN, cStage, pT, pN, pStage, type of gastrectomy, and extent of LN dissection. Consequently, rigorous adjustment for significant differences in the baseline characteristics of patients with propensity-score matching using nearest-neighbor matching without replacement with a caliper width of 0.2 of the standard deviation of the logit of the propensity score was performed. We used the absolute standardized difference (SD) to measure covariate balance, in which an absolute standardized mean difference > 0.1 reflected a meaningful imbalance [18].
Statistical analysis
All analyses were performed using IBM SPSS Statistics 23 (IBM Corporation, Armonk, NY, USA). Between-group comparisons were examined by the χ2 test or the Mann–Whitney U test. Univariate χ2 test and multivariate logistic regression analysis were used to determine the factors contributing to the occurrence of postoperative complications. Data were expressed as medians [range] or odds ratio (OR) [95% confidence interval] unless otherwise noted. A probability (p) value < 0.05 (two-tailed) was considered statistically significant.
Results
Baseline data on patients receiving LG by non-ESSQS-qualified and ESSQS-qualified surgeons overall and by PSM analysis
In total, 33 surgeons participated in this study. Eight surgeons, including I.U., had already been ESSQS-accredited before the study, 11 surgeons had newly acquired ESSQS qualification, and 14 surgeons remained non-ESSQS-qualified surgeons. The length of surgeon experience was significantly shorter among the non-ESSQS-qualified surgeons than among the qualified surgeons (non-ESSQS-qualified, 11 years [3–27] vs. ESSQS-qualified, 15 years [7–34], p < 0.001). The patient characteristics of each cohort are summarized in Table 1. Across the entire cohort, no differences were observed in terms of age, gender, BMI, ASA score, and history of laparotomy between the nonqualified and qualified group; however, significant differences were found in tumor size, cT, cN, cStage, pT, pN, pStage, preoperative chemotherapy, type of resection, extent of lymphadenectomy, and splenectomy. Factors having an SD > 0.1 included tumor size, cT, cN, cStage, pT, pN, pStage, use of preoperative chemotherapy, type of resection, extent of lymphadenectomy, and splenectomy (Table 1). To compensate for such differences, PSM analysis was used. The average and standard deviation of the propensity score were 0.543 and 0.200, respectively, thus yielding a caliper width of 0.04 for this study. After propensity-score matching, 321 patients were included in each group. Propensity-score distributions for each case before and after matching are presented in Fig. 2. After matching, the SD for age, gender, BMI, ASA classification, history of laparotomy, tumor size, cT, cN, cStage, pT, pN, pStage, presence of neoadjuvant chemotherapy, type of resection, extent of LN dissection, and splenectomy decreased to < 0.10, indicating that a sufficient balance was achieved (Table 1).
The surgical outcomes of LG by ESSQS-qualified and non-ESSQS-qualified surgeons overall and by PSM analysis
All procedures were completed by each operating surgeon without any severe intraoperative adverse events in this series. The surgical outcomes and short-term postoperative courses of the entire cohort and the PSM cohort are summarized in Table 2. Before PSM, patients operated by non-ESSQS-qualified surgeons had significantly less estimated blood loss and a significantly shorter duration of hospitalization following surgery compared with the patients operated by the ESSQS-qualified surgeon group, although having a lower number of dissected LNs. No significant differences were observed in total operative time, conversion to open procedure, reoperation rate, morbidity rate, intra-abdominal infectious complications rate, or in-hospital mortality (Table 2). After PSM, the non-ESSQS-qualified surgeon group had a significantly longer total operative time (non-ESSQS-qualified group, 368 [170–779] min vs. ESSQS-qualified group, 316 [147–772] min; p < 0.001) and a significantly larger estimated blood loss (non-ESSQS-qualified group, 28 [0–702] mL vs. ESSQS-qualified group, 25 [0–1069] mL; p = 0.042]). No significant differences were observed in the number of dissected LNs, conversion to open procedure, reoperation rate, morbidity rate, intra-abdominal infectious complications rate, the length of hospital stay following surgery, or in-hospital mortality (Table 2).
Postoperative complications
Postoperative complications are summarized in Table 3. Briefly, across the entire cohort, no significant differences were observed in the incidence of total morbidity, intra-abdominal infectious complications, other local complications, and systemic complications. After propensity-score matching, results similar to those for the entire cohort were obtained (Table 3).
Relationship between surgeon experience, the ESSQS qualified rate and morbidity rate as well as operative time
We investigated whether the overall experience and ESSQS qualification of the surgeons influenced on the incidence of postoperative complications in the entire cohort. No obvious relationship was observed between the incidence of complications and the number of years of surgeon experience for both non-ESSQS-qualified and ESSQS-qualified surgeons (Fig. 3). In addition, there were no significant differences in the incidence of complications between the non-ESSQS-qualified surgeons, nonexpert ESSQS-qualified surgeons, and expert ESSQS-qualified surgeons (Fig. 4). Next, the entire cohort was divided into the following three groups based on each operating surgeon’s number of LG experience irrespective of the presence of the ESSQS qualification: each operating surgeon’s case 1–20 (Case 1–20), 21–50 (Case 21–50), and 51– (Case 51–). Then, case volume stratified by the type of LG procedure (LDG D1+, LDG D2, and LPG/LTG) in each group was shown as a bar graph (Fig. 5). In addition, the proportion of the patients whom the ESSQS-qualified surgeons operated, as well as operative time and morbidity stratified by the type of LG procedure in each group were also demonstrated (Fig. 5A, B). Consequently, as the number of experienced LGs increased, the proportion of ESSQS-qualified surgeons significantly increased (p < 0.001), and operative time significantly decreased in each procedure (total: p < 0.001, LDG-D1+: p < 0.001, LDG-D2: p < 0.001, LPG/LTG: p = 0.008, Fig. 5a). However, morbidity did not significantly change between the groups, even in each procedure (Fig. 5b). Regarding procedure type, over 50% in Case 1–20 consisted of LDG D1+, however, in Case 21–50 and Case 51-, the proportions of more complicated procedures including LDG with D2 dissection and LPG/LTG increased (Fig. 5a, b).
Factors determining postoperative morbidity after LG in the entire cohort
To identify the factors determining postoperative CD-grade IIIa or higher complications of LG in the entire cohort, univariate and multivariate analyses were performed. Univariate analyses revealed six significant determinants, including male, cStage II or higher, proximal or total gastrectomy, splenectomy, operative time ≥ 360 min, and estimated blood loss ≥ 50 mL (Table 4). Multivariate analyses demonstrated that only operative time ≥ 360 min (OR 1.818 [1.069–3.094], p = 0.027) was identified as an independent risk factors for morbidity (Table 4).
Factors determining postoperative morbidity after LG in the PSM cohort
We also investigated the risk factors for postoperative complications of LG in the PSM cohort by uni- and multivariate analyses. Univariate analyses revealed three significant determinants, including cStage II or higher, operative time ≥ 360 min, and estimated blood loss ≥ 50 mL (Table 5). Multivariate analyses demonstrated that only operative time ≥ 360 min (OR 2.206 [1.076–4.521], p = 0.031) was identified as an independent risk factors for morbidity (Table 5).
Discussion
In this retrospective single-center study, the incidence of postoperative complications did not differ according to the presence and absence of ESSQS qualification both before and after PSM analysis. In addition, the surgeons’ experience—both in terms of years and cases—were not significantly associated with an increased incidence of complications. Moreover, multivariate analysis showed that being operated by a non-ESSQS-qualified surgeon was not an independent risk factor for postoperative complications. Therefore, these findings provided evidence that the non-ESSQS-qualified surgeons performed LG safely, without increasing the incidence of postoperative morbidity, in contrast to our hypothesis that ESSQS-qualified surgeons may decrease postoperative morbidity. This result is in line with previous studies showing that the incidence of postoperative morbidity did not differ between highly qualitied and less qualified surgeons [27,28,29,30]. An important similarity between these studies and our study was that the non-ESSQS-qualified surgeons operated under the guidance of the ESSQS-qualified surgeons. Therefore, it appears that the qualified surgeons could successfully remedy any immature surgical skills of the nonqualified surgeons by providing intraoperative instructions, including advice on the prevention of severe postoperative complications. These findings suggest that the ESSQS is relevant both for improving the skills of the operator, but also for securing better supervision, since ESSQS-qualified surgeons are used as instructive assistants during LG. In the future, we hope that further maturation of the ESSQS will help the trainees overcome the learning curve of LG, which involves at least 40–60 cases [31,32,33,34].
With regard to the comparison of the complication rates between the qualified and nonqualified surgeons, all surgeons involved in the operations could understand and carry out the various common surgical concepts and technical principles including outermost layer-oriented LN dissection and intracorporeal anastomosis, which are performed safely and reproducibly in our institute as previously reported [16, 17, 22, 35,36,37]; this is achieved via the sufficient experiences of the first assistant, a scope operator, and regular video conferences. Similarly, some previous studies have also reported that the trainees could efficiently achieve a plateau of the operative time in LG and perform LG without significantly increasing the incidence of morbidity after sufficient experience as an assistant and a scope operator or through the systematic education system [27,28,29,30]. Therefore, we believe that the operative quality might rely more on sharing the common surgical concepts and technical principles within the surgical team than on the individual operator’s skills per se.
Another important finding is that the total operative time in the group of ESSQS-qualified surgeons was significantly shorter than that of the non-ESSQS-qualified surgeons group after compensating for patient demographics, tumor characteristics, and surgical procedure by PSM analysis. In addition, as the number of experienced LGs increased, the proportion of ESSQS-qualified surgeons increased, and operative time decreased in each procedure. These findings suggest that the presence of ESSQS qualification is positively associated with experienced LG cases and the proficiency of LG procedure. Several previous studies also have successfully demonstrated that highly qualified surgeons perform LG in a significantly shorter operative time than the less qualified surgeons [27,28,29]. Our data strengthen the evidence that ESSQS-qualified surgeons have superior skills of LG compared with non-ESSQS-qualified surgeons. Accordingly, our strategy wherein the ESSQS-qualified surgeons are deemed sufficiently skillful and always involved as the instructive assistant when non-ESSQS-qualified surgeons perform LG seems quite reasonable.
On the other hand, the multivariate analyses of this study showed that only operative time ≥ 360 min was identified as an independent risk factor for postoperative morbidity in both the entire cohort and the PSM cohort, confirming observations from our previous studies [17, 18]. This finding also agreed with previous reports observing that prolongation of the operative time leads to an increased risk of postoperative morbidity [38,39,40]. Nevertheless, shortening of the operative time by ESSQS-qualified surgeons did not results in a reduced incidence of postoperative morbidity. This might be explained by the possibility that the ESSQS-qualified surgeons performed LG for more patients with large tumor and more advanced disease, who received preoperative chemotherapy, D2 dissection, total or proximal gastrectomy, and splenectomy in this study. Hence, since ESSQS-qualified surgeons were possibly more likely to perform LG requiring these technically demanding procedures, the morbidity risk might thereby be balanced out. In addition, many of these technically demanding procedures were excluded by the PSM analysis. Therefore, postoperative morbidity risk associated with the qualification of the operating surgeon might have been underestimated in this study. In addition, because we could not identify the factor most associated with an increased incidence of complications among the factors protracting the operative time, further investigation is necessary to clarify the association between protracted operative time and increase of the complications.
In comparison with those previous reports [27,28,29,30], our study has strengths in the following three points: First, this study included a lot more operating surgeons (a total of 33 surgeons) and the ESSQS-qualified surgeons (a total of 19 surgeons), whereas the previous studies included only a couple of the trainers or the ESSQS-qualified surgeons. The outcomes of our study are likely to be more reproducible in regard to the impact of ESSQS-qualified surgeons. Second, this study included more complicated procedures including LPG, LTG, and LG after neoadjuvant therapy, whereas the previous studies focused only on LDG. Therefore, our study has determined the impact of ESSQS-qualified surgeons in more advanced setting. Third, apart from the previous studies, this study used propensity score matched analysis to control for confounding. Therefore, we believe our study has determined the impact of ESSQS-qualified surgeons in more reproducible fashion in more advanced setting using more statistically reliable manner.
There were a couple of limitations to the present study. First, this study was analyzed based on a single-center, retrospective, and nonrandomized design. Therefore, several sources of patient bias, especially patient selection bias, could not be excluded, despite compensating for differences in preoperative patients characteristics by propensity-score matching. Further studies including prospective non-inferiority trials are warranted to provide sufficient evidence to accept or refute our hypothesis. Second, our study was limited by operators’ bias. Because we were not able to obtain the number of LGs which each operating surgeon conducted before he/she joined our institution from our database, association between the lifetime total number of LGs of each operating surgeon and his/her skills was not determined in this study. In addition, detailed information on the instructive assistants was not available for analysis, while the operators’ experience was investigated. Therefore, a detailed analysis of the influence of the joint experience of the operator and the instructive assistant on operative time and postoperative morbidity could not be carried out. As already mentioned, several technically demanding procedures performed by the ESSQS-qualified surgeons were excluded in the PSM analysis in this study. Therefore, the relative contribution of the ESSQS-qualified surgeons to safely performed LG for patients requiring technically demanding procedures should be clarified. Third, the oncological safety could not be investigated in this study because long-term surveillance is still in development. Due to the small differences in morbidity rate between patients operated by the ESSQS-qualified and non-ESSQS-qualified surgeons, respectively, the long-term oncological outcomes might be expected to be equivalent, if the dissected area was evenly kept between the two groups. Further investigation is warranted to determine the oncological safety of this procedure.
In conclusion, ESSQS qualification might contribute to shortening the operative time of LG. In contrast, the incidence of postoperative morbidity appears not to differ between the ESSQS-qualified and non-ESSQS-qualified surgeons, as long as ESSQS-qualified surgeons provide intraoperative instructions.
References
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage i gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 263:28–35
Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W (2009) A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer 22:214–222
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 5:506–513
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S (2017) Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 20:699–708
Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, Koeda K (2020) Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol 5:142–151
Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K, Takiguchi N, Kaji M (2009) Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 22:999–1008
Zou ZH, Zhao LY, Mou TY, Hu YF, Yu J, Liu H, Chen H, Wu JM, An SL, Li GX (2014) Laparoscopic vs open D2 gastrectomy for locally advanced gastric cancer: a meta-analysis. World J Gastroenterol 20:16750–16764
Zeng F, Chen L, Liao M, Chen B, Long J, Wu W, Deng G (2020) Laparoscopic versus open gastrectomy for gastric cancer. World J Surg Oncol 18:20
Yoshida K, Honda M, Kumamaru H, Kodera Y, Kakeji Y, Hiki N, Etoh T, Miyata H, Yamashita Y, Seto Y, Kitano S (2018) Surgical outcomes of laparoscopic distal gastrectomy compared to open distal gastrectomy: a retrospective cohort study based on a nationwide registry database in Japan. Ann Gastroenterol Surg 2:55–64
Hiki N, Honda M, Etoh T, Yoshida K, Kodera Y, Kakeji Y, Kumamaru H, Miyata H, Yamashita Y, Inomata M, Konno H (2018) Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric Cancer 21:162–170
Kodera Y, Yoshida K, Kumamaru H, Kakeji Y, Hiki N, Etoh T, Honda M, Miyata H, Yamashita Y, Seto Y, Kitano S (2019) Introducing laparoscopic total gastrectomy for gastric cancer in general practice: a retrospective cohort study based on a nationwide registry database in Japan. Gastric Cancer 22:202–213
Mori T, Kimura T, Kitajima M (2010) Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minim Invasive Ther Allied Technol 19:18–23
Tanigawa N, Lee SW, Kimura T, Mori T, Uyama I, Nomura E, Okuda J, Konishi F (2011) The endoscopic surgical skill qualification system for gastric surgery in Japan. Asian J Endosc Surg 4:112–115
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I (2019) Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 27:286–294
Nakauchi M, Suda K, Kadoya S, Inaba K, Ishida Y, Uyama I (2016) Technical aspects and short- and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective study. Surg Endosc 30:4632–4639
Uyama I, Suda K, Satoh S (2013) Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer 13:19–25
Nakamura K, Suda K, Suzuki A, Nakauchi M, Shibasaki S, Kikuchi K, Nakamura T, Kadoya S, Inaba K, Uyama I (2018) Intracorporeal isosceles right triangle-shaped anastomosis in totally laparoscopic distal gastrectomy. Surg Laparosc Endosc Percutan Tech 28:193–201
Shibasaki S, Suda K, Nakauchi M, Nakamura K, Kikuchi K, Inaba K, Uyama I (2020) Non-robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra-abdominal infectious complications: a single-center, retrospective and propensity score-matched analysis. World J Gastroenterol 26:1172–1184
Japanese classification of gastric carcinoma: 3rd English edition (2011) Gastric cancer 14:101–112
Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I (2015) Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 29:673–685
Japanese gastric cancer treatment guidelines 2014 (ver. 4) (2017) Gastric cancer 20:1–19
Shibasaki S, Suda K, Nakauchi M, Nakamura T, Kadoya S, Kikuchi K, Inaba K, Uyama I (2018) Outermost layer-oriented medial approach for infrapyloric nodal dissection in laparoscopic distal gastrectomy. Surg Endosc 32:2137–2148
Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S (2012) Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 36:331–337
Shibasaki S, Suda K, Nakauchi M, Kikuchi K, Kadoya S, Ishida Y, Inaba K, Uyama I (2017) Robotic valvuloplastic esophagogastrostomy using double flap technique following proximal gastrectomy: technical aspects and short-term outcomes. Surg Endosc 31:4283–4297
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, Sasako M (2016) Extended Clavien–Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 46:668–685
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kameda C, Watanabe M, Suehara N, Watanabe Y, Nishihara K, Nakano T, Nakamura M (2018) Safety of laparoscopic distal gastrectomy for gastric cancer when performed by trainee surgeons with little experience in performing open gastrectomy. Surg Today 48:211–216
Yamada T, Kumazu Y, Nakazono M, Hara K, Nagasawa S, Shimoda Y, Hayashi T, Rino Y, Masuda M, Shiozawa M, Morinaga S (2020) Feasibility and safety of laparoscopy-assisted distal gastrectomy performed by trainees supervised by an experienced qualified surgeon. Surgi Endosc 34:429–435
Kuroda S, Kikuchi S, Hori N, Sakamoto S, Kagawa T, Watanabe M et al (2017) Training system for laparoscopy-assisted distal gastrectomy. Surg Today 47:802–809
Nunobe S, Hiki N, Tanimura S, Nohara K, Sano T, Yamaguchi T (2013) The clinical safety of performing laparoscopic gastrectomy for gastric cancer by trainees after sufficient experience in assisting. World J Surg 37:424–429
Kim MC, Jung GJ, Kim HH (2005) Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroentero 11:7508–7511
Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, Han SU (2007) Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc 21:28–33
Kunisaki C, Makino H, Yamamoto N, Sato T, Oshima T, Nagano Y, Fujii S, Akiyama H, Otsuka Y, Ono HA, Kosaka T (2008) Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech 18:236–241
Hu WG, Ma JJ, Zang L, Xue P, Xu H, Wang ML, Lu AG, Li JW, Feng B, Zheng MH (2014) Learning curve and long-term outcomes of laparoscopy-assisted distal gastrectomy for gastric cancer. J Laparoendosc Adv S 24:487–492
Kanaya S, Haruta S, Kawamura Y, Yoshimura F, Inaba K, Hiramatsu Y, Ishida Y, Taniguchi K, Isogaki J, Uyama I (2011) Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc 25:3928–3929
Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T (2002) Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 195:284–287
Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I (2010) Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 211:e25-29
Procter LD, Davenport DL, Bernard AC, Zwischenberger JB (2010) General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg 210:60-65.e2
Park YS, Son SY, Oo AM, Jung do H, Shin DJ, Ahn SH, Park DJ, Kim HH (2016) Eleven-year experience with 3000 cases of laparoscopic gastric cancer surgery in a single institution: analysis of postoperative morbidities and long-term oncologic outcomes. Surg Endosc 30:3965–3975
Wang X, Yao Y, Qian H, Li H, Zhu X (2019) Longer operating time during gastrectomy has adverse effects on short-term surgical outcomes. J Surg Res 243:151–159
Acknowledgements
The authors would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Funding
This work was not supported by any grant or funding.
Author information
Authors and Affiliations
Contributions
All the authors have fully met the ICMJE authorship criteria as follows: Study design: SS, KS, IU; Data collection: SS, MN, KN, TT, KK; Statistical analysis and interpretation of results: SS, KS, MN, KI; Drafting of the manuscript: SS, KS; Critical revision of the manuscript for important intellectual content: KS, IU. All authors read and approved the final manuscript. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Disclosures
Ichiro Uyama has received lecture fees from Intuitive Surgical, Inc., outside of the submitted work Koichi Suda, Tsuyoshi Tanaka, and Kenji Kikuchi have been funded by Medicaroid, Inc. in relation to Collaborative Laboratory for Research and Development in Advanced Surgical Technology, Fujita Health University. Koichi Suda has also received advisory fees from Medicaroid, Inc., outside of the submitted work. Susumu Shibasaki, Koichi Suda, Masaya Nakauchi, Kenichi Nakamura, Tsuyoshi Tanaka, Kenji Kikuchi, Kazuki Inaba, and Ichiro Uyama have no commercial association with or financial involvement that might pose a conflict of interest in connection with the submitted article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shibasaki, S., Suda, K., Nakauchi, M. et al. Impact of the Endoscopic Surgical Skill Qualification System on the safety of laparoscopic gastrectomy for gastric cancer. Surg Endosc 35, 6089–6100 (2021). https://doi.org/10.1007/s00464-020-08102-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08102-5