Abstract
Background
Cellular apoptosis plays an important role in ischemia-reperfusion (I/R) injury during organ transplantation. Synthetic small interference RNA (siRNA) targeting apoptotic receptor Fas has proven effective to protect mice against hepatitis and renal I/R injury. The objective of this study is to investigate the silencing impact of Fas siRNA to alleviate I/R injury in rat liver transplantation.
Materials and methods
Rat hepatocytes (BRL cells) were transfected with three pairs of synthesized Fas siRNA; cells untreated and treated with GFP siRNA were taken as blank and siRNA control. The most effective Fas siRNA was chosen for in vivo experiments. Syngeneic orthotopic liver transplantation was performed in Fas siRNA group, siRNA control group, and blank control group of Sprague–Dawley rats. There were 25 pairs of rats in each group. siRNA transfection of donor rats was done with hydrodynamic injection method 48 h before liver procurement. Blood and liver samples were collected for evaluation of serum ALT levels, Fas protein and mRNA expression, and apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining, 1, 3, 6, 12, and 24 h after liver transplantation.
Results
Fas siRNA2, which inhibited Fas gene expression much more than other siRNAs, was chosen for in vivo experiment. The serum ALT levels of Fas siRNA group were much less than those of blank and siRNA control groups 1, 3, 6, 12, and 24 h after blood reperfusion, indicating diminishing ischemia-reperfusion injury. Donor livers in Fas siRNA group had substantially less cell apoptosis. The expression of Fas mRNA and protein was reduced dramatically in the Fas siRNA group compared with the other two groups.
Conclusion
Fas-mediated apoptosis play an important role in I/R injury of rat liver transplantation. Silencing Fas by hydrodynamic injection of siRNA holds therapeutic promise to limit I/R injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia-reperfusion (I/R) injury is a major cause of primary nonfunction after liver transplantation [1]. Several studies have pointed out that apoptosis is activated during I/R injury and is a critical mechanism of reperfusion injury in the ischemic liver [2–4].
Fas(CD95) is one of the most prominent surface receptors transducing death signals into the cell and inducing Fas-dependent pathway of apoptosis. This pathway is initiated by Fas ligand (FasL, CD95L). Hepatocytes have a high expression level of Fas and are very susceptible to Fas-mediated apoptosis [5]. Fas-mediated apoptosis is important in cold preservation and reperfusion injury related to liver transplantation [6, 7]. Moreover, Fas play an important role in organ injection during liver transplantation because CTLs express FasL to induce apoptosis of their targeting cells. Accordingly, Fas hold potential therapeutic value in liver transplantation.
Cellular production of the Fas receptor drives apoptosis when associated with FasL. However, if the receptor is unavailable, even with the ligand present, the apoptotic pathway cannot proceed. Synthetic small interference RNA (siRNA) 21 to 23 nucleotides in length silence gene expression post-transcriptionally. RNA interference (RNAi) targeting Fas has been implied to protect mice from fulminant hepatitis and limit renal ischemia-reperfusion injury in mice [8, 9]. As shown in those previous experiments, hydrodynamic method, a large volume solution injection, was used for in vivo transfection of siRNA. The hepatocytes gave positive results of 88 ± 6% siRNA uptake, providing an efficient method of siRNA delivery [8]. These researches also showed that suppression of Fas mRNA and protein could persist for 10 days in mouse hepatocytes.
Based on these facts, we designed this study to screen effective rat Fas siRNA in vitro and investigate whether siRNAs targeting Fas could suppress Fas expression and protect rat undergoing syngeneic liver transplantation from liver I/R injury.
Materials and methods
Cells and reagents
Rat hepatocytes (BRL cells) were obtained from the Cell Bank of Sun Yat-Sen University (Guangzhou, China). Three 21-nt siRNA duplexes targeting rat Fas gene (GenBank accession no. NM 139194) were designed using the siRNA Target Finder and Design Tool available at http://www.ambion.com and were commercially obtained from Ribobio (Guangzhou, China). The sequence of these siRNAs was: Fas siRNA1, beginning at nt 175, 5′ CGACAACAACUGCUCAGAA dGdT 3′ (sense), 3′ dCdA GCUGUUGUUGACGAGUCUU 5′ (antisense); Fas siRNA2, beginning at nt 315, 5′ ACACGGACAGGAAACACUA dGdT 3′ (sense); 3′ dTdG UGUGCCUGUCCUUUGUGAU 5′ (antisense); Fas siRNA 3, beginning at nt 418, 5′ CACCUCGUGUGGACUUGAA dTdG 3′ (sense); 3′ dGdTGUGGAGCACACCUGAACUU5′ (antisense). Green fluorescent protein (GFP) siRNA, with no homology to Fas gene, was taken as siRNA control. Its sequence was 5′P.GGCUACGUCCAGGAGCGCACC3′ (sense), 5′P.UGCGCUCCUGGACGUAGCCUU3′ (antisense).
siRNA transfection and screening
BRL cells were cultured in 37°C Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum supplemented with glutamine (2 mM) and streptomycin (100 μg/ml). Twenty-four hours before transfection, the cells were seeded into 12-well plates at a density of 0.52 × 105 cells/well and transfected with synthesized siRNA (50 nmol/l) using Lipofectamine 2000 and serum-free medium (OPTI-MEM) according to the manufacturer’s recommendations. Cell transfected with GFP was taken as siRNA control. Blank control cell was treated with Lipofectamine 2000 but not with siRNA. After incubation for 48 h, cells were harvested and expression of Fas at both mRNA and protein levels was detected by real time PCR (RT-PCR) and Western blot. Fas siRNA that had the maximum inhibition rate would be chosen for in vivo experiment.
Liver transplantation
Male specific pathogen-free Sprague–Dawley rats weighing 200–250 g were obtained from the Laboratory Animal Center of Sun Yat-Sen University. Animals received care according to guidelines of the China Association of Laboratory Animal Care. Forty-eight hours before liver procurement, hydrodynamic injection method was used for siRNA transfection in vivo through the penis vein. Donor rats (n = 25) of Fas siRNA group, siRNA control group, and blank control group were injected with phosphate-buffered saline (PBS; 100 ml/kg) containing 200 nmol/kg Fas siRNA, with PBS (100 ml/kg) containing 200 nmol/kg GFP siRNA, and with PBS (100 ml/kg) only, respectively. All the injections were finished within 15 s. The recipient rats were fasted for 12 h before transplantation but had free access to water. All the donor livers were harvested and preserved with 4°C Ringer’s solution. The preservation time was 3 h. Syngeneic orthotopic liver transplantation was performed using the method of Kamada’s et al. [10] without hepatic artery reconstruction. The average anhepatic period was 17.0 ± 3.0 min. Five recipient rats of each group were killed at 1, 3, 6, 12, and 24 h after reperfusion. Blood samples and liver specimens were collected for further measurements.
Assay of serum ALT
Alanine aminotransferase (ALT) was taken as a mark of hepatocellular injury after I/R injury. Blood samples were taken from the inferior vena cava and immediately centrifuged. Plasma samples obtained were preserved at −80°C for determination of liver enzymes. ALT levels were measured with Hitachi clinical analyzer 7180.
TUNEL staining
Part of the specimens was immediately fixed in 4°C buffered formalin (4% paraformaldehyde) and embedded in paraffin. In situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) of fragmented DNA was performed on 5-μm thick sections of paraffin-embedded tissue using the in situ TUNEL assay (Roche Diagnostics) according to the supplier’s instructions. The sections were deparaffinized in xylene and rehydrated before analysis. After treatment with proteinase K (20 mg/ml in 10 mM Tris-HCl, pH 7.6) at 37°C for 30 min, sections were washed in PBS and endogenous peroxidase activity was inhibited by immersing the slides in 3% hydrogen peroxide in methanol for 30 min. Then, the slides were treated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 15 min. For the labeling reaction, a TUNEL mixture containing terminal deoxynucleotidyl transferase (TdT), its buffer solution, and biotinylated dUTP was added to cover the sections, which were then incubated at 37° for 60 min in a humidified chamber. Washed sections were incubated with peroxidase-labeled streptavidin for 30 min and then stained with diaminobenzidine, followed by counterstaining with hematoxylin.
TUNEL-positive nuclei in brown were identified from negatively unstained nuclei in blue. Apoptotic cells were counted for each tissue sample under high-power magnification (×400) in a blinded fashion. Apoptotic index (AI) was used as a quantitative indicator, indicating the percentage of TUNEL-positive hepatocytes by counting the number of positive cells in 30 random microscopic high-power fields.
RT-PCR of Fas mRNA
To assess Fas mRNA expression, total RNA was isolated by using the TRIzol reagent (Invitrogen). Two micrograms of RNA was reversely transcribed in a thermal cycler (Gene Amp PCR 9600, Perkin Elmer Applied Biosystems) with SuperScript II reverse transcriptase (Invitrogen) to obtain cDNA. RT-PCR was performed on an ABI Prism 7000 sequence detector system (Perkin Elmer Applied Biosystems) with SYBR green reagent for detection based on the manufacturer’s instructions. GAPDH was used as an endogenous control to normalize the expression level of Fas gene. Primer sequences for Fas were 5′ forward TGCACCTCGTGTGGACTTGA and 3′ reverse TTTGTTTCTTGCATTTGGTGTTG. Primer sequences of GAPDH were 5′ forward CACTATCGGCAATGAGCGGT and 3′ reverse ATTTGCGGTGCACGATGGA. All reactions were done in a 50-μl reaction volume. Thermal cycling conditions consisted of 3 min at 93°C followed by 40 cycles of 1 min at 93°C, 1 min at 55°C, and 1 min at 72°C. A melt-curve analysis was performed to verify the specificity of the amplification. Standard curves, which were generated using serially diluted cDNA samples of Fas and GAPDH, were used for analysis. Relative expression of Fas mRNA was normalized to GAPDH mRNA.
Western blot analysis of Fas protein
Fas protein was detected using the Western blot method. The liver samples were finely minced and suspended in ice-cold lysis buffer (2 ml/g of tissue), which contained protease inhibitors to minimize protein degradation. The homogenized suspension was then centrifuged at 12,000×g for 10 min at 4°C, and supernatants were recovered and used for analysis. After measurement of the protein concentration in each extract, 20 μg protein in sodium dodecyl sulfate (SDS) loading buffer were solubilized by boiling. They were subjected to 10% SDS polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride (PVDF) membrane by electroblotting. Finally, the PVDF membrane was incubated with rabbit anti-rat-Fas (1:1,000; Boster, China), rabbit-anti-actin antibody (1:1,000; Boster, China), and followed with goat anti-rabbit-IgG (1:2,000; Boster, China). Protein-antibody complexes were detected with the enhanced chemiluminescence system. Protein bands detected were estimated using UVP Labworks Imaging Analysis software (UVP Labworks, Upland, CA, USA). The density measurement was correlated to protein expression and normalized to β-actin.
Statistics
Experimental results are expressed as mean values ± SEM. Statistical difference between different groups was determined by one-way ANOVA with least-significant difference multiple comparisons. Less than 0.05 P values were considered statistically significant.
Results
siRNA screening
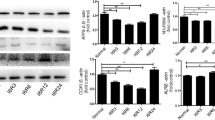
In siRNA screening experiment, the potential of siRNA for inhibition of Fas was measured by RT-PCR and Western blot. At 48 h post-transfection, BRL cells treated with sequence 13 Fas siRNAs suppressed Fas mRNA expression by 53.5 ± 9.0, 69.3 ± 7.8, and 45.0 ± 8.9%, respectively, compared to blank control. Control siRNA treated cells had similar Fas mRNA expression with blank control cells, indicating that control siRNA failed to induce any significant silencing activity. In agreement with the findings on RT-PCR, Western blot analysis of Fas protein showed decreased levels of Fas by 50–75% after transfection with Fas siRNAs and confirmed that sequence 2 siRNA was the most effective in suppressing Fas protein level (Fig. 1a,b, and c). Similarly, treatment of control siRNA had no influence on Fas protein expression. Thus, the results indicated that the downregulation effect of Fas siRNA was sequence-specific and considerable. As Fas siRNA2 was most efficient, we chose it to suppress Fas expression in rat liver transplantation experiment and investigate its biological effects.
a Fas gene expression levels of BRL cells measured by RT-PCR and normalized by GAPDH. Fas siRNA2 was the most effective in suppressing Fas gene expression. *p < 0.01 compared with blank and siRNA control group using one-way ANOVA multiple comparisons. b Western blot analysis for Fas protein in BRL cells indicated that Fas protein were downregulated in the Fas siRNA transfected cells. β-actin was taken as a loading control. c Western blot band density was quantified with UVP Labworks imaging analysis software. Fas siRNA2 was the most efficient
Assay of serum ALT
As shown in Table 1, after liver transplantation, the levels of serum ALT were significantly elevated 1, 3, 6, 12, and 24 h after reperfusion, and the culminated point of ALT levels was 6 h in each group. Fas siRNA pretreatment significantly reduced ALT levels at each checkpoint as compared with that in blank group and siRNA control group (P < 0.05). Compared to blank group, siRNA control group had no significantly different ALT levels (P > 0.05).
TUNEL staining
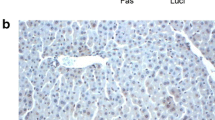
The incidence of apoptosis in the liver was assessed by TUNEL staining. Under light microscope, positive TUNEL cells were recognized by focal nuclear staining, with intact nuclear and cell membrane integrity. Table 2 shows the results of TUNEL staining of liver isografts subject to 1, 3, 6, 12, and 24 h of blood reperfusion. Apoptotic cells increased and were maximized at 6 and 12 h in each group. In Fas siRNA group, AI was significantly diminished compared with blank or siRNA control group (P < 0.01). The difference between blank and siRNA group was not significant. Figure 2 shows the liver TUNEL stain of Fas siRNA group and blank group at 6 h.
RT-PCR of Fas mRNA
After 1, 3, 6, 12, and 24 h of blood reperfusion, Fas mRNA gene relative expression levels were assayed by RT-PCR. As shown in Fig. 3a, Fas mRNA levels were similar in blank and siRNA control groups at each checkpoint. In Fas siRNA group, we detected significant reduction in the relative level of Fas mRNA (about twofold) at all the investigated time compared with that in control group (P < 0.05) or blank group (P < 0.05), respectively.
a Relative Fas mRNA levels of rat liver were suppressed by Fas–siRNA after blood reperfusion. Levels are quantified by RT-PCR and normalized by GAPDH. *p < 0.01 compared with blank and siRNA control group using one-way ANOVA multiple comparisons. b Fas protein expressions were examined by Western blot analysis. β-Actin served as a loading control. Fas protein in Fas siRNA group was much lower than that in siRNA control and blank control groups. c Western blot band density quantified with software demonstrated significant decrease of Fas protein in Fas siRNA group as compared with control groups, *p < 0.01
Western blot analysis of Fas protein
To determine whether the synthesized siRNA could effectively silence Fas protein expression in donor livers, Western blot of Fas protein was assayed. The time-course alteration of Fas protein was in agreement with that of Fas mRNA. From Fig. 3b and c, we can see that the amount of Fas protein in blank and siRNA control livers was not significantly different from each other. Fas siRNA group livers showed a strong reduction in Fas expression compared to the blank and siRNA control livers at each checkpoint.
Discussion
SiRNA must be properly designed against the target mRNA sequence. Hence, rules for siRNA design have been developed [11]. However, not all duplexes matching the design criteria are potent [12]. It is often necessary to design several siRNAs, and these siRNAs still have to be experimentally assessed and optimized for the knockdown of targeting mRNA. In our siRNA screening experiment, three pairs of synthesized Fas siRNAs were transfected into BRL cells. Their gene-silencing efficacy was assessed with RT-PCR for mRNA and Western blot for Fas protein 48 h after transfection. All these siRNAs reduced Fas mRNA expression and protein level in BRL cells, and among them, Fas siRNA2, reducing 75% of Fas mRNA expression, was the most effective. The silencing effect was specific because hepatocytes transfected with control siRNA targeting GFP did not affect Fas mRNA expression. In light of these results, Fas siRNA2 was chosen for in vivo study.
Hydrodynamic injection has been proven effective to facilitate delivery of siRNAs into organs with high blood flow, such as liver and kidney [13]. Delivery of siRNA using this method is achieved using large volumes of aqueous solution injected rapidly, creating a high pressure in the vascular circulation and enhance the vessel’s permeability, enabling passage of large nucleic acid molecules to target cells outside the blood vessel. This approach can make a majority of hepatocytes uptake siRNA. In our experiment, we used this approach to transfect Fas siRNA in donor rat liver. A large volume of 10% rat weigh PBS with a high injection speed did not cause any mortality. All the rats recovered soon after the injection.
I/R injury is not only a major cause of primary graft nonfunction but also increases the immunogenicity of allograft and may lead to a high frequency of early and late rejection occurrence [14]. Several experiments have demonstrated cellular apoptosis as a pivotal mechanism of preservation and ischemia-reperfusion injury in liver transplantation [3, 15]. Free radicals, calcium entry, or inflammation cause cellular dysfunction and may well induce apoptosis in liver grafts [16]. In our study, quantitative analysis of apoptotic cells in donor livers showed apoptotic cells increased after blood reperfusion in all the experiment groups, indicating apoptosis participating the process of I/R injury.
Small interference RNA targeting Fas has been proven effective in mice liver and kidney. We transfected newly designed Fas siRNA in rat donor livers and investigated its function in liver transplantation. After blood reperfusion, ALT level in Fas siRNA group is significantly reduced as compared with blank and siRNA control group at each checkpoint, indicating that Fas siRNA could alleviate I/R injury. In our study, after the reperfusion number of apoptotic cells kept increasing and peaked at 6 h, about 6.68 to 9.43% TUNEL-positive cells were found in blank and siRNA control groups. The fluctuation of AI was similar with that of serum ALT. The results were consistent with other findings [17, 18]. In those reports, the severity of liver tissue apoptosis after transplantation was leveled with serum ALT. The number of apoptotic cells in Fas siRNA group was 2.45 to 4.43%, indicating significant reduction as compared with blank and siRNA control group. Based on these results, we attribute the protective effect of Fas siRNA to the decrease in apoptosis.
FasL and Fas are significantly higher in acute rejection after liver transplantation and other inflammatory conditions [19]. Cursio et al. [20] reported that the proapoptotic molecule FasL is involved in the induction of liver apoptosis after normothermic liver I/R injury. In their study, FasL mRNA and protein expression were increased in ischemic liver, whereas Fas mRNA levels remained unchanged. In contrast, Saxton et al. [21] showed that the upregulation of Fas gene expression occurred during liver reperfusion and resulted in liver apoptosis. In our study, after blood reperfusion, Fas gene mRNA level and Fas protein expression in blank and siRNA control groups continued to increase and peaked at 6 h, indicating Fas was upregulated by I/R injury and lead to apoptotic cells augmentation. The results were also in agreement with other findings on organ I/R injury including heart, brain, and kidney [9, 22, 23].
Death ligand FasL binding to its relevant receptor, Fas, results in recruitment of Fas-associated death domain, which recruits caspase-8. Activated caspase-8 can directly activate downstream caspases or lead to the release of cytochrome c from mitochondria by cleaving Bid, the Bcl2-interacting protein [24, 25]. Thus, the Fas receptor can activate both extrinsic and intrinsic apoptotic pathways [26]. In the Fas siRNA group, the upregulation of Fas mRNA and Fas protein was inhibited by RNAi. Fas expression was much lower than that in blank and siRNA control group. As a result, the number of apoptotic cells was substantially reduced. We can conclude that siRNA targeting Fas protects I/R injury by the knockdown of Fas mRNA. Nevertheless, apoptosis during synergetic liver transplantation is not completely knocked out by Fas siRNA because a small part of the hepatocytes does not uptake hydrodynamic injected siRNA, and there is another apoptotic pathway involving the release of cytochrome c from the mitochondria, which cannot be completely affected by Fas.
Conclusion
In this experiment, we silenced Fas gene expression using siRNA, suppressed the Fas gene expression and protein level of BRL cells in vitro. Furthermore, we successfully transfected siRNA in vivo with hydrodynamic method and alleviated I/R injury in rat liver transplantation by inhibiting the cellular apoptosis. These results should be useful in future studies and suggest the clinical potential of gene therapy for I/R injury by siRNA-mediated gene silence of Fas. More appropriate delivery strategies should be developed to facilitate the application of siRNA in human.
References
Varotti G, Grazi GL, Vetrone G, Ercolani G, Cescon M, Del Gaudio M, Ravaioli M, Cavallari A, Pinna A (2005) Causes of early acute graft failure after liver transplantation: analysis of a 17-year single-centre experience. Clin Transplant 19(4):492–500
Jiang H, Meng F, Li J, Sun X (2005) Anti-apoptosis effects of oxymatrine protect the liver from warm ischemia reperfusion injury in rats. World J Surg 29(11):1397–1401
Cursio R, Filippa N, Miele C, Colosetti P, Auberger P, Van Obberghen E, Gugenheim J (2005) Fas ligand expression following normothermic liver ischemia-reperfusion. J Surg Res 125(1):30–36
Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA (2000) Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 32(6):1280–1288
Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S (1997) Essential roles of the Fas ligand in the development of hepatitis. Nat Med 3(4):409–413
Yin XM, Ding WX (2003) Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med 3(6):491–508
Kasahara I, Saitoh K, Nakamura K (2000) Apoptosis in acute hepatic failure: histopathological study of human liver tissue using the TUNEL method and immunohistochemistry. J Med Dent Sci 47(3):167–175
Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J (2003) RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 9(3):347–351
Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J (2004) Small interfering RNA targeting Fas protects mice against renal ischemia reperfusion injury. Proc Natl Acad Sci 101(41):14883–14888
Kamada N, Davies HS, Roser B (1981) Reversal of transplantation immunity by liver grafting. Nature 292(5826):840–842
Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A (2004) Rational SiRNA design for RNA interference. Nat Biotechnol 22(3):326–330
Hsieh AC, Bo R, Manola J, Vazquez F, Bare O, Khvorova A, Scaringe S, Sellers WR (2004) A library of SiRNA duplexes targeting the phosphoinositide 3-kinase pathway: determinants of gene silencing for use in cell-based screens. Nucleic Acids Res 32(3):893–901
Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D (2004) Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther 11(8):675–682
Ke B, Shen XD, Lassman CR, Gao F, Katori M, Busuttil RW, Kupiec-Weglinski JW (2003) Interleukin-13 gene transfer protects rat livers from antigen-independent injury induced by ischemia and reperfusion. Transplantation 75(8):1118–1123
Yang J, Jones SP, Suhara T, Greer JJ, Ware PD, Nguyen NP, Perlman H, Nelson DP, Lefer DJ, Walsh K (2003) Endothelial cell overexpression of Fas ligand attenuates ischemia-reperfusion injury in the heart. J Biol Chem 278(17):15185–15191
Jaeschke H (2002) Reperfusion injury after warm ischemia or cold storage of the liver: Role of apoptotic cell death. Transplant Proc 34(7):2656–2658
Wei ZZ, Xia SS (2004) Gamma-hydroxybutyrate protects the liver from warm ischemia-reperfusion injury in rat. Hepatobiliary Pancreat Dis Int 3(2):245–249
Zhang Y, Ye QF, Lu L, Xu XL, Ming YZ, Xiao JS (2005) Panax notoginseng saponins preconditioning protects rat liver grafts from ischemia/reperfusion injury via an antiapoptotic pathway. Hepatobiliary Pancreat Dis Int 4(2):207–212
Tannapfel A, Kohlhaw K, Ebelt J, Hauss J, Liebert U, Berr F, Wittekind C (1999) Apoptosis and the expression of Fas and Fas ligand (FasL) antigen in rejection and reinfection in liver allograft specimens. Transplantation 67(7):1079–1083
Cursio R, Filippa N, Miele C, Colosetti P, Auberger P, Van Obberghen E, Gugenheim J (2005) Fas ligand expression following normothermic liver ischemia-reperfusion. J Surg Res 125(1):30–36
Saxton NE, Barclay JL, Clouston AD, Fawcett J (2002) Cyclosporin A pretreatment in a rat model of warm ischaemia/reperfusion injury. J Hepatol 36(2):241–247
Qiu J, Whalen MJ, Lowenstein P, Fiskum G, Fahy B, Darwish R, Aarabi B, Yuan J, Moskowitz MA (2002) Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci 22(9):3504–3511
Kossmehl P, Kurth E, Faramarzi S, Habighorst B, Shakibaei M, Wehland M, Kreutz R, Infanger M, Danser AH, Grosse J, Paul M, Grimm D (2006) Mechanisms of apoptosis after ischemia and reperfusion: role of the rennin–angiotensin system. Apoptosis 11(3):347–358
Zhao Y, Ding WX, Qian T, Watkins S, Lemasters JJ, Yin XM (2003) Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology 125(3):854–867
Schattenberg JM, Galle PR, Schuchmann M (2006) Apoptosis in liver disease. Liver Int 26(8):904–911
Feldmann G, Haouzi D, Moreau A, Durand-Schneider AM, Bringuier A, Berson A, Mansouri A, Fau D, Pessayre D (2000) Opening of the mitochondrial permeability transition pore causes matrix expansion and outer membrane rupture in Fas-mediated hepatic apoptosis in mice. Hepatology 31(3):674–683
Acknowledgment
The project was supported by the Major State Basic Research Development Program of China, 973 Program, No. 2003CB515507 and the Ph.D. Programs Foundation of Ministry of Education of China, No. 20040558060.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Zhang, J.F., Lu, M.Q. et al. Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbecks Arch Surg 392, 345–351 (2007). https://doi.org/10.1007/s00423-006-0142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-006-0142-5