Abstract
Hepatic ischemia/reperfusion injury (IRI) still remains an unavoidable problem in hepatectomy. The inflammatory response plays an important role in its pathogenesis. The plasma membrane-bound G protein-coupled bile acid receptor (TGR5), as one of G protein-coupled receptor (GPCR) families, has been proved to serve a protective role in several liver diseases. However, the exact function of TGR5 in modulating IRI remains obscure. We injected wild mice with a small interfering RNA of TGR5 (si-TGR5) or TGR5 agonist (INT-777) and established liver partial warm ischemia/reperfusion model. The results showed that knockdown of TGR5 significantly aggravated hepatic tissue injury, but treatment with INT-777 could reverse it, as evidenced by serum ALT and AST tests, liver histological injury, cytokines expressions, liver immunohistochemical analysis, and TUNEL staining. The apoptosis-associated proteins were evaluated after reperfusion. Moreover, we used primary bone marrow–derived macrophages (BMDMs) to establish hypoxia/reoxygenation (H/R) model to verify the anti-inflammation effect of TGR5. In in vivo experiments, we used TGR5-siRNA and TGR5 agonist (INT-777) to determine that TGR5 significantly attenuated liver damage after IRI through activating the Keap1-Nrf2 pathway. In addition, we found that overexpression of INT-777-activated TGR5 could reduce oxidative stress and inflammatory response in H/R-induced BMDMs through regulation of Keap1-Nef2 pathway during in vitro experiment. Importantly, these results were completely reversed in si-TGR5 BMDMs. In conclusion, the results indicated that TGR5 could effectively alleviated inflammation response via accelerating the activation of Keap1-Nrf2 signaling pathway during hepatic IRI, which may be meaningful in reducing related inflammatory molecules and adjusting inherent immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ischemia-reperfusion injury (IRI) remains a significant complication after liver resection and liver transplantation [1, 2], which leads to liver dysfunction and failure [3], and it is a major challenge for liver surgery [4]. IRI is a highly complex process that involves the activation of innate immune reactions, oxidative stress, and cell apoptosis [3]. Recent studies have shown that the Keap1-Nrf2 signaling pathway serves an important role in IRI and the activation of Keap-Nrf2 pathway could alleviate IRI [5]. During liver reperfusion, the acute inflammation response has two phases, which is acute and subacute responses, respectively. Obviously, the activation of Kupffer cells plays a major role in hepatocyte damage during the acute phase at 3–6 h after reperfusion [6, 7]. Thus, TGR5 in Kupffer cells may play an important role in liver IRI, but the explicit effects of TGR5 on acute inflammation responses during liver IRI are still unclear.
The transmembrane G protein-coupled bile acid receptor (TGR5) is a novel cell membrane bile acid receptor [8]. TGR5 is expressed in various organs and tissues including the liver, spleen, placenta, stomach, heart, and kidney [9]. Increasing evidence has reported the important role of TGR5 in the regulation of many biological functions, including energy homeostasis and glucose metabolism. In addition, another special function of TGR5 is its powerful anti-inflammatory effect. In hepatic tissue, TGR5 expression is detected in Kupffer cells. Recent studies have proved that TGR5 contributes important protective actions against inflammation by suppressing the NF-κB signaling pathway [10, 11]. 6-Ethyl-23(S)-methyl-cholic acid (INT-777), a specific TGR5 agonist, can effectively decrease the LPS-triggered inflammatory response and apoptosis in hepatic tissue [10, 12]. In our previous study, we have demonstrated that TGR5 can inhibit inflammatory responses in IRI through suppression of the TLR4-NF-κB pathway [13]. In addition, TGR5 activation on hepatocytes has positive effects on hepatocellular apoptosis inhibition [14, 15]. However, the physiological function of TGR5 in inflammatory responses, and its immunoregulatory mechanism remains unknown.
In the present study, we sought to determine whether TGR5 could ameliorate inflammation and hepatocellular apoptosis due to liver IRI, proving the protective effects of TGR5 in the liver inflammation response through the activation of the Keap1-Nrf2 signaling pathway.
MATERIALS AND METHODS
Animals
Eight-week-old WT male mice (16–18 g) (C57BL/6 J; Laboratory Animal Resources Center of Nanjing Medical University, Nanjing, China) were maintained in a pathogen-free animal facility under a standard 12-h light-dark cycle and free access to standard rodent food and water. Procedures were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals. The Institutional Animal Care & Use Committee (IACUC) of Nanjing Medical University (Protocol Number NJMU08-092) approved the animal protocol.
Surgical Procedure and Treatment
A well-established mouse model of segmental (70%) warm hepatic IRI was used in this study [16]. Briefly, a midline laparotomy was performed under 10% chloral hydrate (0.3 g/kg, intraperitoneally) anesthesia in mice. Mice were injected with heparin (100 U/kg), artery/portal vein blood supply to the left/median hepatic lobes and bile duct in left were interrupted with an atraumatic bulldog clamp. After 90 min of ischemia, the clip was removed, and a continuous 4-0 silk was used to close the abdomen immediately. The mice were sacrificed at required times after reperfusion, and blood and liver tissue samples were harvested for analysis. Six groups of mice were included: sham, sham + si-TGR5, sham + INT-777, IR6, IR6 + si-TGR5, and IR6 + INT-777. Three mice were used in each group. Mice in sham + si-TGR5 group and IR6 + si-TGR5 group received a single injection of si-TGR5 (2 mg/kg, tail vein injection, Invitrogen, Carlsbad, CA) 2 days earlier before the surgery. Mice in sham + INT-777 group and IR6 + INT-777 group were fed with INT-777 (30 mg/kg/day, Invitrogen, Nanjing, China) for 2 days prior to IR injury. Mice were killed by dislocation of the neck after 6 h of reperfusion.
Cell Culture and Hypoxia/Reoxygenation Model
Murine bone marrow–derived macrophages (BMDMs) were dissociated from the bone marrow from 8-week-old C57BL/6 J mice after injection of sodium pentobarbital (30 mg/kg) prior to euthanasia and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco BRL, USA), 1% penicillin/streptomycin, and 10% L929-conditioned medium for 6 days. Cell purity was assayed to range from 94 to 99% CD11b+. The experiments were divided into six groups: control, control + si-TGR5, control + INT-777, H/R, H/R + si-TGR5, and H/R + INT-777. The cells of control + INT-777 and H/R + INT-777 were pretreated with INT-777 (3 μm for 1 h, Invitrogen, Nanjing, China), and cells of control + si-TGR5 and model + si-TGR5 were transfected with TGR5-siRNA by using Lipofectamine® RNAiMax (Life technologies, USA). Cells of three model groups were placed in a chamber with AnaeroPack-Anaero system (MGC, Tokyo, Japan) working as an oxygen absorber and CO2 generator. When the O2 concentration was < 5%, the chamber was placed in cell culture incubator for 90 min and then returned to normoxic (20% O2) conditions for 6 h to establish the hypoxia/reoxygenation (H/R) model.
Serum Biochemical Examination
Blood samples (1 ml) were collected 6 h after ischemia/reperfusion from orbit and centrifuged to obtain serum. The degree of hepatic damage was assessed by the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using an automated chemical analyzer (Olympus Automated Chemistry Analyzer AU5400, Tokyo, Japan).
Quantitative Real-Time PCR Analyses
Total RNA was extracted with TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and then used as a template for reverse transcription into cDNA using the reverse transcription kit (BioTek, Beijing, China). The mRNA levels were amplified by quantitative PCR using SYBR Green (Platinum SYBR Green qPCR Kit; Invitrogen). The relative expression of each mRNA was normalized against β-actin. The results were analyzed by the 2-ΔΔCt method for relative quantization. All experiments were performed three times (Table 1).
Western Blot Analysis
Tissues and cells were lysed using an RIPA lysis buffer containing a protease inhibitor (Thermo Fisher Scientific, China) according to the manufacturer’s instructions. The total protein concentration in supernatants was detected and evaluated using a BCA protein assay kit (Bio-Rad, Hercules, CA). Proteins were separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membrane. These membranes were blocked with 5% skim milk in TBST for 2 h and then incubated with primary antibodies for TGR5 (Abcam Trading Shanghai Company, Shanghai, China), Bax, Bcl-2, cleaved caspase-3, cleaved caspase-9, Keap1, nuclear Nrf2, HO-1, tubulin, and histone H3 (Cell Signaling Technology, Danvers, MA) at 4 °C overnight. Following 3 washes with PBS-T, the membranes were incubated with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA) for 2 h at room temperature. Finally, bands were visualized by the enhanced chemiluminescence system (Pierce, NJ).
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Staining
Paraffin sections of liver tissue (6 μm in thickness) were deparaffinized in toluene and then rehydrated in a graded series of ethanol solutions. A TUNEL apoptosis assay kit (in situ cell death detection kit, Roche-Boehringer Mannheim, Germany) was employed to detect the apoptotic cells.
Measurement of Cytokines
The serum and cells culture medium were centrifuged, and then the supernatant was used for evaluation of cytokines. The levels of interleukin-6 (IL-6), interleukin-10 (IL-10) and tumor necrosis factor alpha (TNF-α) were assessed using enzyme-linked immunosorbent assay (ELISA) kits (Abcam, England). Concentration was calculated using a microplate reader (Bio-Rad, USA) according to the standard curve.
Histopathological Examination
To assess the histopathology of the liver, hematoxylin and eosin (H&E) staining were performed. Paraffin slices of 5-μm tissue sections were stained with an HE. The liver tissues were evaluated randomly with light microscopy. Sections were scored from 0 to 4 for sinusoidal congestion, vacuolization of hepatocyte cytoplasm, and parenchymal cells as described by Suzuki et al. [14].
Immunohistochemical Staining
For IHC staining, formalin-fixed and paraffin-embedded liver tissue were sectioned at 4 mm, deparaffinized, and rehydrated through graded alcohols. Slides were washed three times with PBS and inhibited by 3% hydrogen peroxide in methanol for 20 min followed by Avidin-Biotin blocking using a Biotin-Blocking Kit (Dako, Hamburg, Germany). After that, tissues were incubated with primary antibody (TGR5 and Nrf2) overnight at 4 °C. Following a washing step, sections were incubated with biotinylated secondary antibody. Slides were stained with diaminobenzidine (DAB) (Beyotime Institute of Biotechnology) and counterstained with hematoxylin. The sections were subjected to histopathological analysis by light microscopy.
Immunofluorescence Assay
Murine bone marrow–derived macrophages (BMDMs) (5 × 105 cells) of different groups in vitro were seeded on sterile coverslips in a six-well plate after treatment. They were washed with PBS three times; then, the cells were fixed in 4% paraformaldehyde and permeabilized in 0.3% (v/v) Triton X-100 for 5 min at room temperature. The coverslips were incubated with primary antibodies (Keap1, Nrf2) at 4 °C overnight. After washing with PBS three times, the coverslips were incubated with a secondary antibody for 2 h at room temperature. Each coverslip was observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical Analysis
GraphPad Prism 6.0 was used to assess the data. All data analyzed are expressed as the means ± standard deviation. Student’s two-tailed t-test was used for comparison differences between the two groups. One-way analysis of variance (ANOVA) was performed for multiple comparisons. All experiments were performed at least in triplicate. P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
TGR5 Protects Hepatic Tissues against Liver IRI
First, we analyzed the levels of TGR5 in the WT mice liver following a sham operation or 90 min of ischemia with different reperfusion times (time point: 1, 2, 4, 6, 8, 10, and 12 h). Importantly, the expression of TGR5 markedly increased in the early phase of reperfusion and reached a peak after 6 h for reperfusion, following that TGR5 mRNA and protein expression levels were gradually reduced (Fig. 1a–c).
TGR5 protects liver tissues against liver IRI. qRT-PCR (a, b) and western blot (c) analysis of the TGR5 expression after 90 min of ischemia at different times of reperfusion (1, 2, 4, 6, 8, 10, 12 h, n = 3 mice of each time point), value versus sham group. The experimental group mice (IR6, IR6 + si-TGR5, IR6 + INT-777) were subjected to 90 min of partial liver ischemia followed by 6 h of reperfusion. d, e Liver function evaluated by ALT (U/L) and AST (U/L) of 6 groups of mice. Mean ± SD; ***P < 0.001; **P < 0.01; *P < 0.05. f, g Histopathologic analysis of livers harvested 6 h after reperfusion. h The severity of liver IRI was evaluated by Suzuki’s histological grading. **P < 0.01. Mice in sham + si-TGR5 group and IR6 + si-TGR5 group received the injection of si-TGR5 (2 mg/kg/day, tail vein injection) for 2 days before the surgery. Mice in control + INT-777 group and model + INT-777 group were fed with INT-777 (30 mg/kg/day) for 2 days prior to IR injury. Mean ± SD; ***P < 0.001; **P < 0.01.
To determine the effect of TGR5 on liver IRI, we first examined serum ALT and AST levels in each group. As shown in Fig. 1d–e, the ALT and AST levels markedly increased in the IR6 group compared with those in the sham group. Moreover, the levels of ALT and AST in IR6 + si-TGR5 mice were significantly elevated compared with those in the IR6 + INT-777 mice, which means that TGR5 activation by its agonist INT-777 in vivo could further decrease hepatic IR injury. Consistent with serum results, similar results were followed by hepatic histopathological analysis (Fig. 1f–g) and liver injury grading according to Suzuki score (Fig. 1h). This data demonstrated that TGR5 significantly attenuates hepatic IRI.
TGR5 Alleviates Hepatocellular Apoptosis in Hepatic IRI
To further investigate whether TGR5 reduced liver apoptosis, we first used immunohistochemistry analysis to detect TGR5 in IRI livers, which showed that the number of TGR5 positive cells was obviously raised in IR6 groups compared with sham groups. Moreover, the rate of TGR5 was higher in the IR6 + INT-777 group than other IR6 groups (Fig. 2a–b). Differences in expression of TGR5 among 6 groups in vivo indicated that TGR5 was markedly activated in liver tissues after IR. Next, we analyzed hepatocellular apoptosis in ischemic livers by TUNEL assay at 6 h after reperfusion. As shown in Fig. 2c–d, the number of TUNEL positive cells in IR6 groups was dramatically higher than sham groups, but the extent of apoptosis in IR6 + INT-777 group was significantly lower compared with those in IR6 + si-TGR5 groups, which demonstrates that TGR5 obviously reduces liver apoptosis. In addition, apoptosis-related protein levels of Bax, Bcl-2, cleaved casepase-3, and cleaved caspase-9 were measured by western blot in liver tissues (Fig. 2e). The results showed that the activity of Bax, cleaved caspase-3, and cleaved caspase-9 were markedly increased in IR6 groups when compared with the control groups. In contrast, the level of Bcl-2 was significantly reduced after I/R injury. Meanwhile, treatment with INT-777 significantly reduced the level of Bax, cleaved caspase-3, and cleaved caspase-9 in IR6 + INT-777 group when compared with IR6 + si-TGR5 group. These results indicated that TGR5 could remarkably attenuate liver apoptosis in liver IRI.
TGR5 alleviates hepatocellular apoptosis in hepatic IRI. a,b Immunohistochemistry analysis of TGR5 were evaluated in high-power fields (× 200) in 6 groups. Mean ± SD; ***P < 0.001. c,d Apoptotic cells were quantified in × 200 fields and expressed as percentages of apoptotic cells among total cells. Mean ± SD; ***P < 0.001; **P < 0.01; *P < 0.05. e Western blot analysis of cleaved casepase-3, cleaved casepase-9, Bcl-2, Bax, TGR5, and tubulin in livers of 6 groups.
TGR5 Suppresses I/R-Induced Inflammatory Response In Vivo
As previous research has shown, an IR-caused acute inflammatory response can aggravate liver injury [17]. Inflammatory cytokines (IL-6 and TNF-α), which are engaged in hepatocellular injury, show pro-inflammatory and pro-apoptotic roles in ischemia liver tissues post-reperfusion. However, as an anti-inflammatory and anti-apoptotic cytokine, IL-10 displays a protective effect in IR-stressed liver. As shown in Fig. 3a–c, compared with sham groups, IR6 groups had significantly higher levels of IL-6 and TNF-α, but lower levels of IL-10. Moreover, the levels of pro-inflammatory cytokines (IL-6 and TNF-α) in IR6 + si-TGR5 group was much higher than that in IR6 + INT-777 group. However, the levels of anti-inflammatory cytokines (IL-10) were reversed completely. Similar results were found in serum levels of IL-6, TNF-α, and IL-10 detected by ELISA (Fig. 3d–f). This data demonstrated that TGR5 not only can restrain pro-inflammatory cytokine generation but also promote anti-inflammatory cytokine generation in ischemia livers after reperfusion.
TGR5 suppressed I/R-induced inflammatory response in vivo. a–c TNF-α, IL-6, and IL-10 gene expression in livers harvested 6 h after reperfusion by qRT-PCR analysis. Mean ± SD; *P < 0.05; **P < 0.01. d–f TNF-a, IL-6, and IL-10 secretion by ELISA in serums harvested 6 h after reperfusion. Mean ± SD; ***P < 0.001; **P < 0.01; *P < 0.05.
TGR5 Alleviated Hepatic IRI Through the Keap1-Nrf2 Pathway In Vivo
In order to further investigate the molecular mechanisms underlying the protective effects of TGR5 on liver IRI, we evaluated the regulation of TGR5 on the Keap1-Nrf2 signaling pathway. First, we tested an immunohistochemical analysis of nuclear Nrf2 in ischemia liver tissues after reperfusion. The frequency of nuclear Nrf2 positive cells obviously increased in the IR6 groups compared with the sham groups. Moreover, the frequency of nuclear Nrf2 positive cells in the IR6 + INT-777 group was higher than those in the IR6 + si-TGR5 group (Fig. 4a–b). As shown in Fig. 4c–e, qRT-PCR results showed that hepatic IR distinctly caused nuclear Nrf2 and HO-1 activation, which increased along with the expression of TGR5. The expression of Keap1 was reduced by IRI, however, that could be reversed completely by TGR5 activation (Fig. 4f). Similar results could be found in protein levels of IR-stressed liver tissues through western blot analysis (Fig. 4g–h). These data demonstrated that inflammatory responses during IR-stressed injury could be alleviated by TGR5 via a keap1-Nrf2 signaling pathway.
TGR5 alleviated hepatic IRI through the Keap1-Nrf2 pathway in vivo. a,b Immunohistochemistry analysis of Nrf-2 (× 200) in livers of 6 groups. c–f The expression of Nrf-2, HO-1, TGR5, and Keap1in livers harvested 6 h after reperfusion by qRT-PCR analysis. Mean ± SD; ***P < 0.001; **P < 0.01; *P < 0.05. g Western blot analysis of Keap1, HO-1, TGR5, and tubulin in livers harvested 6 h after reperfusion. h The expression of Nrf-2 and histone H3 in the nucleus by western blot analysis in livers of 6 groups.
TGR5 Suppressed Inflammatory Response and Cellular Apoptosis in H/R Model of BMDM In Vitro
To verify the impact of TGR5 on inflammatory response in vitro, BMDMs isolated from WT mice were transfected with the small interfering RNA (siRNA) to silence TGR5 expression. The results of qRT-PCR and western blot analysis confirmed that siRNA had been successfully transfected into BMDM (Fig. 5a–b). The BMDMs from different groups were exposed to 90 min of hypoxia (5% O2) followed by 6 h of re-oxygenation (20% O2). INT-777 (TGR5-specific agonist) was pretreated 1 h before H/R. The mRNA and inflammatory cytokines secretion of IL-6, TNF-α, and IL-10 were tested by qRT-PCR (Fig. 5c–e) and ELISA (Fig. 5f–h), respectively. Compared with control groups, the levels of IL-6 and TNF-α were much higher in H/R groups, with lower levels of IL-10. However, INT-777 pretreatment in the H/R + INT-777 group significantly reduced IL-6 and TNF-α expression and enhanced IL-10 expression compared to the H/R + si-TGR5 group. Next, we analyzed the apoptosis-related protein levels of Bax, Bcl-2, cleaved casepase-3, and cleaved caspase-9 by western blot in H/R model of BMDM in vitro (Fig. 5i). The results showed that the activity of Bax, cleaved caspase-3, and cleaved caspase-9 were markedly increased in H/R model groups compared with control groups. In contrast, the level of Bcl-2 was significantly reduced after H/R injury. Meanwhile, treatment with INT-777 significantly reduced the level of Bax, cleaved caspase-3, and cleaved caspase-9 in the H/R + INT-777 group when compared with the H/R + si-TGR5 group. The results indicated TGR5 could remarkably attenuate apoptosis in H/R model of BMDM in vitro. Taken together, these data demonstrated TGR5 could effectually reduce the inflammatory response in vitro.
TGR5 suppressed inflammatory response and cellular apoptosis in H/R model of BMDM in vitro. The experimental groups were subjected to hypoxia condition (O2 < 5%) for 90 min and then returned to normoxic condition (O2 = 20%) for 6 h to establish the hypoxia/reoxygenation (H/R) model. a,b The TGR5 expression of control groups, control, control + si-TGR5, and control + INT-777, and the experimental groups, H/R, H/R + si-TGR5, and H/R + INT-777, by qRT-PCR and western blot analysis. The cells of control + INT-777 and H/R + INT-777 were precubated with INT-777 (3 μm) for 1 h, and cells of control + si-TGR5 and H/R + si-TGR5 were transfected with TGR5-siRNA for 2 days before they were harvested. c–e TNF-α, IL-6, and IL-10 gene expression in BMDMs harvested 6 h after reoxygenation by qRT-PCR analysis. f–h TNF-a, IL-6, and IL-10 secretion by ELISA in BMDMs harvested 6 h after reoxygenation. g Western blot analysis of cleaved casepase-3, cleaved casepase-9, Bcl-2, Bax, TGR5, and tubulin in BMDMs harvested 6 h after reoxygenation.
TGR5 Alleviate Inflammatory Response Through Keap1-Nrf2 Pathway In Vitro
In order to further investigate the molecular mechanisms underlying the protective effects of TGR5, we evaluated the regulation of TGR5 on the Keap1-Nrf2 signaling pathway. We first subjected BMDM to H/R, and then Keap1, nuclear Nrf2, and HO-1 were tested by qRT-PCR and western blot. As shown in Fig. 6a–e, the mRNA level of Nrf2 and HO-1 and the protein level of nuclear Nrf2 and HO-1 were increased in H/R groups when compared to control groups. In contrast, Keap1 was decreased in H/R group. Interestingly, TGR5 activation by INT-777 pretreatment further increased nuclear Nrf2 and HO-1 expression, but decreased Keap1 expression. However, these results above could be reversed by knockdown of TGR5. Moreover, immunofluorescence analysis was performed to measure Keap1-Nrf2 pathway-associated factors, respectively. As displayed in Fig. 6f, the expressions of Keap1 dramatically was higher in the H/R + si-TGR5 group compared with the H/R + INT-777 group, which were markedly reduced by INT-777 pretreatment. In contrast, the expression of nuclear Nrf2 was decreased in the H/R + si-TGR5 group, but increased in the H/R + INT-777 group (Fig. 6g). These observations suggested that TGR5 could alleviate inflammatory response through Keap1-Nrf2 pathway in vitro.
TGR5 alleviate inflammatory response through Keap1-Nrf2 pathway in vitro. a–c The expression of Nrf-2, HO-1, and Keap1in BMDMs harvested 6 h after reoxygenation by qRT-PCR analysis. d Western blot analysis of Keap1, HO-1, TGR5, and tubulin in BMDMs harvested 6 h after reoxygenation. e The expression of Nrf-2 and histone H3 in the nucleus by western blot analysis in BMDMs of 6 groups. f–g Immunofluorescence analysis of Keap1 and Nrf-2 (× 200) in BMDMs harvested 6 h after reoxygenation.
DISCUSSION
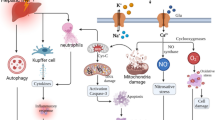
As the number of the largest protein family of trans-membrane receptors, TGR5 could regulate several molecules and activate internal signal pathways. This novel receptor plays an important role in inflammatory response and metabolism balance, for example, innate immunity [18], glucose metabolism and insulin regulation [15], and liver cancer [16, 17]. Previous studies have shown that activation of TGR5 could reduce LPS-caused expression of cytokines and TGR5 could downregulate liver inflammatory response after LPS treatment [10]. Our previous study also indicates that TGR5 attenuates liver IRI via the inhibition of a TLR4 signaling pathway [13]. Numerous studies have showed that the Keap1-Nrf2 signaling pathway serves an important role in ischemia-reperfusion injury [5, 19]. When stimulated by inflammatory response or oxidative stress, Nrf2 could be released from Keap1 and translocate into the nucleus to activate the ARE, which regulates the transcription of genes, including HO-1 [20]. However, the regulation of Keap1-Nrf2 signaling by TGR5 in hepatic IR remains unknown. In the current study, we focused on the role of TGR5 in the regulation of macrophage Keap1-Nrf2 signaling pathway as well as its effect on hepatic IRI (Fig. 7).
We first detected the protective function of TGR5 on hepatic tissues with warm IRI. The activation of TGR5 was raised by IR and reached the peak at 6 h after reperfusion. TGR5 knockdown obviously worsened hepatic IR damage, whereas TGR5 overexpression by its special agonist INT-777 mitigated liver damage in WT mice. HE staining of liver tissues and the level of serum ALT and AST showed the protective role of TGR5. TGR5 was further detected by immunohistochemistry, and the results proved that the number of positive cells was higher in the IR6 + INT-777 group compared with the sham groups and IR6 + si-TGR5 group. As one of the modalities in cell death, necrosis and apoptosis are extremely significant during hepatic IRI. Compared with the IR6 + si-TGR5 group, the number of the apoptotic cells measured by TUNEL assay was less in the IR6 + INT-777 group. Moreover, we detected apoptosis-related protein (Bax, Bcl-2, cleaved casepase-3, and cleaved caspase-9) to further confirm the protective function of TGR5 by western blot assay. Inflammatory response plays a significant role in IR-induced hepatic injury, especially innate immune responses, including many cytokines, like IL-6, IL-10, and TNF-α [21]. In vivo we tested the RNA level and serum level of IL-6, IL-10, and TNF-α using qRT-PCR and ELISA assay. Our data revealed that upregulation of TGR5 could reduce the expression of IL-6 and TNF-α, but enhance the expression of IL-10. To further verify the relation between TGR5 and the Keap1-Nrf2 signaling pathway, we used immunohistochemistry to study nuclear Nrf2 performance. Compared with the sham groups and the IR6 + si-TGR5 group, nuclear Nrf2 level was higher in the IR6 + INT-777 group. Furthermore, Keap1, nuclear Nrf2, and Ho-1 expression were detected in IR hepatic tissues by qRT-PCR and western blot assay. The results proved that activation of TGR5 by INT-777 increased nuclear Nrf2 and Ho-1 expression, but decreased Keap1 expression. The knockdown of TGR5 by si-TGR5 could completely reverse expression of Keap1, Nuclear Nrf2, and Ho-1. These data demonstrated that TGR5 significantly inhibit IR-caused hepatic damage by promoting the generation of Nrf2 and the translocation of Nrf2 to nucleus in IR livers.
Secondly, we established a BMDM H/R model in vitro to further detect the function of TGR5 in macrophage inflammatory response. We used qRT-PCR and western blot to detect whether small interfering RNA of TGR5 had been transfected successfully. The activation of TGR5 in other groups was also tested by the same methods. To further analyze the role of TGR5 in the regulation of macrophage inflammatory activation in vitro, we used qRT-PCR and ELISA assay to detect the RNA level and serum level of IL-6, IL-10, and TNF-α. Consistent with the results in vivo, activation of TGR5 could reduce the expression of IL-6 and TNF-α, but enhance the expression of IL-10. As the result in vivo showed that upregulation of TGR5 could inhibit inflammatory response through the Keap1-Nrf2 signaling pathway in IR liver. To further study the role of TGR5 in macrophage inflammatory response, we used qRT-PCR and western blot assay to test the RNA level and protein level of Keap1, nuclear Nrf2, and Ho-1 in BMDM H/R model. Our data revealed that compared with the control groups and H/R + si-TGR5 group, activation of TGR5 by INT-777 increased nuclear Nrf2 and Ho-1 expression, but decreased Keap1 expression; however, downregulation of TGR5 by si-TGR5 could completely reverse it. Moreover, the immunofluorescence analysis of Keap1 and nuclear Nrf2 further proved that the Keap1-Nrf2 signaling pathway might serve a role in the protective effects of TGR5 against inflammatory response. Although, we have not found out whether TGR5 directly target Keap1 or Nrf2 yet. But interestingly, under normal conditions, Keap1 combines with CUL3-RBX1-E2 complex to ubiquitinates Nrf2 and promotes Nrf2 degradation. In the state of oxidative stress, cells express Sestrin2 protein in large quantities and cooperate with selective autophagy adaptor protein SQSTM1 and protein kinase ULK1 to promote the dissociation of Nrf2 and Keap1 and transfer Nrf2 to the nucleus, thus playing an antioxidant role. We hypothesized that TGR5 may regulate the Keap1-Nrf2 signaling pathway by targeting Sestrin2 expression.
In conclusion, our findings have demonstrated that activation of the Keap1-Nrf2 signaling pathway is important for TGR5 protection against hepatic IR-stressed injury. TGR5 could not only reduce hepatocellular apoptosis but also inhibit inflammatory response through regulating Keap1-Nrf2 signaling pathways. These findings might provide a novel and potential therapeutic approach to prevent inflammatory response and hepatic I/R injury. Thus, targeting TGR5 molecules could benefit liver surgery outcomes.
References
Pols, T.W., M. Nomura, T. Harach, et al. 2011. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metabolism 14: 747–757.
Rao, J., J. Qin, X. Qian, L. Lu, P. Wang, Z. Wu, Y. Zhai, F. Zhang, G. Li, and X. Wang. 2013. Lipopolysaccharide preconditioning protects hepatocytes from ischemia/reperfusion injury (IRI) through inhibiting ATF4-CHOP pathway in mice. PLoS One 8: e65568.
Chen, F., Y.M. Zhang, J.T. Wang, J. Wang, Z.L. Cui, and Z.R. Liu. 2019. Pre-treatment with FK506 reduces hepatic ischemia-reperfusion injury in rats. Clinics and Research in Hepatology and Gastroenterology 43: 161–170.
Zhai, Y., H. Petrowsky, J.C. Hong, R.W. Busuttil, and J.W. Kupiec-Weglinski. 2013. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nature Reviews. Gastroenterology & Hepatology 10: 79–89.
Lee, L.Y., C. Harberg, K.A. Matkowskyj, S. Cook, D. Roenneburg, S. Werner, J. Johnson, and D.P. Foley. 2016. Overactivation of the nuclear factor (erythroid-derived 2)-like 2-antioxidant response element pathway in hepatocytes decreases hepatic ischemia/reperfusion injury in mice. Liver Transplantation 22: 91–102.
Kaczorowski, D.J., A. Tsung, and T.R. Billiar. 2009. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 1: 91–98.
Qiao, Y.L., J.M. Qian, F.R. Wang, Z.Y. Ma, and Q.W. Wang. 2014. Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. The Journal of Surgical Research 187: 653–659.
Pols, T.W., L.G. Noriega, M. Nomura, J. Auwerx, and K. Schoonjans. 2011. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. Journal of Hepatology 54: 1263–1272.
Deutschmann, K., M. Reich, C. Klindt, C. Dröge, L. Spomer, D. Häussinger, and V. Keitel. 2018. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochimica et Biophysica Acta - Molecular Basis of Disease 1864: 1319–1325.
Wang, Y.D., W.D. Chen, D. Yu, B.M. Forman, and W. Huang. 2011. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology 54: 1421–1432.
Guo, C., H. Qi, Y. Yu, et al. 2015. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) inhibits gastric inflammation through antagonizing NF-kappaB signaling pathway. Frontiers in Pharmacology 6: 287.
de Oliveira, M.C., E.H. Gilglioni, B.A. de Boer, J.H. Runge, D.R. de Waart, C.L. Salgueiro, E.L. Ishii-Iwamoto, R.P.J. Oude Elferink, and I.C. Gaemers. 2016. Bile acid receptor agonists INT747 and INT777 decrease oestrogen deficiency-related postmenopausal obesity and hepatic steatosis in mice. Biochimica et Biophysica Acta 1862: 2054–2062.
Yang, H., H. Zhou, L. Zhuang, J. Auwerx, K. Schoonjans, X. Wang, C. Feng, and L. Lu. 2017. Plasma membrane-bound G protein-coupled bile acid receptor attenuates liver ischemia/reperfusion injury via the inhibition of toll-like receptor 4 signaling in mice. Liver Transplantation 23: 63–74.
Zhuang, L., Y. Fan, L. Lu, W. Ding, C. Ni, X. Wang, F. Zhang, and J. Rao. 2016. Ischemic preconditioning protects hepatocytes from ischemia-reperfusion injury via TGR5-mediated anti-apoptosis. Biochemical and Biophysical Research Communications 473: 966–972.
Makri, E., E. Cholongitas, and K. Tziomalos. 2016. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World Journal of Gastroenterology 22: 9039–9043.
Guo, C., J. Su, Z. Li, R. Xiao, J. Wen, Y. Li, M. Zhang, X. Zhang, D. Yu, W. Huang, W.D. Chen, and Y.D. Wang. 2015. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell proliferation and migration through antagonizing STAT3 signaling pathway. Oncotarget 6: 34402–34413.
Casaburi, I., P. Avena, M. Lanzino, D. Sisci, F. Giordano, P. Maris, S. Catalano, C. Morelli, and S. Andò. 2012. Chenodeoxycholic acid through a TGR5-dependent CREB signaling activation enhances cyclin D1 expression and promotes human endometrial cancer cell proliferation. Cell Cycle 11: 2699–2710.
Asgharpour, A., D. Kumar, and A. Sanyal. 2015. Bile acids: emerging role in management of liver diseases. Hepatology International 9: 527–533.
Kudoh, K., H. Uchinami, M. Yoshioka, E. Seki, and Y. Yamamoto. 2014. Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Annals of Surgery 260: 118–127.
Wang, W., C. Guan, X. Sun, Z. Zhao, J. Li, X. Fu, Y. Qiu, M. Huang, J. Jin, and Z. Huang. 2016. Tanshinone IIA protects against acetaminophen-induced hepatotoxicity via activating the Nrf2 pathway. Phytomedicine 23: 589–596.
Rao, J., X. Qian, G. Li, X. Pan, C. Zhang, F. Zhang, Y. Zhai, X. Wang, and L. Lu. 2015. ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. American Journal of Transplantation 15: 76–87.
Funding
This work was supported by the medical research guidance project of Jiangsu Provincial Health Committee (No: Z2019027), major science and technology projects of Changzhou City (No: ZD201719), Clinical Medical Science and Technology Development Foundation of Jiangsu University (No: JLY20180084), science and technology development projects of Wujin District (No: WS201708 WS201808 WS201611), and the project funding from Young Talent Development Plan of Changzhou Health Commission (CZQM2020120).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhuang, L., Ding, W., Zhang, Q. et al. TGR5 Attenuated Liver Ischemia-Reperfusion Injury by Activating the Keap1-Nrf2 Signaling Pathway in Mice. Inflammation 44, 859–872 (2021). https://doi.org/10.1007/s10753-020-01382-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01382-y