Abstract
Endothelial function is commonly determined via the ultrasound-based flow-mediated dilation (FMD) technique which assesses arterial dilation in response to a hyperemia response following distal cuff occlusion. However, the low-flow-mediated constriction (L-FMC) response during cuff-induced ischemia is often overlooked. L-FMC provides unique information regarding endothelial function, but vascular researchers may be unclear on what this metric adds. Therefore, the objective of this review was to examine the mechanistic determinants and participant-level factors of L-FMC. Existing mechanistic studies have demonstrated that vasoreactivity to low flow may be mediated via non-nitric oxide vasodilators (i.e., endothelial hyperpolarizing factors and/or prostaglandins), inflammatory markers, and enhancement of vasoconstriction via endothelin-1. In general, participant-level factors such as aging and presence of cardiovascular conditions generally are associated with attenuated L-FMC responses. However, the influence of sex on L-FMC is unclear with divergent results between L-FMC in upper versus lower limb vessels. The ability of aerobic exercise to augment L-FMC (i.e., make more negative) is well supported, but there is a major gap in the literature concerning the mechanistic underpinnings of this observation. This review summarizes that while larger L-FMC responses are generally healthy, the impact of interventions to augment/attenuate L-FMC has not included mechanistic measures that would provide insight into non-nitric oxide-based endothelial function. Clarifications to terminology and areas of further inquiry as it relates to the specific pharmacological, individual-level factors, and lifestyle behaviors that impact L-FMC are highlighted. A greater integration of mechanistic work alongside applied lifestyle interventions is required to better understand endothelial cell function to reductions in local blood flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of adverse cardiovascular conditions is preceded by dysfunction of the vascular endothelium which is responsible for the release of vasoactive substances that promote vasoconstriction and vasodilation in response to chemical or physical stimuli (Konukoglu and Uzun 2017). Endothelial function of the conduit arteries can be determined via the flow-mediated dilation (FMD) technique (Thijssen et al. 2019), whereby the vasodilation in response to a brief reactive hyperemia following the release of a cuff-induced period of ischemia is calculated. This ultrasound-based determination of endothelial function has been widely adopted and generally reflects the ability of endothelial cells to release nitric oxide (NO) in response to influxes of blood flow and the corresponding shear stress stimuli (Green et al. 2014). During the distal cuff-induced ischemia, a conduit artery constrictor response is typically observed, referred to as low-flow-mediated constriction (L-FMC) (Gori et al. 2008) (Fig. 1). A greater constrictor response is generally healthier, whereas an attenuated L-FMC response may be less favorable. Despite FMD guidelines encouraging the vessel of interest to be imaged throughout the entire technique (Thijssen et al. 2019), considerably less is known about L-FMC. However, the notion of assessing the vasoreactivity to changes in local shear stress is analogous to the FMD technique, but to a reduction- rather than an influx of shear stress.
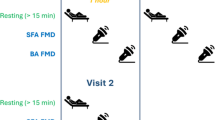
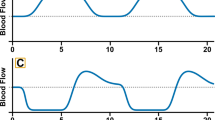
Illustration of how flow-mediated dilation (FMD) and low-flow-mediated constriction (L-FMC) are derived. A The FMD and L-FMC tests are ultrasound-derived where the probe is connected to a high-resolution ultrasonography machine that provides images of the artery of interest. A pressure cuff is placed distally to the probe (blue). B After a 2-min baseline, the pressure cuff is inflated to suprasystolic levels to induce the low-flow phase. Following 5 min, the cuff is deflated to elicit a reactive hyperemia. L-FMC can be calculated as percentage change in diameter from baseline during the last 30 s the low-flow phase. Peak constriction may be derived by calculating the average diameter during the last 30 s of cuff occlusion or based on the nadir diameter from ~ 3 s averages. The current figure represents L-FMC derived from the nadir diameter. FMD is characterized as a percent increase in diameter above the baseline following cuff deflation
Although observations that the brachial artery constricts in response to cuff-induced ischemia have been established in the late 1980s (Anderson and Mark 1989), L-FMC was termed in 2008, following the seminal study by Gori et al. who examined the mechanisms of radial L-FMC and interestingly suggested L-FMC may reflect resting endothelial function (Gori et al. 2008). Since then, the number of publications investigating L-FMC has grown. L-FMC may provide unique and complementary clinically relevant information compared to FMD alone (Gori et al. 2012). Specifically, while L-FMC is associated with and provides insight into the magnitude of FMD response in a healthy and clinical population (Aizawa et al. 2016), it may also be an indicator of the production of vasoactive substances that are not dominant in the FMD response (e.g., prostaglandins and endothelial-derived hyperpolarizing factors) and increases the predictive ability of FMD to detect endothelial dysfunction, such as that in a diseased population (e.g., coronary artery disease) (Gori et al. 2012). While reviews on the mechanistic determinants and participant-level factors (e.g., age (Seals et al. 2011), sex (Lew et al. 2022), and exercise (Ramos et al. 2015)) on FMD have been conducted, the impact of such factors on L-FMC are unclear. A vague understanding of what information is gained by this metric is likely a primary barrier preventing cardiovascular and specifically FMD researchers from integrating L-FMC as part of their ultrasound assessments.
The objective of this narrative review was to examine the mechanistic and participant-level factors that impact L-FMC and emphasize the interpretation of it as a complimentary measure to FMD. Areas of further mechanistic study, considerations for what information is gained by this metric, and our knowledge of exercise/movement as determinants of L-FMC are highlighted.
Mechanisms of low-flow-mediated constriction
The FMD response following cuff deflation has been shown to be primarily nitric oxide (NO) mediated, as evident by an attenuated response following the infusion of NG-monomethyl-l-arginine (L-NMMA) (Green et al. 2014). L-NMMA is an endothelial nitric oxide synthase (eNOS) inhibitor, whereby eNOS mediates the conversion of L-arginine to NO and L-citrulline. However, unlike FMD, the infusion of L-NMMA to block NO production did not alter L-FMC responses in the radial artery (Gori et al. 2008), indicating that vasoreactivity to low flow is not dictated by the potent vasodilator. Subsequent work examining the impact of trans-radial catheterization and thus endothelial denudation, observed attenuated L-FMC responses in the catheterized radial artery (− 2.1 ± 0.8 to 0.4 ± 0.8%), but a preserved L-FMC response in the non-intervention radial artery (Dawson et al. 2012). Therefore, despite not being NO-mediated, the L-FMC response in the radial artery is endothelial-dependent. Alternative vasoactive chemicals that may dictate the magnitude of the L-FMC response are discussed.
Endothelial-derived hyperpolarizing factors
Endothelial-derived hyperpolarizing factors (EDHFs) are vasoactive substances [epoxyeicosatrienoic acid (EET), hydrogen peroxide, and potassium ions (K+)] (Ozkor and Quyyumi 2011). EETs are the primary EDHF and largely involved in endothelial-derived hyperpolarization that results in an activation of transmembrane K+ channels on vascular smooth muscle cells and blunts contraction. Specifically, acetylcholine, bradykinin, and shear stress increase endothelial intracellular Ca2+ concentrations. Ca2+ activates cytosolic phospholipase A2, which releases arachidonic acid. EETs are created from oxygenated arachidonic acid via a cytochrome P450-2C9 epoxygenase pathway.
To understand whether endothelial-released EDHFs are involved in the L-FMC response, L-FMC tests were conducted following the oral administration of 150 mg fluconazole, which blocks the cytochrome P450-2C9 epoxygenase pathway. In both the radial and popliteal arteries, fluconazole blunted L-FMC responses (Gori et al. 2008; Petterson et al. 2021), supporting that the L-FMC response is mediated via EDHFs.
Similar to EET, a cytochrome P450 pathway (cytochrome P450 4A3 hydrolase) and arachidonic acid may create hydroxyeicosatetraenoic acid, which is a potent vasoconstrictor (Ozkor and Quyyumi 2011). Fluconazole is primarily a CYP epoxygenase inhibitor, but also may inhibit other CTY isoforms (e.g., cytochrome P450-4A3 hydrolase) (Ozkor and Quyyumi 2011). The specific mechanisms regarding how EDHFs are involved in the L-FMC response are not fully understood, but it is hypothesized that the L-FMC response involves CYP pathways. Specifically, the potential disruption of competing CYP isoforms (CYP2C9 vs CYP4A3) may cause vasodilation versus vasoconstriction. More targeted EDHF inhibitors are needed to better understand the role of EDHFs in mediating L-FMC responses (Ozkor and Quyyumi 2011). For example, unlike fluconazole, Sulfaphenazole is a target of CYP2CP specifically and may serve a useful role in uncovering the divergent effects of these cytochrome P450 pathways on L-FMC. Understanding the specific EDHF pathways involved in mediating the vasoreactivity to low flow may lead to more targeted pharmacological interventions in studying and improving endothelial cell function.
Prostaglandins
The endothelium also releases prostaglandins, which may be a critical modulator of vascular tone (Yi et al. 2000). Similar to EDHFs, arachidonic acid is the most common precursor of prostaglandins, and is released from the cell membrane phospholipids, primarily by phospholipase A2 (Moncada and Vane 1978). Prostaglandins and thromboxane A2 are formed when arachidonic acid is metabolized by prostaglandin G/H synthase or cyclooxygenase (COX1 and COX2 isoforms) (Félétou et al. 2011). COX1 and COX2 isoforms are the common substrates for multiple unique prostaglandin isoforms (Ricciotti and FitzGerald 2011). COX2 may produce PGI2 in response to increases in local shear stress (Koller et al. 1993), which activates adenylyl cyclase via a stimulatory G-protein that increases cyclic adenosine monophosphate (Ricciotti and FitzGerald 2011) and causes vascular smooth muscle cell relaxation.
Unlike EDHFs, whether prostaglandins influence the L-FMC response is less clear. Specifically, a 500 mg oral administration of aspirin, an inhibitor of cyclooxygenase products (e.g., prostaglandins), impaired the L-FMC response in the radial (Gori et al. 2008), but not the brachial or popliteal arteries (Petterson et al. 2021). Furthermore, a separate study that administered 1200 mg of ibuprofen (prostaglandin inhibitor) did not alter L-FMC responses in the brachial artery (Carter et al. 2014). While both aspirin and ibuprofen (and any non-steroidal anti-inflammatory drugs) inhibit COX, aspirin has an irreversible (versus a reversible with ibuprofen) antiplatelet effect, thinning blood to a greater extent than ibuprofen. The impact on platelets is unlikely to explain the differential observations given that a three-arm trial of antiplatelet agents of different potencies did not alter radial L-FMC acutely or after 28 days of repeated dosage in patients with unstable angina or requiring coronary intervention (Schnorbus et al. 2020). Of note, these studies are limited by the use of either aspirin or ibuprofen as they are non-selective inhibitors making it unclear whether they are affecting vasodilatory or vasoconstrictor prostaglandins. However, reduced production of specifically PGI2 and PGE2 may attenuate L-FMC, as these prostaglandins are responsible for vascular smooth muscle cell relaxation. It is unclear whether L-FMC is mediated via the inhibition of prostaglandins-vasodilatory pathway, or if it is dependent upon the artery of interest (radial versus brachial or popliteal). Large-scale studies that are sufficiently powered to simultaneously compare prostaglandin inhibitors in multiple vascular beds are needed to better define this mechanistic pathway.
Endothelin-1
In addition to local vasodilators (NO, EDHFs, prostaglandins), the endothelium also regulates local vascular tone by releasing the potent vasoconstrictor, endothelin-1. Endothelin-1 is a short (21-amino acid) peptide released continuously by endothelial cells and vascular smooth muscle cells (Böhm and Pernow 2007). In response to increases in laminar shear stress, preproendothelin-1 messenger RNA is transiently upregulated in a dose-dependent manner. Preproendothelin-1 (212 amino acids) undergoes proteolytic cleavage to form big endothelin-1 (39 amino acids) (Davenport et al. 2016). At the endothelial cell membrane, endothelin converting enzyme converts big endothelin-1 to endothelin-1 in the myoendothelial space. The biological effects of endothelin-1 are mediated through the activation of ETA and ETB receptor subtypes, with ETB assisting in the clearance of endothelin-1 from the vasculature and stimulating eNOS enzyme activity and NO formation (Rubanyi and Polokoff 1994). ETB receptors are located on both the endothelium and smooth muscle. Conversely, binding to the more dominant ETA receptor on vascular smooth muscle stimulates the formation of inositol triphosphate from phosphatidylinositol biphosphate by phospholipase C via a Gq-protein (Bourque et al. 2011; Davenport et al. 2016). Increased inositol triphosphate within the vascular smooth muscle stimulates Ca2+ release from the sarcoplasmic reticulum causing vasoconstriction.
In the radial artery, the contribution of endothelin-1 to the L-FMC response has been investigated by conducting the occlusion-hyperemia test following the intra-arterial infusion of BQ-123 (vs. saline) (Spieker et al. 2003). BQ-123 is a selective ETA endothelin receptor antagonist. In this sample of healthy young adults, ETA inhibition attenuated radial L-FMC (-6.8% to -2.7%), but did not influence the FMD response, indicating that the L-FMC response is largely endothelin-1 mediated (Spieker et al. 2003).
Tumor necrosis factor-α
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine that mediates inflammatory, proliferative, and cytotoxic effects in endothelial cells and vascular smooth muscle cells. TNF-α may promote endothelial dysfunction via inhibiting eNOS, CYP450, and enhancing the removal of NO through the increase in NADPH-dependent O2- production, creating peroxynitrite (ONOO–) (Greenberg et al. 1993; Gao et al. 2007; Zhang et al. 2009). Providing a neutralizing antibody to TNF-α reduces the formation of reactive oxygen species (e.g., O2–, ONOO–, and H2O2) and increases NO-mediated dilation (Zhang et al. 2009).
The only study to investigate the impact of inflammation on L-FMC, administered 8–12 weeks of TNF-α inhibitors [adalimumab (40 mg once/2 week) or etanercept (50 mg once/week] in patients with moderate–severe psoriasis. After the anti-inflammatory intervention, brachial L-FMC was augmented and brachial FMD was unaffected (Wegner et al. 2022). Despite the inability to discern the impact of repeated measures variability or timeline on L-FMC, as there was not a concurrent control group, Wegner et al. provided support that brachial L-FMC may be favorably improved through repeated anti-inflammatory treatment in patients with psoriasis (Wegner et al. 2022). The actions of TNF-α on the endothelium are on NO production, reactive oxygen species, and inhibiting CYP450. Since L-FMC is not NO-mediated, the impact of TNF-α inhibition on L-FMC may be specifically due to improved CYP450 pathway and thus EDHF production, which has been consistently implicated in the L-FMC response (Gori et al. 2008; Petterson et al. 2021). Conversely, prior work demonstrating that L-FMC was not NO-mediated was conducted in the radial artery of healthy young adults (Gori et al. 2008). Therefore, the contribution of vasoactive chemicals to the L-FMC response may vary by artery and/or disease status. Further work is needed to understand the impact of inflammation on measures of endothelial vasomotor function in multiple arteries in healthy adults and those with varying disease conditions.
Summary
Existing work has investigated the endothelial-based mechanisms governing the L-FMC response primarily in healthy persons and in the radial artery (Table 1). While endothelial-dependent, L-FMC responses may be mediated through the inhibition of EDHF signaling(Gori et al. 2008; Petterson et al. 2021) and/or prostaglandin pathways (Gori et al. 2008), as well as enhanced vasoconstrictor signaling via endothelin-1 (Spieker et al. 2003). In addition, longitudinal anti-inflammatory, TNF-α inhibition augmented L-FMC (Wegner et al. 2022). Therefore, the vasoreactivity to low flow may be determined by the inhibition of non-NO vasodilators (EDHF/prostaglandins), endothelin-1 signaling, and the inflammatory state of the endothelium. However, to comprehensively understand the L-FMC response future work must be conducted to understand the combine effect of these mechanism (i.e., using multiple concurrent inhibitors) and their influence in varying adult populations (i.e., age, sex, and disease status).
Resting versus recruitable endothelial function
The interpretation of what information is gained by examining the L-FMC response is generally heterogeneous across researchers. It has been positioned by some that L-FMC and FMD represent resting and recruitable endothelial function, respectively (Gori et al. 2010, 2012, 2017). The position that L-FMC reflects resting endothelial-dependent vascular tone may be attributed to the observation that inhibiting vasodilators (e.g., EDHFs or prostaglandins) resulted in blunted vasoconstrictor responses. The notion that inhibiting vasodilator production results in an attenuated constriction may be peculiar on the surface. However, the administration of inhibitors ~ 60 min before beginning the FMD protocol lowers EDHF or prostaglandin production, including during the baseline portion of the occlusion-hyperemia test. Therefore, there may fewer vasodilators to inhibit or ability to further constrict from baseline. Then, in response to low flow, there is a lesser capacity for further constriction (i.e., floor effect). We provide an argument discussing some of the pitfalls of describing L-FMC was a measure of resting endothelial function or tone.
Endothelial tone is primarily regulated by the potent vasodilator, NO (Loscalzo and Jin 2010). Given that the L-FMC response has been shown to be unaffected by blocking the NO precursor (eNOS) (Gori et al. 2008), it may be a misnomer to describe L-FMC as a measure of endothelial tone if one of the main vasoactive substances is not reflected by this assessment. Second, the notion that baseline vasoactive substances influences L-FMC also applies to the FMD test (positioned as recruitable function) in that an artery that is in a more dilated resting state is likely to exhibit a smaller FMD response. This is typically observed following acute exercise, whereby a reduction in FMD is observed when accompanied by an exercise-induced increase in resting diameter (Dawson et al. 2013). Third, the vasoconstrictor response is a result of a reduction in local shear stress and not to a resting state, as implied by the L-FMC name. Without a hypoemia stimulus (e.g., distal cuff inflation), there is not a concomitant vasoconstriction. Such ideas challenge the terminology of how L-FMC is described and suggests that L-FMC might be optimally described as endothelial-dependent vasoconstrictor function rather than as a measure of resting endothelial function.
Related to L-FMC terminology, the description implies that vasoconstrictor responses are always observed in responses to low flow. However, there is considerable variability in whether arteries dilate, are unchanged, or constrict to reductions in local flow (Gori et al. 2008; Weissgerber et al. 2010; O’Brien et al. 2021b). For example, specific to the brachial artery, evidence consistently suggests that upwards of 60% of participants demonstrate a constrictor response during cuff inflation, while ~ 40% vasodilate or do not change (Harrison et al. 2011; Spiro et al. 2011; Aizawa et al. 2016; Shenouda et al. 2020). However, the radial artery exhibits an L-FMC response notably more consistent than the brachial artery (Weissgerber et al. 2010). Nevertheless, factors that are established to be associated with better cardiovascular function (e.g., younger age and no chronic diseases) are typically associated with an augmented L-FMC response. While an FMD is typically observed in response to hyperemia, some observe very modest increases or no changes in diameter (Holder et al. 2021). Accordingly, while not all people exhibit an L-FMC or an FMD response, the name of the assessment is directed towards the anticipated generally healthy response. Aizawa et al. (2016) observed a large proportion of participants exhibited a vasodilatory response during cuff occlusion (~ 40%), they observed that increased vasoconstriction was associated with a smaller subsequent FMD response in the brachial artery (Aizawa et al. 2016). Although the opposite L-FMC to FMD relationship has been observed in the popliteal artery (O’Brien et al. 2021b). It should be recognized that the positions presented herein on the terminology are not static and that with emerging evidence as to the importance of L-FMC and the specific information gained in various populations and between vessel beds, the terminology and best description of the technique may be refined.
Between-artery considerations
There are known structural and functional differences between differing conduit arteries, as it relates to the L-FMC response. Known heterogeneous responses exist between the brachial and lower limb vessels, such as the popliteal artery (Thijssen et al. 2011). Specifically, the size of upper limb arteries are inversely correlated to the FMD response, but the same was not true for the superficial and common femoral artery (Thijssen et al. 2011). Such disparities in responses may be due to artery diameter being inversely related to FMD, with larger arteries dilating less than smaller arteries (e.g., brachial or radial) (Thijssen et al. 2008). Specifically, Thijssen et al. (2008) compared FMD in the brachial, radial, common femoral, superficial femoral and popliteal arteries in young adults and observed that resting arterial diameter was inversely related to the peak FMD responses (Thijssen et al. 2008). Lower limb arteries are more susceptible to the development of atherosclerosis and peripheral vascular disease (Debasso et al. 2004). Despite this, there is evidence that FMD responses in the radial, brachial, and superficial femoral arteries are NO-mediated, as reviewed in (Green et al. 2014). The limb-specific differences in FMD may be attributed to the increased hydrostatic pressure in the lower limb due (Newcomer et al. 2004). With that, the lower limb arteries supply a greater muscle mass and experience sustained increases in blood flow during locomotion. Conversely, the popliteal artery experiences a greater reduction in local blood flow than upper limb vessels during sedentary postures.
While some evidence exists supporting that divergent FMD responses exist between the brachial and popliteal arteries (Nishiyama et al. 2008; Thijssen et al. 2011), brachial vasoconstrictor responsiveness is also greater than that of the popliteal artery, which is exposed to larger shear stress fluctuations during bouts of sedentary behavior and traditional lower limb modes of exercise. Importantly, most mechanistic insights into the L-FMC response have been conducted in the radial or brachial arteries (Spieker et al. 2003; Gori et al. 2008; Carter et al. 2014). Furthermore, another key difference between upper and lower limb vessels may related to the impact of artery structure and its impact of vasoactive responses. It is well established that regional heterogeneity exists between arteries in terms of wall architecture and thickness (Tinken et al. 2008). Specifically, subjects with enlarged wall-to-lumen ratios exhibit generalized increased vasoactive responses. Therefore, smaller arteries, such as those found in the upper limbs possess more smooth muscle relative to elastic laminae and have enlarged wall-to-lumen ratios facilitating their hyperresponsiveness (Thijssen et al. 2008).
Participant factors on L-FMC
The local endothelial-based mechanisms discussed above contribute to the magnitude of the L-FMC response, but such mechanisms are impacted by participant-level factors. Compared to FMD, L-FMC is relatively understudied, but the limited work has begun to advance our understanding of participant characteristics and lifestyle behaviors on L-FMC. We briefly highlight important participant-level characteristics (e.g., age and sex) factors on L-FMC and emphasize areas that warrant further investigation.
Impact of age
Aging is associated with several unfavorable vascular adaptations that perpetuate endothelial dysfunction, as characterized by attenuated endothelial-dependent and endothelial-independent dilation (Black et al. 2009). In a large sample of 584 patients (67 ± 11 years), age was an independent positive predictor of radial L-FMC in multivariate analysis that considered sex, smoking, coronary artery disease and diabetes (Gori et al. 2017). In healthy participants matched for aerobic fitness, age, and sex-specific percentiles, popliteal artery L-FMC was also attenuated with age in both males and females (O’Brien et al. 2021a). This attenuated L-FMC response with age occurred despite aging being associated with a greater baseline lumen diameter (O’Brien et al. 2021a). This is relevant as a larger conduit artery diameter may have a greater capacity to constrict, with baseline diameter being shown to be inversely associated with the magnitude of L-FMC in the brachial and radial arteries of young adults (Sen et al. 2020). This further substantiates the need for mechanistic and longitudinal research to be conducted in younger and older adults to provide insight into the this observation that aging, even in the absence of chronic disease, impairs L-FMC.
Impact of sex
The impact of biological sex on vascular function has been well studied in the FMD literature, with mixed results as to whether females exhibit augmented or similar FMD responses to males (Holder et al. 2019, 2021; O’Brien et al. 2019b; Johns et al. 2020). In young adults, popliteal L-FMC has been demonstrated to be similar between males and females matched for habitual physical activity levels (O’Brien et al. 2019b) or age/sex-specific aerobic fitness percentiles (O’Brien et al. 2021a). Similarly, in a larger sample size of popliteal L-FMC (n = 110), sex was not a predictor of L-FMC in univariate or multivariate controlled analyses (O’Brien et al. 2021b). Conversely, in a large sample of radial L-FMC (n = 584), females exhibited an augmented L-FMC response compared to males (Gori et al. 2017). While early work failed to initially observe brachial L-FMC differences between males and females, normalizing L-FMC to the decrease in shear rate resulted in a more pronounced normalized L-FMC among females (Levenson et al. 2001). Therefore, it is hypothesized that a greater consideration of the shear rate changes during the cuff occlusion on the L-FMC response may reveal sex differences. Of note, while normalizing FMD to shear rate area under the curve (i.e., the stimulus for the FMD response) is still a controversial practice that is not recommended unless statistical assumptions are met (Atkinson et al. 2009), the applicability of normalizing L-FMC to its shear rate stimulus during the cuff-inflation period should be further investigated. Specifically, it may be possible that the assumptions required to normalize FMD to SR (i.e., correlation between the shear rate stimulus and the FMD response is statistically significant, and the 95% confidence intervals of the y-intercept encompass zero) may be extrapolated to L-FMC and provide a more accurate interpretation of the metric, but this warrants further study.
Furthermore, sex differences may be limb-dependent with some work demonstrating smaller L-FMC responses in the popliteal versus brachial arteries (O’Brien et al. 2019a). How sex and the associated differences between males and females (e.g., hormonal, genetic, etc.) impact L-FMC is unclear. Notably, when females are tested throughout their menstrual cycle may impact results (Williams et al. 2001), but previous work in the popliteal artery was conducted when hormones were lowest to control for this phenomenon (O’Brien et al. 2019b, 2021a, b). An inclusion of these methodological factors that account for the shear rate stimulus are, therefore, needed to advance our understanding on sex differences in L-FMC.
Impact of chronic disease
Impaired endothelial function is typically a preceding characteristic to the development of cardiovascular diseases (Drexler and Hornig 1996; Paterick and Fletcher 2001). Compared to young adults or healthy age-matched controls, attenuated radial L-FMC responses are observed in patients with coronary artery disease, congestive heart failure, and hypertension (Gori et al. 2010). In multivariable analyses, coronary artery disease, but not diabetes mellitus, was predictive of attenuated radial L-FMC (Gori et al. 2017). Similarly, radial L-FMC is further attenuated among multi-vessel versus single vessel coronary artery disease (Gori et al. 2012). In the brachial artery, coronary artery disease patients and moderate-severe psoriasis patients exhibited smaller L-FMC responses than healthy young adults (Wegner et al. 2022). In contrast, Spiro et al. observed that brachial artery L-FMC were attenuated in patients with unstable vs. stable (larger L-FMC) coronary atherosclerosis and smaller following percutaneous coronary intervention (Spiro et al. 2011). While Gori et al. frequently observed attenuated L-FMC responses in the radial artery of cardiovascular compromised patients (Gori et al. 2008, 2011), observations in the brachial artery by Spiro et al. (Spiro et al. 2011) are inconsistent. An obvious rationale for the divergent results is not clear. Certainly, a better understanding as to how much a larger/smaller L-FMC response represents an (un)healthy response, its associated mechanistic underpinnings, and clinical relevance is needed.
Summary
Although limited research has been conducted on L-FMC responses relative to FMD responses, existing work has demonstrated that healthy aging and presence of cardiovascular conditions generally are associated with attenuated L-FMC responses. Whether sex differences in L-FMC exist may be limb-dependent, where future research should address that observations in the lower limb do not necessarily reflect the more commonly assessed upper limb. Nevertheless, participant-level factors generally influence the magnitude of L-FMC.
Exercise and movement factors and L-FMC
The cardiovascular benefits of regular exercise are well established, with numerous reviews supporting the efficacy of exercise to improve FMD (Ashor et al. 2015; Ramos et al. 2015; Early et al. 2017). As outlined in a recent review of the topic, higher aerobic fitness is associated with- and engaging in aerobic exercise training augments L-FMC responses (O’Brien et al. 2022). Such results were observed across the radial, brachial, and popliteal arteries. However, conflicting evidence exists as to whether resistance training or prolonged sitting impacts L-FMC. Accordingly, such information suggests that exercise type may result in a differential impact of endothelial-dependent vasoconstriction, with aerobic exercise specifically augmenting this response.
Among aerobic exercise studies, cross-sectional work documented an inverse relationship between aerobic fitness and brachial L-FMC in young males (Bell et al. 2017) and older adults (O’Brien et al. 2019c), but a stronger relationship with popliteal L-FMC in older adults (O’Brien et al. 2019a). Given that traditional forms of aerobic exercise involve the lower limb, it is unsurprising that lower limb arteries are more related with aerobic fitness. Acutely, 30 min of cycling augmented radial L-FMC in young males (Elliott et al. 2018). The intervention by Van Craenenbroeck et al. did not observe changes in L-FMC but consisted of at home cycling in patients with stage 3 or 4 chronic kidney disease (Van Craenenbroeck et al. 2015). It is plausible that a lack of supervision of participants or health status of the participants are responsible for the divergent results with other work. Conversely, a 6-week training intervention conducted by Rakobowchuk et al. (2012) observed increased L-FMC (i.e., more constriction) following both moderate and vigorous intensity interval training in healthy adults (Rakobowchuk et al. 2012). While the clinical or prognostic value of L-FMC has yet to be established, the notion of examining vasoreactivity to changes in local blood flow follows a similar line of thinking as the FMD technique but under divergent mechanisms. Given the higher aerobic fitness and engagement of exercise training are associated with better cardiovascular function, indirectly, this is suggestive of an augmented vasoconstrictor response to low-flow being a healthier response. Of relevance, most work relating movement and L-FMC has been conducted in the last 5 years, indicating some adoption by vascular exercise researchers (O’Brien et al. 2022). Establishing the clinical relevance may further propagate this metric among researchers.
The mechanisms responsible for the augmented L-FMC with aerobic exercise are unclear, and this is a call for conducting studies in this worthy area of investigation. Endothelin-1 levels are typically lower in older adults who are more aerobically fit(Nyberg et al. 2013) and if basal endothelin-1 levels are lower pre-occlusion then there may be a greater capacity to produce vasoconstrictors in response to low-flow, contributing to the augmented L-FMC among persons who are more aerobically fit. In addition, aerobic exercise training improves vasodilatory signaling of endothelial-derived hyperpolarizing factors (Minami et al. 2002) and prostaglandins (Spier et al. 2007) in rodent models. Lastly, a long-term adaptation to regular exercise is an improved anti-inflammatory response, suppressing TNF-α (Petersen and Pedersen 2005). Systemic TNF-α is reduced following moderate and high-intensity exercise training in Wistar rats (Jiménez-Maldonado et al. 2019). Integrating this mechanistic information alongside cross-sectional and exercise training studies in humans are needed to advance our understanding of the mechanistic contributions of lifestyle factors on endothelial function. Researchers probing the impact of exercise training on vascular function occlusion-reaction ultrasound assessments are encouraged to integrate these relatively straightforward mechanistic measurements to strengthen their research studies and our understanding of vascular physiology.
Of note, other lifestyle factors such as diet may influence the L-FMC response. However, only one study has investigated the impact of diet on L-FMC and observed that high dietary sodium intake attenuated L-FMC in salt-resistant adults (Shenouda et al. 2020). Future studies should consider diet to uncover the other effects it may have on L-FMC.
Summary
Existing work supports that aerobic exercise may augment L-FMC (O’Brien et al. 2022), but our understanding of the mechanistic underpinnings of this observation are not well studied. To improve our understanding of the endothelial impact of exercise and the possible benefits of including L-FMC alongside FMD, studies incorporating inhibitors of L-FMC within their movement interventions are essential. Such information would provide mechanistic insight and may advance our knowledge on the non-NO-mediated changes in endothelial function. Our existing understanding of the factors that might be determinants of L-FMC are outlined in Fig. 2.
Summary of mechanistic determinants and participant-level factors impact on the low-flow-mediated constrictor response. Low-flow-mediated constriction (L-FMC) is mediated via the inhibition of prostaglandins, endothelial-derived hyperpolarizing factors (EDHFs), and tumor necrosis factor-α (TNF-α) but augmented via endothelin-1. Aging and presence of cardiovascular conditions are known to attenuate L-FMC, but the impact of sex is unknown. Aerobic exercise has been shown to augment L-FMC, but future work is needed to understand the impact of resistance training and prolonged sitting. ↑, augment or make more negative; ↓, attenuate or make less negative
Conclusion
L-FMC represents a unique measure of endothelial function that provides information beyond that gained by FMD in isolation but is often not reported. Existing work supports that L-FMC is mediated via the inhibition of non-NO vasodilators (EDHF/prostaglandins), endothelin-1 signaling, and the inflammatory state of the endothelium. Participant factors (e.g., younger age and absence of chronic disease) and engaging in aerobic exercise are associated with augmented L-FMC, but the mechanistic underpinnings of these observations are unclear. A greater integration of mechanistic work alongside applied lifestyle interventions is required to better understand endothelial cell function to reductions in local blood flow.
Abbreviations
- Ca2+ :
-
Calcium ion
- COX:
-
Cyclooxygenase
- CYP:
-
Cytochromes P450
- EET:
-
Epoxyeicosatrienoic acid
- EDHF:
-
Endothelial-derived hyperpolarizing factor
- eNOS:
-
Endothelial nitric oxide synthase
- FMD:
-
Flow-mediated dilation
- K+ :
-
Potassium ion
- L-FMC:
-
Low-flow-mediated constriction
- L-NMMA:
-
NG-Monomethyl-l-arginine
- NO:
-
Nitric oxide
- TNF-α:
-
Tumor necrosis factor-α
References
Aizawa K, Elyas S, Adingupu DD et al (2016) Reactivity to low-flow as a potential determinant for brachial artery flow-mediated vasodilatation. Physiol Rep 4:e12808
Anderson E, Mark A (1989) Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation 79:93–100
Ashor AW, Lara J, Siervo M et al (2015) Exercise modalities and endothelial function: a systematic review and dose–response meta-analysis of randomized controlled trials. Sports Med 45:279–296. https://doi.org/10.1007/s40279-014-0272-9
Atkinson G, Batterham A, Black M et al (2009) Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J Appl Physiol 107:1893–1899
Bell PL, Kelley ET, McCoy SM, Credeur DP (2017) Influence of aerobic fitness on vasoreactivity in young men. Eur J Appl Physiol 117:2075–2083. https://doi.org/10.1007/s00421-017-3698-6
Black M, Cable N, Thijssen D, Green D (2009) Impact of age, sex, and exercise on brachial artery flow-mediated dilation. Am J Physiol Heart Circ Physiol 297:H1109–H1116
Böhm F, Pernow J (2007) The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76:8–18. https://doi.org/10.1016/j.cardiores.2007.06.004
Bourque SL, Davidge ST, Adams MA (2011) The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol 300:R1288–R1295. https://doi.org/10.1152/ajpregu.00397.2010
Carter SE, Faulkner A, Rakobowchuk M (2014) The role of prostaglandin and antioxidant availability in recovery from forearm ischemia–reperfusion injury in humans. J Hypertens 32:339–351. https://doi.org/10.1097/HJH.0000000000000033
Davenport AP, Hyndman KA, Dhaun N et al (2016) Endothelin. Pharmacol Rev 68:357–418. https://doi.org/10.1124/pr.115.011833
Dawson EA, Alkarmi A, Thijssen DH et al (2012) Low-flow mediated constriction is endothelium-dependent: effects of exercise training after radial artery catheterization. Circ Cardiovasc Interv 5:713–719. https://doi.org/10.1161/CIRCINTERVENTIONS.112.971556
Dawson EA, Green DJ, Timothy Cable N, Thijssen DHJ (2013) Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol 115:1589–1598. https://doi.org/10.1152/japplphysiol.00450.2013
Debasso R, Åstrand H, Bjarnegård N et al (2004) The popliteal artery, an unusual muscular artery with wall properties similar to the aorta: implications for susceptibility to aneurysm formation? J Vasc Surg 39:836–842. https://doi.org/10.1016/j.jvs.2003.12.005
Drexler H, Hornig B (1996) Importance of endothelial function in chronic heart failure. J Cardiovasc Pharmacol 27(Suppl 2):S9-12. https://doi.org/10.1097/00005344-199600002-00003
Early KS, Stewart A, Johannsen N et al (2017) The effects of exercise training on brachial artery flow-mediated dilation. J Cardiopulm Rehabil Prev 37:77–89. https://doi.org/10.1097/HCR.0000000000000206
Elliott R, Alsalahi S, Fisher J (2018) Impact of acute dynamic exercise on radial artery low-flow mediated constriction in humans. Eur J Appl Physiol. https://doi.org/10.1007/s00421-018-3876-1
Félétou M, Huang Y, Vanhoutte PM (2011) Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol 164:894–912. https://doi.org/10.1111/j.1476-5381.2011.01276.x
Gao X, Belmadani S, Picchi A et al (2007) Tumor necrosis factor-α induces endothelial dysfunction in Lepr db mice. Circulation 115:245–254. https://doi.org/10.1161/CIRCULATIONAHA.106.650671
Gori T, Dragoni S, Lisi M et al (2008) Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51:1953–1958. https://doi.org/10.1016/j.jacc.2008.01.049
Gori T, Grotti S, Dragoni S et al (2010) Assessment of vascular function: flow-mediated constriction complements the information of flow-mediated dilatation. Heart 96:141–147. https://doi.org/10.1136/hrt.2009.167213
Gori T, Parker JD, Munzel T (2011) Flow-mediated constriction: further insight into a new measure of vascular function. Eur Heart J 32:784–787. https://doi.org/10.1093/eurheartj/ehq412
Gori T, Muxel S, Damaske A et al (2012) Endothelial function assessment: flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur Heart J 33:363–371
Gori T, von Henning U, Muxel S et al (2017) Both flow-mediated dilation and constriction are associated with changes in blood flow and shear stress: Two complementary perspectives on endothelial function. Clin Hemorheol Microcirc 64:255–266. https://doi.org/10.3233/CH-168102
Green DJ, Dawson EA, Groenewoud HMM et al (2014) Is flow-mediated dilation nitric oxide mediated? Hypertension 63:376–382. https://doi.org/10.1161/HYPERTENSIONAHA.113.02044
Greenberg S, Xie J, Wang Y et al (1993) Tumor necrosis factor-alpha inhibits endothelium-dependent relaxation. J Appl Physiol 74:2394–2403. https://doi.org/10.1152/jappl.1993.74.5.2394
Harrison M, Parkhurst K, Tarumi T et al (2011) Low flow-mediated constriction: prevalence, impact and physiological determinant. Clin Physiol Funct Imaging 31:394–398. https://doi.org/10.1111/j.1475-097X.2011.01032.x
Holder SM, Brislane Á, Dawson EA et al (2019) Relationship between endothelial function and the eliciting shear stress stimulus in women: changes across the lifespan differ to men. J Am Heart Assoc. https://doi.org/10.1161/JAHA.118.010994
Holder SM, Bruno RM, Shkredova DA et al (2021) Reference intervals for brachial artery flow-mediated dilation and the relation with cardiovascular risk factors. Hypertension 77:1469–1480. https://doi.org/10.1161/HYPERTENSIONAHA.120.15754
Jiménez-Maldonado A, Montero S, Lemus M et al (2019) Moderate and high intensity chronic exercise reduces plasma tumor necrosis factor alpha and increases the Langerhans islet area in healthy rats. J Musculoskelet Neuronal Interact 19:354–361
Johns JA, O’Brien MW, Bungay A, Kimmerly DS (2020) Sex and light physical activity impact popliteal, but not brachial artery flow-mediated dilation in physically active young adults. Appl Physiol Nutr Metab 45:1387–1395. https://doi.org/10.1139/apnm-2020-0308
Koller A, Sun D, Kaley G (1993) Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res 72:1276–1284. https://doi.org/10.1161/01.RES.72.6.1276
Konukoglu D, Uzun H (2017) Endothelial dysfunction and hypertension. Adv Exp Med Biol 956:511–540. https://doi.org/10.1007/5584_2016_90
Levenson J, Pessana F, Gariepy J et al (2001) Gender differences in wall shear–mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol 38:1668–1674. https://doi.org/10.1016/S0735-1097(01)01604-7
Lew LA, Williams JS, Stone JC et al (2022) Examination of sex-specific participant inclusion in exercise physiology endothelial function research: a systematic review. Front Sports Act Living 4:860356. https://doi.org/10.3389/fspor.2022.860356
LoscalzoJin J (2010) Vascular nitric oxide: formation and function. J Blood Med 1:147. https://doi.org/10.2147/JBM.S7000
Minami A, Ishimura N, Harada N et al (2002) Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis 162:85–92
Moncada S, Vane JR (1978) Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 30:293–331
Newcomer SC, Leuenberger UA, Hogeman CS et al (2004) Different vasodilator responses of humen arms and legs. J Physiol 556:1001–1011
Nishiyama SK, Wray DW, Richardson RS (2008) Sex and limb-specific ischemic reperfusion and vascular reactivity. Am J Physiol Heart Circ Physiol 295:H1100–H1108. https://doi.org/10.1152/ajpheart.00318.2008
Nyberg M, Mortensen SP, Hellsten Y (2013) Physical activity opposes the age-related increase in skeletal muscle and plasma endothelin-1 levels and normalizes plasma endothelin-1 levels in individuals with essential hypertension. Acta Physiol 207:524–535. https://doi.org/10.1111/apha.12048
O’Brien MW, Johns JA, Robinson SA et al (2019a) Relationship between brachial and popliteal artery low-flow-mediated constriction in older adults: impact of aerobic fitness on vascular endothelial function. J Appl Physiol 127:134–142. https://doi.org/10.1152/japplphysiol.00092.2019
O’Brien MW, Johns JA, Williams TD, Kimmerly DS (2019b) Sex does not influence impairments in popliteal endothelial-dependent vasodilator or vasoconstrictor responses following prolonged sitting. J Appl Physiol 127:679–687. https://doi.org/10.1152/japplphysiol.00887.2018
O’Brien MW, Mekary S, Robinson SA et al (2019c) The relationship between aerobic fitness and low-flow-mediated constriction in older adults. Eur J Appl Physiol 119:351–359. https://doi.org/10.1007/s00421-018-4044-3
O’Brien MW, Johns JA, Petterson JL et al (2021a) The impact of age and sex on popliteal artery endothelial-dependent vasodilator and vasoconstrictor function. Exp Gerontol 145:111221. https://doi.org/10.1016/j.exger.2020.111221
O’Brien MW, Petterson JL, Johns JA et al (2021b) A larger low-flow-mediated constrictor response is associated with augmented flow-mediated dilation in the popliteal artery. Clin Physiol Funct Imaging 41:497–504. https://doi.org/10.1111/cpf.12725
O’Brien MW, Petterson JL, Wu Y et al (2022) What is the impact of aerobic fitness and movement interventions on low-flow-mediated vasoconstriction? A systematic review of observational and intervention studies. Vasc Med 27:193–202. https://doi.org/10.1177/1358863X211073480
Ozkor MA, Quyyumi AA (2011) Endothelium-derived hyperpolarizing factor and vascular function. Cardiol Res Pract 2011:1–12. https://doi.org/10.4061/2011/156146
Paterick TE, Fletcher GF (2001) Endothelial function and cardiovascular prevention: role of blood lipids, exercise, and other risk factors. Cardiol Rev 9:282–286. https://doi.org/10.1097/00045415-200109000-00008
Petersen AMW, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98:1154–1162. https://doi.org/10.1152/japplphysiol.00164.2004
Petterson JL, O’Brien MW, Johns JA et al (2021) Influence of prostaglandins and endothelial-derived hyperpolarizing factors on brachial and popliteal endothelial-dependent function in young adults. J Appl Physiol 130:17–25. https://doi.org/10.1152/japplphysiol.00698.2020
Rakobowchuk M, Harris E, Taylor A et al (2012) Heavy and moderate interval exercise training alters low-flow-mediated constriction but does not increase circulating progenitor cells in healthy humans. Exp Physiol 97:375–385. https://doi.org/10.1113/expphysiol.2011.062836
Ramos JS, Dalleck LC, Tjonna AE et al (2015) The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med 45:679–692. https://doi.org/10.1007/s40279-015-0321-z
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000. https://doi.org/10.1161/ATVBAHA.110.207449
Rubanyi GM, Polokoff MA (1994) Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46:325–415
Schnorbus B, Daiber A, Jurk K et al (2020) Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: a randomized, blinded, parallel study. Eur Heart J 41:3144–3152. https://doi.org/10.1093/eurheartj/ehz917
Seals DR, Jablonski KL, Donato AJ (2011) Aging and vascular endothelial function in humans. Clin Sci 120:357–375. https://doi.org/10.1042/CS20100476
Sen S, Chandran DS, Jaryal AK, Deepak KK (2020) The low–flow-mediated arterial constriction in the upper limbs of healthy human subjects are artery specific and handedness independent. Ultrasound Med Biol 46:1949–1959. https://doi.org/10.1016/j.ultrasmedbio.2020.04.008
Shenouda N, Ramick MG, Lennon SL et al (2020) High dietary sodium augments vascular tone and attenuates low-flow mediated constriction in salt-resistant adults. Eur J Appl Physiol 120:1383–1389. https://doi.org/10.1007/s00421-020-04370-0
Spieker LE, Lüscher TF, Noll G (2003) ETA receptors mediate vasoconstriction of large conduit arteries during reduced flow in humans. J Cardiovasc Pharmacol 42:315–318
Spier S, Delp M, Stallone J et al (2007) Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292:H3119–H3127. https://doi.org/10.1152/ajpheart.00588.2006
Spiro JR, Digby JE, Ghimire G et al (2011) Brachial artery low-flow-mediated constriction is increased early after coronary intervention and reduces during recovery after acute coronary syndrome: characterization of a recently described index of vascular function. Eur Heart J 32:856–866
Thijssen D, Dawson EA, Black MA et al (2008) Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295:H1927–H1934
Thijssen DHJ, Rowley N, Padilla J et al (2011) Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol 111:244–250. https://doi.org/10.1152/japplphysiol.00290.2011
Thijssen D, Bruno RM, van Mil ACCM et al (2019) Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40:2534–2547. https://doi.org/10.1093/eurheartj/ehz350
Tinken TM, Thijssen DH, Black MA et al (2008) Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586:5003–5012
Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K et al (2015) Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3–4: a randomized controlled trial. Am J Kidney Dis 66:285–296. https://doi.org/10.1053/j.ajkd.2015.03.015
Wegner J, Karbach S, Drosos I et al (2022) TNF-α blockade may lead to improvement of vascular function in psoriasis patients. Exp Dermatol 31:237–241. https://doi.org/10.1111/exd.14452
Weissgerber TL, Davies GA, Tschakovsky ME (2010) Low flow-mediated constriction occurs in the radial but not the brachial artery in healthy pregnant and nonpregnant women. J Appl Physiol 108:1097–1105
Williams MRI, Westerman RA, Kingwell BA et al (2001) Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86:5389–5395. https://doi.org/10.1210/jcem.86.11.8013
Yi G-H, Zwas D, Wang J (2000) Chronic exercise training preserves prostaglandin-induced dilation of epicardial coronary artery during development of heart failure in awake dogs☆. Prostaglandins Other Lipid Mediat 60:137–151. https://doi.org/10.1016/S0090-6980(00)00057-5
Zhang H, Park Y, Wu J et al (2009) Role of TNF-α in vascular dysfunction. Clin Sci 116:219–230. https://doi.org/10.1042/CS20080196
Funding
MES was supported by a Nova Scotia Graduate Scholarship and a Heat & Stroke BrightRed Scholarship. MWO was supported by a CIHR Post-Doctoral Fellowship Award (#181747) and a Dalhousie University Department of Medicine University Internal Medicine Research Foundation Research Fellowship Award.
Author information
Authors and Affiliations
Contributions
MWO: conceived research question and design. MES and MWO: wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Brien, M.W., Shivgulam, M.E. Mechanistic, participant, and movement-related factors that contribute to low-flow-mediated constriction. Eur J Appl Physiol 123, 2687–2697 (2023). https://doi.org/10.1007/s00421-023-05332-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05332-y