Abstract
Purpose
Caffeine is a stimulant with well-recognized performance and metabolic benefits, however, there is a lack of studies investigating the time-of-day influence in the properties of caffeine to enhance fat oxidation in women. Thus, the aim of the present study was to evaluate the influence of the time of the day on the effect of caffeine on the maximal rate of fat oxidation during aerobic exercise in trained women.
Methods
Fourteen female athletes (25.5 ± 7.1 years) took part in a randomized, crossover, double-blind study. All participants undertook four different experimental trials combining the ingestion of 3 mg/kg caffeine and a placebo either in the morning (8.00–10.00 h) and in the evening (17.00–19.00 h) realizing an incremental test on a cycle ergometer with 3 min stages at workloads from 30 to 70% of maximal oxygen uptake (VO2max). Substrate oxidation rates were measured by indirect calorimetry. In each trial, the maximum rate of fat oxidation (MFO) and the intensity that elicited MFO (Fatmax) were measured.
Results
In comparison to placebo, MFO was significantly higher with caffeine both in the morning (0.24 ± 0.13 vs 0.30 ± 0.14 g/min; p < 0.001; ES = 0.79) and in the evening (0.21 ± 0.08 vs 0.28 ± 0.10 g/min; p = 0.002; ES = 0.72). No time-of-day effect on the capacity of caffeine to increase MFO was found (all p = 0.336)
Conclusion
The intake of 3 mg/kg of caffeine increased the use of fat as a fuel during exercise independently of the time-of-day in trained women.
Trial registration
The study was registered in ClinicalTrials.gov with the following ID: NCT05880186 by 15 May 2023.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The circadian system, controlled by a central pacemaker called suprachiasmatic nucleus, is a key regulator not only of daily sleep–wakefulness timing but also other physiological and behavioral processes that occur in the human body (Maier et al. 2022). Specifically, the circadian system controls metabolism at both central and local levels including clocks within several peripheral tissues such as the liver, pancreas, skeletal muscle, intestine, and adipose tissue (Marcheva et al. 2013). In these body tissues, the circadian system has the capacity to regulate glucose and lipid metabolism via endocrine hormones secretion (Bass and Takahashi 2010). In the last years, there has been an increase in the scientific interest on how the circadian system affects metabolism during exercise (Pickel and Sung 2020). Previous investigations on circadian rhythms showed that there is a diurnal variation in the rate of fat utilization during exercise with a higher contribution of fat as substrate in the evening than in the morning in men (Amaro-Gahete et al. 2019) but not in women (Robles-Gonzalez et al. 2022). However, it is less well-understood if dietary supplements can also contribute to the time-of-day variations in substrate oxidation during exercise.
Caffeine (1, 3, 7 trimethylxanthine) is a natural substance commonly included to dietary supplements due to their purported effect to enhance physical and mental performance. After oral intake, caffeine is distributed throughout body water and readily crosses cell membranes which produces that this substance targets several body tissues with several physiological outcomes such as increased blood pressure, diuresis, and wakefulness (Benowitz 1990). However, caffeine’s ergogenic effect during exercise is believed to be mediated through the action of this substance exclusively in the central nervous system (CNS), thanks to its ability to cross the blood brain barrier (Fredholm 1995). Previous work has demonstrated caffeine’s antagonistic effect on adenosine receptors (A1 and A2A), reversing the fatiguing effects of adenosine while promoting the liberation of other neurotransmitters (Kalmar et al. 2004). Hence, although some effect of caffeine has been found at the muscle level (Ruiz-Moreno et al. 2020, Domaszewski, et al. 2021) caffeine may be considered as a substance with a potent CNS action to enhance aspects of exercise performance (Guest et al 2018).
In the sports context, caffeine is presented in different natural and artificial nutritional forms (e.g., coffee, black tea, or capsules/tablets, etc.) and is commonly ingested for enhancing sports performance as the form of administration has minimal influence on the benefits obtained (Guest et al. 2018). The use of caffeine in sport has been progressively increased since 2004 as caffeine was removed from the World Anti-Doping Agency’s list of prohibited substances (Aguilar-Navarro et al. 2019). Aside its performance-enhancing effect, caffeine has the potential of increasing fat utilization during aerobic exercise at submaximal intensities, lowering-down the contribution of carbohydrate as a fuel. This property of caffeine may provoke a glycogen-sparing effect in the skeletal muscle and liver for exercise situations where carbohydrate availability may be a challenge (Collado-Mateo et al. 2020). Additionally, the capacity of caffeine to enhance fat utilization during exercise could be of interest for improving health outcomes as it may increase the rate of change in body composition in exercise programs (Ramirez-Maldonado et al. 2021).
Maximal fat oxidation rate (MFO) during exercise is a remarkable physiological indicator associated with metabolic flexibility/body weight loss and endurance performance, such as Ironman triathlon (Frandsen et al. 2017). Interestingly, previous investigations have found that MFO can be increased just by the oral intake of caffeine in doses between 3 and 6 mg/kg of body mass (Gutiérrez-Hellín et al. 2023, Varillas-Delgado et al. 2023). Additionally, it has been demonstrated that caffeine increases MFO during exercise performed in the morning and in the evening although such effect has been found only in males (Ramírez-Maldonado et al. 2021). To date, it is unknown if caffeine increases MFO in the same proportion during morning and evening exercise trials in women according to what has been previously established that women have a greater reliance on fat oxidation than men during submaximal exercise (Cano et al. 2022; Chenevière et al. 2011). In addition, the study of the effect of caffeine specifically in women at different times of the day is needed as the translation of data reported in male participants should be made with caution due to differences in body fat (Blaak 2001), circulating catecholamines (Friedlander et al. 1998) and oxidation rates of free fatty acids of women vs men (Roepstorff et al. 2002) in conjunction that caffeine intake has demonstrated and influenced human circadian timing (Burke et al. 2015). For this reason, the aim of the present study was to evaluate the influence of the time of the day on the effect of caffeine on MFO in women. We hypothesized that caffeine would increase MFO during morning and evening exercise and this effect would be of similar magnitude at both times of day.
Materials and methods
Participants
Fourteen trained women (25.5 ± 7.1 years) voluntarily participated in this investigation. All of them performed at least 60 min of exercise per day for at least 4 days per week for the 2 years prior to the investigation. Inclusion criteria were: (a) to be non-smokers, (b) to have a low caffeine intake (i.e., < 50 mg of caffeine per day in the previous 2 months) as previously defined (Goncalves et al. 2017), (c) to show no previous history of cardiopulmonary diseases or having suffered musculoskeletal injuries in the previous 6 months, (d) to have a regular duration of their menstrual cycle for the previous 6 months, and (e) to confirm no existence of any type of menstrual disorders such as dysmenorrhea, amenorrhea, or strong symptoms associated with pre-menstrual syndrome. This information was obtained from a pre-participation screening that included a medical and training history as well as a food frequency questionnaire. Participants signed an informed written consent form to participate in the investigation. The study was approved by the Francisco de Vitoria University Research Ethics Committee (Institutional Review Board) (18-2020) following the Declaration of Helsinki (last update 2013) and registered with ClinicalTrials.gov, U.S. National Institutes of Health (Identifier: NCT05880186).
Experimental design
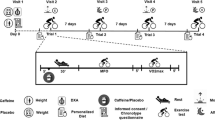
A randomized, crossover, double-blind, and placebo-controlled study design was used. Each participant completed four identical exercise sessions following identical procedures under four different experimental conditions: a) during the morning (8:00–10.00 h) after the intake of 3 mg/kg of caffeine (Bulk Powders, Essex, United Kingdom), b) during the morning (8:00–10.00 h) after the ingestion of 3 mg/kg of a placebo (Cellulose, Guinama, Valencia, Spain), c) during the evening (17.00–19:00 h) after the intake of 3 mg/kg of caffeine, d) during the evening (17.00–19:00 h) after the ingestion of 3 mg/kg of the placebo. An interval period of 7 days between experimental sessions was used to allow the recovery derived from exercise testing sessions. The substances were administered in an opaque and unidentifiable capsule and ingested with 150 mL of tap water 60 min after the onset of exercise. The order assignment of the trials was performed by an independent investigator using a randomizer software (randomizer.org). To ensure the measurements’ standardization, all tests were completed at the same location (i.e., exercise, physiology, and laboratory) using the same testing devices and handled by the same researchers. During all experimental sessions, we controlled air temperature (21.4 ± 0.6 °C) and relative humidity (30 ± 4%) using a digital temperature and humidity monitor (Ambistik, Bad Kissingen, Germany). All participants completed the experimental tests in the mid-luteal phase of their menstrual cycle (Frandsen et al. 2020) and duration of the menstrual cycle were monitored for each participant through a mobile application (Mycalendar, Period-tracker, US) (Janse de Jonge 2003).
Pre-experimental procedure
One week before the first trial, participants were weighed and morphologically analyzed (e.g., body fat), by bioimpedance (Tanita InnerScan Dual, RD-901BK36, Japan). Once the bioimpedance measurement was finished, the participants filled a morningness-evening questionnaire for detecting individual chronotype to determine the participants’ chronotype (morning, intermediate, or evening). The questionnaire consists of 19 questions related to sleep/wake behavior and yields scores ranging from 16 to 86. Based on their scores, individuals were placed into one of five chronotype categories: 16–41: evening type, 42–58: intermediate, and 59- 86: morning type (Horne and Ostberg 1976). Afterwards, they underwent a standardized warm-up that included 10 min at 50 W on a cyclergometer (Ergoline, Ergoselect 4, Sanro Electromedicine, Spain). A ramp exercise test was subsequently completed programming 15-W increments every 1 min until volitional fatigue. During the test, participants chose a cadence between 70 and 90 rpm. The test was ended when participants were unable to maintain a cadence > 50 rpm. During the incremental exercise test, oxygen uptake (VO2) and carbon dioxide production (VCO2) were measured through indirect calorimetry. Participants’ gas exchange was continuously monitored by a breath-by-breath analyzer (Ergostik-Geratherm Respiratory, Bad Kissingen, Germany). The ramp exercise test was considered maximal and valid when participants reached at least three of the following criteria: (a) to show VO2 stabilization despite increases in ergometric power, (b) to obtain a respiratory exchange ratio (RER) higher than 1.10, (c) to have a participant’ rate of perceived exertion-measured higher than 19 points in the 6–20 Borg scale, (d) to attain a heart rate was superior to 90% of maximal heart rate age-predicted estimate (HRmax = 220-age).
Experimental trials
For each experimental trial, the participants were instructed to refrain from strenuous exercise (24 h before) and to follow a similar diet pattern and fluid intake regimen. Participants were also instructed to avoid alcohol, caffeine, and other stimulants consumption 24 h before each trial. They were requested to complete a 24-h exercise and dietary record on the day before the first trial and to follow the patterns during the remaining visits. The compliance of each standardization was checked by the reviewing the exercise and dietary diaries. In the day of the trials, the participants arrived at the laboratory in a fasted state (at least 8 h after their last meal) and 2 hours after ingesting 7 mL/kg of water. Upon arrival, euhydration status was estimated through urine samples and urine specific gravity was measured with a refractometer (MASTER-S28M, Atago company, Tokyo, Japan). To possess a urine specific gravity < 1020 was a mandatory condition for starting the exercise testing. Then, participants ingested the capsule and rested in a supine position for 50 min. In the last 10-min of this period, the tympanic temperature was measured by triplicate using a portable thermometer (Thermoscan 6520, Braun, Germany) and heart rate (Wearlink, Polar, Kempele, Finland) and blood pressure (M6 Comfort, Omron, Kyoto, Japan) were measured. Just 60 min after substance ingestion, participants started the exercise testing which consisted of 10 min at 30% VO2max (as a warming-up) followed by 10% VO2max increments every 3 min until the RER was higher than 1.00 (Jeukendrup and Wallis 2005). All participants obtained RER > 1.00 at VO2max and the workloads included in this study varied from 30 to 70% of VO2max at steady state for 3 min. Expired gasses were collected with the same stationary breath-by-breath device used for the pre-experimental testing, which is a reliable tool to assess substrate oxidation during exercise (Robles-González et al. 2021) and heart rate was measured during exercise using the heart rate band. For the last 15-s of each stage, participants were asked to rate their perception of effort (RPE) using the Borg 6–20-point scale as previously described (Borg 1982). Calibration of the fluxmeter and gas analyzer was carried out according to the manufacturer’s instructions before each experimental trial. The use of 3-min stages was selected to obtain stable VO2 and VCO2 in each workload and an average of gas exchange data for the last minute of each 3-min stage was selected for statistical analysis based on previous methodological studies (Amaro-Gahete et al. 2019a, Amaro-Gahete et al. 2019b). Rates of energy expenditure and substrate oxidation (fat and carbohydrate) were calculated using the non-protein respiratory quotient (Frayn 1983).
Magnitude of side effects
The magnitude of side effects derived from the substances’ ingestion was measured using a questionnaire that included typical drawbacks associated to caffeine intake such as, nervousness, vigor, irritability, gastrointestinal, and insomnia among others. The magnitude of each side effect was measured with a 1- to 10-point scale following the recommendations laid out by (Salinero et al. 2014). Participants were previously informed that 1 point meant a minimal amount and 10 points referred to a maximal amount. This questionnaire was filled out 24 h after substances ingestion.
Statistical analysis
Data are presented as mean and standard deviation. The Shapiro–Wilk test was used to check the normality of all variables. Since all variables were normally distributed, parametric tests were applied to examine differences among conditions. Two-way analysis of variance (time-of-day x substance) was employed to compare MFO, Fatmax, and magnitude of side effects among the different study conditions. A three-way ANOVA (time-of-the day × substance × exercise intensity) was used to compare energy expenditure, fat and carbohydrate oxidation rates, heart rate, and the rating of perceived exertion during exercise. When a significant F value was obtained, a LSD post hoc analysis was performed to determine pairwise differences. Cohen’s formula for effect size (ES) was used to examine potential differences in the comparisons for time-of-day (morning vs evening) and substances (caffeine vs placebo) classifying these effects based on the following criteria: trivial (0–0.19), small (0.20–0.49), medium (0.50–0.79), and large (0.80 and greater). The significance level was set at p ≤ 0.05. Graphs were plotted using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
Results
Participants’ age and anthropometric characteristics are depicted in Table 1. They were classified as intermediate (n = 9), morning (n = 4), and evening type (n = 1) based on the morningness-eveningness chronotype questionnaire. For MFO, there was a main effect of substance (F = 35.230; p < 0.001) but not a significant time-of-day effect (F = 0.487; p = 0.497) or a substance x time-of-day interaction (F = 0.249; p = 0.626) (Fig. 1). Compared to the placebo, caffeine increased MFO by 25.0 ± 7.7% in the morning (0.24 ± 0.13 vs. 0.30 ± 0.14 g/min; p < 0.001; ES = 0.79) and by 33.3 ± 25.0% in the evening (0.21 ± 0.08 vs. 0.28 ± 0.10 g/min; p = 0.002; ES = 0.72; Fig. 1a). There was a main effect of substance (F = 8.273; p = 0.013; η2 = 0.39) on Fatmax but were not a significant time-of-day effect (F = 1.000; p = 0.336) or substance x time-of-day interaction (F = 1.000; p = 0.336). Caffeine intake increased Fatmax in the morning (p = 0.028; ES = 0.57) but there was no effect of caffeine in the evening (p = 0.752; Fig. 1b).
a Maximal fat oxidation individual response (MFO; upper panel), b Fatmax (lower panel) recorded during exercise of increasing intensity one hour after the acute ingestion of 3 mg·kg−1 of caffeine or a placebo in the morning, and in the evening. Data are mean ± SD for 14 participants. (*) Differences with placebo (P < 0.05)
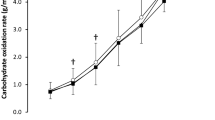
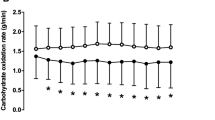
Figure 2 presents data on the effect of caffeine on fat and carbohydrate oxidation rates, and energy expenditure rate during the incremental exercise test. For fat oxidation rate, there was a main effect of substance (F = 16.955; p < 0.001, η2 = 0.56) and exercise intensity (F = 15.318; p < 0.001η2 = 0.86), with no main effect of time-of-day (F = 2.454; p = 0.141) or interaction (F = 4.000; p = 0.614). The post hoc analysis revealed that caffeine increased the rate of fat oxidation over the placebo for both, the morning and evening trials, at 30%, 40%, 50%, and 60% of VO2max (all p < 0.05; η2 from 0.56 to 0.75). Regarding carbohydrate oxidation rate, there were significant main effect of substance (F = 24.750; p < 0.001; η2 = 0.65), exercise intensity (F = 84.601; p < 0.001; η2 = 0.97), and time-of-day (F = 5.359; p = 0.038; η2 = 0.29; Fig. 2b), while no interaction between variables (F = 4.000; p = 0.749). The post hoc analysis revealed that caffeine increased the rate of fat oxidation at all exercise intensities in the morning and from 30 to 60% of VO2max in the evening (all p < 0.05; η2 from 0.68 to 0.71). There were no main effects of substance, time-of-day, or interaction for energy expenditure with only a main effect of energy expenditure (F = 121.034; p < 0.001η2 = 0.97; Fig. 2c).
a Fat oxidation rate (upper panel), b carbohydrate oxidation rate (middle panel) and c) energy expenditure (lower panel) during exercise of increasing intensity one hour after the acute ingestion of 3 mg·kg−1 of caffeine or a placebo in the morning, and in the evening. Data are mean ± SD for 14 participants. (*) Differences with placebo (P < 0.05)
Figure 3 depicts the effect of caffeine ingestion on RPE and heart rate for the trials performed in the morning and in the evening. For RPE, there was a main effect of exercise intensity (F = 60.636; p < 0.001; η2 = 0.96) but not substance (F = 0.898; p = 0.361), time-of-day (F = 1.320; p = 0.271) or interaction (F = 0.435; p = 0.781) (Fig. 3a). The post hoc analysis revealed that caffeine intake reduced RPE values at 50% and 70% of VO2max only in the evening (p = 0.028–0.004; η2 = 0.57–0.70). Referring to heart rate, there was a main effect of exercise intensity (F = 97.957; p < 0.001) but no main effect of substance (F = 1.215; p = 0.290), time-of-day (F = 3.176; p = 0.098) or interaction (F = 1.240; p = 0.355) (Fig. 3b). The post hoc analysis revealed no differences for any pairwise caffeine-placebo comparison.
Finally, differences in side effects reported by questionnaire were reported between caffeine and placebo consumption (Table 2).
Discussion
The aim of the present study was to evaluate the influence of the time of the day on the effect of caffeine on MFO measured during a graded exercise test in trained women. This study was based on a previous investigation carried out with men that demonstrated that oral caffeine intake increases MFO during exercise performed in the morning and in the evening (Ramírez-Maldonado et al. 2021). The current investigation is also pertinent as women do not have a higher contribution to energy derived from fat in the evening, contrary to time-of-day effect on fat oxidation during exercise found in men (Robles-Gonzalez et al. 2022). Overall, caffeine, ingested orally 60 min before exercise in a dose of 3 mg/kg, was effective to enhance fat oxidation from 30% to 60 of VO2max for both morning and evening trials. As a result, MFO was increased with caffeine in both trials while the statistical analysis revealed that there was not time-of-day x substance interaction for MFO. These results suggest that caffeine intake can be considered an effective strategy to increase fat oxidation and MFO during both in the morning and in the evening in trained women. In this context, the magnitude of the caffeine-derived effect will be of similar magnitude, implying an increase of 12.5–13% in the amount of fat oxidized during exercise, independently of the time of the day.
In women, it has been found that the acute intake of 3 and 6 mg of caffeine per kg of body mass increased MFO during exercise (Varillas-Delgado et al. 2023) slightly lower to the data presented in this current study. The study by Varillas-Delgado was performed in women that carried out a graded exercise test in the morning after a period of fasting, mimicking the protocols employed in the current investigation. However, no previous study had been carried out to indicate if the benefits of caffeine on fat oxidation during exercise is also present in the evening in women. Interestingly, a previous study carried in active men reported improvements in MFO with caffeine between 10.7 and 29.0% while this effect was similarly present during exercise performed in the morning and in the evening (Gutierrez-Hellin and Del Coso 2018; Ramirez-Maldonado et al. 2021). Referring to Fatmax (%VO2max) was increased in the morning after caffeine comparing with placebo conditions, while no differences were reported in the evening. It could be explained due to time-of-day effect is lower in women’s athletes comparing with men as previously reported (Robles-Gonzalez et al. 2022).
In comparison to the placebo, the rate of fat oxidation was higher with 3 mg/kg of caffeine at 30, 40, 50, and 60% of VO2max (Fig. 2). Additionally, the increase of fat oxidation at these intensities coincides with a reduction in the use of carbohydrate, with no effect on energy expenditure. This concomitant effect of caffeine to increase fat and to reduce carbohydrate oxidation during exercise of low to moderate intensity has been found in studies with male participants (Gutierrez-Hellin et al. 2022), suggesting that both men and women may benefit from caffeine intake to enhance fat oxidation and to spare carbohydrate during exercise. Interestingly, the effect of caffeine on fat oxidation was not present at 70% VO2max, as the contribution of fat as a fuel was reduced at this intensity. In turn, the results show that the metabolic respiratory quotient (RQ) at steady state exercise is lower with caffeine intake (Fig. 2). This has interesting practical applications for women seeking enhanced fat oxidation during exercise with caffeine as indicate that there has to be a combination of an exercise intensity < 70% VO2max a pre-exercise caffeine intake to optimize the use of fat as fuel during the exercise session.
RPE is a valid and reliable practical tool for monitoring the internal load imposed on the athlete during a training session (Haddad et al. 2017). Previous investigations have established that caffeine supplementation may result in a decrease in the RPE during aerobic and submaximal exercise when exercise intensity is fixed (Guest et al. 2021). This may be the result of the reduced leg-muscle pain induced by caffeine which ultimately allows to perceive less effort and exertion for the same exercise intensity (Astorino et al. 2011). Our data agree that the caffeine-induced lower RPE although this effect was only present in the 50% and 70% VO2max. These data concurred with previous studies that reported between a 2.5 and 4.0% lower rate of perception exertion after caffeine ingestion in elite and recreational male athletes (Dominguez et al. 2021; Jodra et al. 2020). This interesting result could be explained by the caffeine ability to block the adenosine receptors in the central nervous system counteracting the negative effect of adenosine on central fatigue (Smith et al. 2005).
Finally, according to side effects, caffeine ingestion reported statistical differences in nervousness and vigor against placebo conditions, while a significant differences of time-of-day when placebo/caffeine were consumed was reported on irritability and sleep quality parameters. Lastly, an interaction between treatments was reported in diuresis. Referring similar findings to previous studies on previous studies while no differences were reported in heart rate between protocol has been reported (Ruiz-Moreno et al. 2021; de Souza et al. 2022).
The results of this study provide further evidence regarding the lack of diurnal variation (morning vs. evening) on MFO and Fatmax in trained women. Concretely, the present data confirmed those provided by Robles-Gonzalez (Robles-Gonzalez et al. 2022) that reported no time-of-day effects in MFO and Fatmax during a submaximal aerobic exercise test in active females. However, our results did not concur with previous works which reported time-of-day differences in MFO and Fatmax levels, with higher values during the evening (Darvakh et al. 2014; Amaro-Gahete et al. 2019). These controversies can be explained because the last studies were conducted in non-athletic men (Darvakh et al. 2014) or male endurance athletes (Amaro-Gahete et al. 2019) (instead of in trained women) and using different experimental protocols (i.e., using a sub-maximal walking test or running test). Sex differences between studies may be attributed to trained women having a higher fat metabolism contribution during submaximal endurance exercise compared with men, a point that could explain the lack of time-of-day differences in our study (Tate and Holtz 1998; Horton et al. 1998) and is mainly attributed to greater free fatty acids during the luteal phase characterized by increments in estrogens and progesterone levels that provoked greater use of fat and reduced amount of CHO compared to other phases (i.e., follicular phases).
Limitations
Although the numerous strengths of the present study—including the study design and the rigorous protocol for data collection—the current investigation has several limitations that should be addressed. First, despite we organized all exercise trials during the luteal phase of the menstrual cycle, no sexual hormones and free-fatty acids concentrations were measured. Second, we used a relatively small dose of caffeine ingestion (3 mg/kg), thus, future studies should corroborate our findings with higher quantities in order to determine whether a dose–response effect is present. Third, only trained women were included in the investigation and, therefore, future studies should be developed in elite sportswomen and untrained women. Fourth, the present study was conducted in eumenorrheic women, and the results should not be extrapolated to postmenopausal women. Fifth, all the participants were low caffeine consumers (i.e., < 50 mg), establishing that caffeine effects could be different in women athletes with higher caffeine ingestion in their diets. Last, the graded exercise protocol employed in this experiment is a valid tool to examine MFO and Fatmax in trained and untrained individuals within an experimental session (Maunder et al. 2018) but it does not represent a real exercise scenario for those seeking weight loss or change body composition through exercise training. For this reason, the effect of caffeine on fat oxidation should be confirmed in more ecologically valid exercise protocols (e.g., prolonged, and fixed-load exercise and submaximal, intermittent exercise) to definitively demonstrate the effectiveness of this substance for morning and evening exercise routines.
Conclusion
In conclusion, the intake of 3 mg/kg of caffeine increased the use of fat as a fuel during exercise independently of the time of day in trained women. The effect of caffeine to enhance fat oxidation during aerobic exercise was of similar magnitude in the morning and evening, and it was enabled by the capacity to maximize fat oxidation at a higher relative intensity with caffeine (i.e., higher Fatmax), at least in the morning. From a practical perspective, the outcomes of this investigation suggest that caffeine can be used by women seeking to enhance fat oxidation in both morning and evening exercise sessions. To obtain this benefit, caffeine should be ingested in a moderate dose of ~ 3 mg/kg, approximately 60 min before endurance exercise set at 30–60% of VO2max. Additionally, no diurnal variation in MFO was obtained in this cohort of trained women suggesting that the effect of the time of day on fat utilization during exercise may not be present in women.
References
Aguilar-Navarro M, Munoz G, Salinero JJ, Munoz-Guerra J, Fernandez-Alvarez M, Plata MDM, Del Coso J (2019) Urine caffeine concentration in doping control samples from 2004 to 2015. Nutrients. https://doi.org/10.3390/nu11020286
Amaro-Gahete FJ, Jurado-Fasoli L, Trivino AR, Sanchez-Delgado G, De-la OA, Helge JW, Ruiz JR (2019) Diurnal variation of maximal fat-oxidation rate in trained male athletes. Int J Sports Physiol Perform 14(8):1140–1146. https://doi.org/10.1123/ijspp.2018-0854
Astorino TA, Terzi MN, Roberson DW, Burnett TR (2011) Effect of caffeine intake on pain perception during high-intensity exercise. Int J Sport Nutr Exerc Metab 21(1):27–32. https://doi.org/10.1123/ijsnem.21.1.27
Benowitz NL (1990) Clinical pharmacolly of caffeine. Ann Rev Med 41:277–288. https://doi.org/10.1146/annurev.me.41.020190.001425
Blaak E (2001) Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4(6):499–502. https://doi.org/10.1097/00075197-200111000-00006
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, Jung CM, O’Neill JS, Wright KPJR (2015) Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aac5125
Cano A, Ventura L, Martinez G, Cugusi L, Caria M, Deriu F (2022) Analysis of sex-based differences in energy substrate utilization during moderate-intensity aerobic exercise. Eur J Appl Physiol 122(1):29–70. https://doi.org/10.1007/s00421-021-04802-5
Chenevière X, Borrani F, Sangsue D, Gojanovic B, Malatesta D (2011) Gender differences in whole-body fat oxidation kinetics during exercise. Appl Physiol Nutr Metab 36(1):88–95. https://doi.org/10.1139/H10-08
Collado-Mateo D, Lavin-Perez AM, Merellano-Navarro E, Coso JD (2020) Effect of acute caffeine intake on the fat oxidation rate during exercise: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu12123603
Darvakh H, Nikbakht M, Shakerian S, Mousavian A (2014) Effect of circadian rhythm on peak of maximal fat oxidation on non-athletic men. Zahedan Journal of Research in Medical Sciences: 8–11
de Souza JG, Del Coso J, Fonseca FS, Silva BVC, de Souza DB, da Silva Gianoni RL, Filip-Stachnik A, Serrao JC, Claudino JG (2022) Risk or benefit? Side effects of caffeine supplementation in sport: a systematic review. Eur J Nutr. https://doi.org/10.1007/s00394-022-02874-3
Domaszewski P, Pakosz P, Konieczny M, Bączkowicz D, Sadowska-Krępa E (2021) Caffeine-induced effects on human skeletal muscle contraction time and maximal displacement measured by tensiomyography. Nutrients. https://doi.org/10.3390/nu13030815
Dominguez R, Veiga-Herreros P, Sanchez-Oliver AJ, Montoya JJ, Ramos-Alvarez JJ, Miguel-Tobal F, Lago-Rodriguez A, Jodra P (2021) Acute effects of caffeine intake on psychological responses and high-intensity exercise performance. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18020584
Frandsen J, Dahl Vest S, Larsen S, Dela F, Helge JW (2017) Maximal fat oxidation is related to performance in an Ironman triathlon. Int J Sports Med 38(13):975–982. https://doi.org/10.1055/s-0043-117178
Frandsen J, Pistoljevic N, Quesada JP, Amaro-Gahete FJ, Ritz C, Larsen S, Dela F, Helge JW (2020) Menstrual cycle phase does not affect whole body peak fat oxidation rate during a graded exercise test. J Appl Physiol (1985) 128(3):681–687. https://doi.org/10.1152/japplphysiol.00774.2019
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 55(2):628–634. https://doi.org/10.1152/jappl.1983.55.2.628
Fredholm BB (1995) Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol 76:93–101. https://doi.org/10.1111/j.1600-0773.1995.tb00111.x
Friedlander AL, Casazza GA, Horning MA, Huie MJ. Piacentini MF, Trimmer JK Brooks, GA (1998). Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J. Appl. Physiol. 85 (3): 1175–1186 https://doi.org/10.1152/jappl.1998.85.3.1175
Goncalves LS, Painelli VS, Yamaguchi G, Oliveira LF, Saunders B, da Silva RP, Maciel E, Artioli GG, Roschel H (2017) Dispelling the myth that habitual caffeine consumption influences the performance response to acute caffeine supplementation. J Appl Physiol 123(1):213–220. https://doi.org/10.1152/japplphysiol.00260.2017
Guest N, Corey P, Vescovi J, El-Sohemy A (2018) Caffeine, CYP1A2 genotype, and endurance performance in athletes. Med Sci Sports Exerc 50(8):1570–1578. https://doi.org/10.1249/MSS.0000000000001596
Guest N, VanDusseldorp TA, Nelson MT, Grgic J, Schoenfeld BJ, Jenkins NDM, Arent SM, Antonio J, Stout JR, Trexler ET, Smith-Ryan AE, Goldstein ER, Kalman DS, Campbell BI (2021) International society of sports nutrition position stand: caffeine and exercise performance. J Int Soc Sports Nutr 18(1):1. https://doi.org/10.1186/s12970-020-00383-4
Gutierrez-Hellin J, Del Coso J (2018) Effects of p-synephrine and caffeine ingestion on substrate oxidation during exercise. Med Sci Sports Exerc 50(9):1899–1906. https://doi.org/10.1249/MSS.0000000000001653
Gutiérrez-Hellín J, Aguilar-Navarro M, Ruiz-Moreno C, Muñoz A, Varillas-Delgado D, Amaro-Gahete FJ, Del Coso J (2023) Effect of caffeine intake on fat oxidation rate during exercise: is there a dose-response effect? Eur J Nutr 62(1):311–319. https://doi.org/10.1007/s00394-022-02988-8
Haddad M, Stylianides G, Djaoui L, Dellal A, Chamari K (2017) Session-RPE method for training load monitoring: validity, ecological usefulness, and influencing factors. Front Neurosci 11:612. https://doi.org/10.3389/fnins.2017.00612
Horne JA, Ostberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4(2):97–110
Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO (1998) Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol 85(5):1823–1832. https://doi.org/10.1152/jappl.1998.85.5.1823
Janse de Jonge XA (2003) Effects of the menstrual cycle on exercise performance. Sports Med 33(11):833–851. https://doi.org/10.2165/00007256-200333110-00004
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26(Suppl 1):S28-37. https://doi.org/10.1055/s-2004-830512
Jodra P, Lago-Rodriguez A, Sanchez-Oliver AJ, Lopez-Samanes A, Perez-Lopez A, Veiga-Herreros P, San Juan AF, Dominguez R (2020) Effects of caffeine supplementation on physical performance and mood dimensions in elite and trained-recreational athletes. J Int Soc Sports Nutr 17(1):2. https://doi.org/10.1186/s12970-019-0332-5
Kalmar JM, Cafarelli E (2004) Caffeine: a valuable tool to study central fatigue in humans. Exerc Sports Sci Rev. 32:143–147. https://doi.org/10.1097/00003677-200410000-00004
Maier T, Kühnel J, Zimmermann B (2022) How did you sleep tonight? The relevance of sleep quality and sleep-wake rhythm for procrastination at work. Front Psychol 12:785154. https://doi.org/10.3389/fpsyg.2021.785154
Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J (2013) Circadian clocks and metabolism. In: Kramer A, Merrow M (eds) Circadian clocks. Handbook of experimental pharmacology, vol 217. Springer, Berlin, Heidelberg, pp 127–155. https://doi.org/10.1007/978-3-642-25950-0_6
Maunder E, Plews DJ, Kilding AE (2018) Contextualising maximal fat oxidation during exercise: determinants and normative values. Front Physiol 9:599. https://doi.org/10.3389/fphys.2018.00599
Pickel L, Sung HK (2020) Feeding rhythms and the circadian regulation of metabolism. Front Nutr 7:39. https://doi.org/10.3389/fnut.2020.00039
Ramirez-Maldonado M, Jurado-Fasoli L, Del Coso J, J RR, Amaro-Gahete FJ, (2021) Caffeine increases maximal fat oxidation during a graded exercise test: is there a diurnal variation? J Int Soc Sports Nutr 18 (1):5. doi:https://doi.org/10.1186/s12970-020-00400-6
Robles-Gonzalez L, Aguilar-Navarro M, Lopez-Samanes A, Ruiz-Moreno C, Munoz A, Varillas-Delgado D, Gutierrez-Hellin J, Helge JW, Ruiz JR, Amaro-Gahete FJ, (2022) No diurnal variation is present in maximal fat oxidation during exercise in young healthy women: A cross-over study. Eur J Sport Scihttps://doi.org/10.1080/17461391.2022.2067007
Robles-González L, Gutiérrez-Hellín J, Aguilar-Navarro M, Ruiz-Moreno C, Muñoz A, Del Coso J, Ruiz JR, Amaro-Gahete FJ (2021) Inter-day reliability of resting metabolic rate and maximal fat oxidation during exercise in healthy men using the Ergostik gas analyzer. Nutrients 13(12):4308. https://doi.org/10.3390/nu13124308
Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B (2002) Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects Am. J Physiol Endocrinol Metab 282(2):E435–E447. https://doi.org/10.1152/ajpendo.00266.2001
Ruíz-Moreno C, Lara B, Brito de Souza D, Gutiérrez-Hellín J, Romero-Moraleda B, Cuéllar-Rayo Á, Del Coso J (2020) Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br J Clin Pharmacol 86(5):861–867. https://doi.org/10.1111/bcp.14189
Ruiz-Moreno C, Gutierrez-Hellin J, Amaro-Gahete FJ, Gonzalez-Garcia J, Giraldez-Costas V, Perez-Garcia V, Del Coso J (2021) Caffeine increases whole-body fat oxidation during 1 h of cycling at Fatmax. Eur J Nutr 60(4):2077–2085. https://doi.org/10.1007/s00394-020-02393-z
Salinero JJ, Lara B, Abian-Vicen J, Gonzalez-Millan C, Areces F, Gallo-Salazar C, Ruiz-Vicente D, Del Coso J (2014) The use of energy drinks in sport: perceived ergogenicity and side effects in male and female athletes. Br J Nutr 112(9):1494–1502. https://doi.org/10.1017/S0007114514002189
Smith A, Sutherland D, Christopher G (2005) Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. J Psychopharmacol 19(6):620–626. https://doi.org/10.1177/0269881105056534
Tate CA, Holtz RW (1998) Gender and fat metabolism during exercise: a review. Can J Appl Physiol 23(6):570–582. https://doi.org/10.1139/h98-032
Varillas-Delgado D, Aguilar-Navarro M, Muñoz A, López-Samanés A, Ruiz-Moreno C, Posada-Ayala M, Amaro-Gahete FJ, Del Coso J, Gutiérrez-Hellín J (2023) Effect of 3 and 6 mg/kg of caffeine on fat oxidation during exercise in healthy active females. Biol Sport 40(3):827–834. https://doi.org/10.5114/biolsport.2023.121321
Acknowledgements
The authors wish to thank the subjects for their invaluable contribution to the study.
Funding
This research was funded by Francisco de Vitoria University (grant number UFV-18/2020).
Author information
Authors and Affiliations
Contributions
MAN and JGH had the idea for the investigation, DVD, CRM, and ALS collected the research data, and all authors contributed to writing and/or critically revising the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muñoz, A., Aguilar-Navarro, M., Ruiz-Moreno, C. et al. Influence of the time of day in the effect of caffeine on maximal fat oxidation during exercise in women: a randomized, crossover, double-blind, and placebo-controlled study. Eur J Appl Physiol 124, 849–859 (2024). https://doi.org/10.1007/s00421-023-05312-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05312-2