Abstract
Purpose
The present study was performed to determine the impact of hypoxia on working muscle oxygenation during incremental running, and to compare tissue oxygenation between the thigh and calf muscles.
Methods
Nine distance runners and triathletes performed incremental running tests to exhaustion under normoxic and hypoxic conditions (fraction of inspired oxygen = 0.15). Peak pulmonary oxygen uptake (\({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\)) and tissue oxygen saturation (StO2) were measured simultaneously in both the vastus lateralis and medial gastrocnemius.
Results
Hypoxia significantly decreased peak running speed and \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) (p < 0.01). During incremental running, StO2 in the vastus lateralis decreased almost linearly, and the rate of decrease from warm-up (180 m min−1) to \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) was significantly greater than in the medial gastrocnemius under both normoxic and hypoxic conditions (p < 0.01). StO2 in both muscles was significantly decreased under hypoxic compared with normoxic conditions at all running speeds (p < 0.01). The rate at which StO2 was decreased by hypoxia was greater in the vastus lateralis as the running speed increased, whereas it changed little in the medial gastrocnemius.
Conclusions
These results suggest that the thigh is more deoxygenated than the calf under hypoxic conditions, and that the effects of hypoxia on tissue oxygenation differ between these two muscles during incremental running.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies have shown that hypoxia influences exercise performance and cardiopulmonary responses during whole-body exercises such as running (Ogawa et al. 2007), cycling (Osawa et al. 2011; Subudhi et al. 2007), swimming (Ogita and Tabata 1992), and rowing (Peltonen et al. 1995). Under hypoxic conditions, pulmonary oxygen uptake (\({\dot{\text{V}}\text{O}}_{ 2}\)) at a given exercise intensity does not change, while peak \({\dot{\text{V}}\text{O}}_{ 2} \,({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}} )\) at maximal exercise decreases. In addition to the influence on cardiopulmonary responses, hypoxia affects regional muscle circulation and metabolism. Near infrared spectroscopy (NIRS) is a useful method to evaluate tissue oxygen metabolism and can be used to continuously and non-invasively monitor tissue oxygenation from various parts of the body during dynamic exercise (Bhambhani 2012; Hamaoka et al. 2007). Previous studies have indicated that muscle oxygenation under hypoxic conditions does not change at rest and during single-knee extension (DeLorey et al. 2004), but decreases during intermittent sprint (Bowtell et al. 2014), constant-load (Spencer et al. 2012a, b), and incremental cycling (Osawa et al. 2011; Subudhi et al. 2007). However, few studies have investigated the effects of hypoxia on working muscles during running, which is a major whole-body exercise mode.
During incremental cycling, systemic \({\dot{\text{V}}\text{O}}_{ 2}\) increases almost linearly until \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) (Day et al. 2003), but working muscle deoxygenation monitored by NIRS changes non-linearly and is attenuated before \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\), despite increases in \({\dot{\text{V}}\text{O}}_{ 2}\) (Boone et al. 2015; Osawa et al. 2011; Subudhi et al. 2007). However, tissue oxygenation in the thigh muscles differs during running compared with cycling. Quaresima et al. (2002) showed that the difference in tissue oxygenation between walking and running was larger in the vastus lateralis muscle than in the medial gastrocnemius muscle. Another study showed that during walking and running at incremental speeds, tissue oxygenation decreased in the vastus lateralis until exhaustion, but this did not occur in the lateral gastrocnemius (Hiroyuki et al. 2002).
The decrease in arterial oxygen content between muscles induced by systemic hypoxia would not be different, while the microvascular responses and recruitment pattern of muscle fibers are different (Heinonen et al. 2010). During high-speed running there was a higher contribution to workload by the thigh as compared with the calf (Simpson and Bates 1990), and tissue deoxygenation of the working muscle was exacerbated by systemic hypoxia (Osawa et al. 2011; Subudhi et al. 2007). The purpose of this study was thus to compare the impact of hypoxia on tissue oxygenation between the thigh and calf muscles during incremental running. We hypothesized that hypoxia-induced tissue deoxygenation would be greater in the thigh than in the calf during high-speed running.
Methods
Subjects
Nine male distance runners and triathletes (age = 24 ± 3 years, height = 1.69 ± 0.05 m, body mass = 57.1 ± 3.6 kg, body mass index = 20.1 ± 1.3 kg·m−2 [means ± standard division (SD)]) volunteered to participate in this study. The health status of each subject was assessed based on medical history and physical examination. All procedures and any potential risks were explained to the participants before starting the study, and all provided written informed consent. This study was approved by the local ethics committee, and all experiments were performed in accordance with the Declaration of Helsinki.
Experimental procedure and protocol
After familiarization with the experimental procedures and the treadmill machine, the participants performed two incremental running tests on the treadmill [inclination = 1% (Jones and Doust 1996)] in an environmentally controlled, simulated room under normoxic or normobaric hypoxic (fraction of inspired oxygen (F IO2) = 0.15, balanced with nitrogen) conditions in random order. Ambient conditions were controlled automatically (temperature = 23.0°, relative humidity = 40%). The participants performed the two sessions 72 h apart and the tests were at the same time each day. The participants were instructed to refrain from caffeine consumption for 12 h and stressful exercise for 24 h before each test. After breathing in each gas mixture at rest for at least 30 min to equilibrate body gas stores of oxygen and carbon dioxide, the participants performed incremental running tests under either normoxic or hypoxic conditions. The protocol included at least 5 min of standing rest, 3 min of running at 180 m·min−1 (warm-up), and then a 10 m·min−1 increase in pace every minute until exhaustion. The actual speeds on the treadmill were measured every minute with a handheld tachometer (DT-105 N; Nidec-Shimpo Corporation, Kyoto, Japan), as the actual treadmill speeds varied slightly compared with the set speeds during running.
Cardiopulmonary responses
Breath-by-breath measurements of pulmonary gas exchange and minute ventilation were obtained using a computerized metabolic cart (AE300; Minato Medical Science, Osaka, Japan). \({\dot{\text{V}}\text{O}}_{ 2}\) was expressed in standard temperature, pressure, and dry, and minute ventilation was expressed in body temperature, ambient pressure, and saturated with water vapor. Heart rate was determined using a heart rate monitor (Polar RS800CX; Polar Electro, Kempele, Finland). Arterial oxygen saturation (SpO2) was monitored non-invasively using a pulse oximeter (OLV-3100; Nihon Kohden, Tokyo, Japan) on the index finger. To ensure the accuracy of the data, pulse data were acquired simultaneously and confirmed to match the heart rate. We were not able to collect complete data from two participants, so the results of SpO2 were averaged in the other seven participants. Respiratory responses, heart rates, and SpO2 were measured throughout the exercise bouts. Blood was acquired from fingertip sticks and blood lactate concentration were measured using a blood lactate test meter (Lactate-Pro; Arkray, Kyoto, Japan) before exercise and at the 1st and 3rd minutes after exercise; the highest value was recorded as the blood lactate at \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\). The participants were allowed to stop running when they fell more than one meter behind on the treadmill despite verbal encouragement.
Tissue oxygenation
Changes in oxygenated (O2Hb) and deoxygenated (HHb) hemoglobin and myoglobin concentrations were calculated using modified Beer–Lambert methods (Hamaoka et al. 2007), in which the concentration of absorber was calculated by the optical density and pathlength. Tissue oxygen saturation (StO2) was assessed with spatially resolved spectroscopy and measured using a multi-distance spatially resolved tissue oximeter (NIRO-200NX; Hamamatsu Photonics, Shizuoka, Japan). The baseline values of O2Hb and HHb were obtained while the participants stood on the treadmill for at least 5 min before the test. Changes in total hemoglobin (Hb) were calculated by adding the changes in O2Hb and HHb, and these were considered to reflect changes in blood volume of the measurement site. Optodes were placed on the lower third of the vastus lateralis and the middle of the medial gastrocnemius using double-sided and surgical tape and housed in plastic holders. The thigh and calf were wrapped with elastic bandages. The light source consisted of three laser diodes of different wavelengths (775, 810, and 850 nm). The intensity of the transmitted light was measured at 5 Hz. The distance between the emitter and receiver was 3 cm. The thickness of the fat layer in the regions of interest was <5 mm (mean ± SD = 2.7 ± 0.7 mm), ensuring that the NIRS signals would be minimally influenced by the fat layer (Niwayama et al. 2007).
Statistical analyses
All data were averaged in the last 30 s of exercise at each speed and all values were calculated as mean ± SD. Statistical analyses were performed using statistical software (IBM SPSS Statistics 19; IBM Cooperation, Chicago, IL). Exercise-induced changes were compared by two-way analysis of variance, and the variables included the muscles, F IO2 conditions, and running speeds. When significant effects were observed, the Bonferroni post hoc test was performed. In all analyses, p < 0.05 was taken to indicate statistical significance.
Results
Running speeds and cardiopulmonary responses at rest and at \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) under normoxic and hypoxic conditions are shown in Table 1. Compared with normoxic conditions, running speed and \({\dot{\text{V}}\text{O}}_{ 2}\) at \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) under hypoxic conditions were significantly reduced by 8.9 ± 2.4% and 12.8 ± 7.0%, respectively. SpO2 was lower in hypoxic than in normoxic conditions by 8.8 ± 4.2 percentage points at rest and 20.0 ± 5.2 percentage points at \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\). Heart rates at \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) were significantly different between the two conditions, but there were no significant difference in minute ventilation and blood lactate concentration.
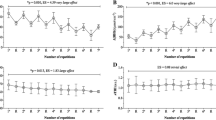
StO2 in the vastus lateralis and medial gastrocnemius at rest did not differ significantly between normoxic and hypoxic conditions. However, the StO2 was significantly decreased by both changes in FIO2 and running speed, and there was a significant interaction between these changes (Fig. 1). The decreases in StO2 from warm-up (180 m·min−1) to \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) in the vastus lateralis (19.5 ± 6.5% points and 18.7 ± 4.8% points under normoxic and hypoxic conditions, respectively) were significantly larger than those in the medial gastrocnemius (6.6 ± 8.9% points and 8.2 ± 6.6% points under normoxic and hypoxic conditions, respectively), but no significant differences were found between normoxic and hypoxic conditions. At \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\), StO2 was lower in the vastus lateralis than in the medial gastrocnemius, and the vastus lateralis, but not the medial gastrocnemius, was more deoxygenated in hypoxic than in normoxic conditions.
StO2 in the vastus lateralis (upper) and the medial gastrocnemius (lower) under normoxic (shaded squares) and hypoxic (clear squares) conditions. Values are mean ± SD. Significantly different from rest under each condition († p < 0.05, ‡ p < 0.01). Significantly different between normoxic and hypoxic conditions (**p < 0.01)
HHb in the vastus lateralis was significantly increased both by hypoxic conditions (compared with normoxic conditions) and increasing running speeds, and there was an interaction between these changes. Similarly, HHb in the medial gastrocnemius interacted significantly with the changes in F IO2 and running speed (Fig. 2).
HHb in the vastus lateralis (upper) and the medial gastrocnemius (lower) under normoxic (shaded squares) and hypoxic (clear squares) conditions. Values are mean ± SD. Significantly different from rest under each condition († p < 0.05, ‡ p < 0.01). Significantly different between normoxic and hypoxic conditions (*p < 0.05, **p < 0.01)
Figure 3 shows the average responses of total Hb in the vastus lateralis and medial gastrocnemius with progression from rest to \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\). The change in the vastus lateralis was similar between normoxic and hypoxic conditions, but that of the medial gastrocnemius interacted significantly with changes in F IO2 and speeds. At \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\), total Hb values were not significantly different in either the vastus lateralis or the medial gastrocnemius in normoxic compared with hypoxic conditions.
At rest, the rate at which StO2 was decreased from normoxic to hypoxic conditions did not differ between the vastus lateralis and medial gastrocnemius. However, there was a significant interaction in the decrease in StO2 under hypoxic compared normoxic conditions between the muscles and speeds (Fig. 4 upper). Similarly, the increase in HHb from normoxic to hypoxic conditions was significantly greater in the vastus lateralis during incremental running compared with at rest, whereas that in the medial gastrocnemius it was not (Fig. 4 lower).
Decrease in StO2 (upper) and increase in HHb (lower) in the vastus lateralis (black bars) and medial gastrocnemius (gray bars) at increasing absolute running speeds. Values are mean ± SD. Significantly different from rest under each condition († p < 0.05, ‡ p < 0.01). Significantly different between normoxic and hypoxic conditions (*p < 0.05, **p < 0.01)
Discussion
We examined the difference in the effects of hypoxia on tissue oxygenation between the thigh and calf muscles during incremental running. The main findings of this study were that hypoxia decreased the tissue oxygenation of both the vastus lateralis and medial gastrocnemius while running, and that the decreases were more pronounced in the vastus lateralis than in the medial gastrocnemius. These results suggest that the effect of hypoxia on tissue oxygenation in the two working muscles was influenced by the running speed, and that the decreases in tissue oxygenation induced by hypoxia were greater in the vastus lateralis than in the medial gastrocnemius, especially during high-speed running. The simulated altitude in this study (calculated from inspired oxygen pressure) was ~2700 m, and this is commonly used as high-altitude training by elite athletes. The data from our study suggest that hypoxic training has disparate effects on different muscles.
Previous studies using positron emission tomography have shown that carbohydrate metabolism is greater in the calf than in other muscles while running (Fujimoto et al. 2000). Likewise, glycogen depletion rates after ~2.5 h of intense running have been shown to be almost 1.7-fold greater in the calf than in the thigh muscles (Krssak et al. 2000). It is possible that carbohydrate metabolism in the medial gastrocnemius—a muscle that is less oxidative than the vastus lateralis (Johnson et al. 1973)—should increase during high-speed running since whole-body carbohydrate usage increases when running speed increases (Zavorsky et al. 1998). Thus, it has been thought that more energy is consumed by the calf than the thigh muscles while running, and thus, the medial gastrocnemius is more deoxygenated than the vastus lateralis during high-speed running. Moreover, the effects of hypoxia on tissue oxygenation are thought to be more pronounced in the medial gastrocnemius than in the vastus lateralis because hypoxia-induced tissue deoxygenation in working muscles is accelerated by increased exercise intensity (Osawa et al. 2011; Subudhi et al. 2007). However, we showed that tissue deoxygenation increased more in the vastus lateralis than in the medial gastrocnemius during incremental running under both normoxic and hypoxic conditions. These results could be partly attributable to changes in the relative contribution rates of the muscles to propulsive force generation (Novacheck 1998). Previous studies have shown that the movement parameters of the hip, knee, and ankle joints increase as running speed increases, but that the contribution rates of various muscles at these joints differ (Simpson and Bates 1990). For example, the relative contribution of the calf muscles to propulsive forces decreased gradually as running speed increased, while the contribution in the thigh muscles increased. This is partly supported by previous studies showing that the knee velocity increased as the running speed and stride length increased (Collins et al. 2000). The vastus lateralis became deoxygenated nearly linearly until \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\), whereas the medial gastrocnemius became deoxygenated at a slower rate.
NIRS responses
Tissue oxygenation (as monitored by NIRS) is thought to reflect the balance between oxygen supply and consumption at the measured sites (Grassi and Quaresima 2016). The decrease in tissue oxygenation during exercise is attributed not only to increased oxygen consumption, but also to decreased oxygen supply. In other words, there is a relative decrease in the oxygen supply/consumption ratio during exercise. A decrease in StO2 of the vastus lateralis during incremental running may be primarily caused by increased muscle oxygen consumption, but it could also be affected by increases or decreases in oxygen supply. Decreases in oxygen supply can be caused by a decrease in arterial oxygen content resulting from exercise-induced hypoxemia and a decrease in F IO2 (Miyachi and Tabata 1992). In addition, tissue blood flow can be decreased by vasoconstriction due to increases in sympathetic nerve activity (Hansen et al. 2000). Increases in oxygen supply can be caused by increases in cardiac output and leg blood flow as well as vasodilation induced by endothelium-derived nitric oxide (Rådegran and Hellsten 2000). Tissue oxygenation is thus regulated both by control systems affecting oxygen supply to the muscles and by changes in muscle oxygen consumption.
Tissue oxygenation in hypoxia
As seen in cycling (Osawa et al. 2011; Subudhi et al. 2007), working muscles became deoxygenated during incremental running under hypoxic conditions, and this was not completely attributed to decreases in SpO2. At rest, StO2 was similar between the two conditions despite a significant decrease in SpO2 in hypoxia. This was likely because the decrease in SpO2 was modulated by increases in microvascular blood flow. Indeed, muscle oxygenation was unchanged at low \({\dot{\text{V}}\text{O}}_{ 2}\), such as at rest or during low-intensity exercise, and in exercises utilizing a small muscle mass (DeLorey et al. 2004). Therefore, decreases in StO2 in the vastus lateralis and medial gastrocnemius while running in hypoxic conditions are likely related to an inability to compensate for the decrease in SpO2.
The balance between tissue oxygen delivery and consumption determines tissue oxygenation, which mainly reflects the microcirculation (Hamaoka et al. 2007). It has traditionally been thought that the effects of hypoxia on muscle oxygen supply are not different between muscles and that the decrease in StO2 in the vastus lateralis and medial gastrocnemius are similar. However, our results indicate that the effects of hypoxia on tissue oxygenation do indeed differ between these muscles. In addition to physiological responses affected by hypoxia, including sympathetic nerve (Saito et al. 1988) and microcirculatory responses (Bourdillon et al. 2009; Heinonen et al. 2010), intramuscular metabolites likely accumulate more in hypoxic than in normoxic conditions (Wadley et al. 2006), which stimulates the recruitment of fast-twitch muscle fibers (Moritani et al. 1992). The muscle fiber compositions in both the vastus lateralis and medial gastrocnemius are mixed, although the vastus lateralis is slightly more oxidative than the medial gastrocnemius (Johnson et al. 1973). The relative contribution of the thigh to propulsive forces increases more than the calf during incremental running (Simpson and Bates 1990), so the recruitment of fast-twitch muscle fibers, which are thought to influence oxygen delivery and consumption (McDonough et al. 2005), may be greater in the vastus lateralis.
Tissue oxygenation during running
During incremental running, StO2 in the vastus lateralis decreased almost linearly. The deoxygenation dynamics differed from those seen in cycling, in which tissue deoxygenation in working muscles becomes attenuated at high intensity as \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) is approached (Boone et al. 2015, 2016 (review); Fontana et al. 2015; Keir et al. 2015; Osawa et al. 2011; Spencer et al. 2012a, b; Subudhi et al. 2007). The difference in tissue deoxygenation dynamics between running and cycling could be related to various factors, including the range of knee joint motion, the types of muscle contraction, and the duration of muscle contraction per step (running) and per pedal (cycling). However, oxygenation of the medial gastrocnemius formed an inverted arc compared with the vastus lateralis, and the change in amplitude from warm-up to \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) was smaller than that of the vastus lateralis. This is similar to what has been observed in previous reports (Hiroyuki et al. 2002). The small change in tissue oxygenation observed in the medial gastrocnemius during incremental running was likely not mediated by unchanged or decreased muscle oxygen consumption, but instead by concurrent increases in both muscle oxygen supply and consumption. This could explain the near tissue oxygen balance during running. Electromyographic activity (Bijker et al. 2002) and muscle oxygen consumption in the calf both increase during incremental running, although electromyographic activity and muscle deoxygenation do not always correlate (Osawa et al. 2011). Moreover, as tissue oxygenation is partially related to the Bohr Effect (Grassi et al. 1999; Wang et al. 2006), it is possible that intramuscular metabolites and pH are different between the vastus lateralis and medial gastrocnemius.
Previous reports have shown that tissue circulation and metabolism during exercise (Cannon et al. 2013; Fujimoto et al. 2000; Heinonen et al. 2010) differed between muscles, as did muscle oxygenation (Chin et al. 2011) and electromyographic activities (Hug et al. 2004). Moreover, heterogeneous oxygenation within a single muscle has also been reported (Kime et al. 2010; Vogiatzis et al. 2015). However, the present study assessed only the vastus lateralis and medial gastrocnemius as being representative of the thigh and calf muscles, respectively. Therefore, the possibility that tissue oxygenation in other muscles differs from that in the vastus lateralis and medial gastrocnemius cannot be excluded. Further studies are required to elucidate the heterogeneity among working muscles.
Conclusions
We showed that the effects of hypoxia on tissue oxygenation varied between working muscles during incremental running, and that hypoxia-induced tissue deoxygenation was exacerbated by faster running speeds in the vastus lateralis but not in the medial gastrocnemius. It is likely that the disparate contributions to running propulsive forces play a role in the differences in tissue oxygenation between the thigh and calf during incremental running. These results help to understand the microcirculatory and metabolic alterations to different muscles that are induced by hypoxia during whole-body exercise.
Abbreviations
- FIO2 :
-
Fraction of inspired oxygen fraction
- Hb:
-
Hemoglobin
- HHb:
-
Deoxygenated hemoglobin and myoglobin
- NIRS:
-
Near infrared spectroscopy
- O2Hb:
-
Oxygenated hemoglobin and myoglobin
- SD:
-
Standard division
- SpO2 :
-
Arterial oxygen saturation
- StO2 :
-
Tissue oxygen saturation
- \({\dot{\text{V}}\text{O}}_{ 2}\) :
-
Oxygen uptake
- \({\dot{\text{V}}\text{O}}_{{ 2 {\text{peak}}}}\) :
-
Peak oxygen uptake
References
Bhambhani Y (2012) Application of near infrared spectroscopy in evaluating cerebral and muscle haemodynamics during exercise and sport. J Near Infrared Spectrosc 20:117–139
Bijker KE, de Groot G, Hollander AP (2002) Differences in leg muscle activity during running and cycling in humans. Eur J Appl Physiol 87:556–561
Boone J, Barstow TJ, Celie B, Prieur F, Bourgois J (2015) The impact of pedal rate on muscle oxygenation, muscle activation and whole-body VO2 during ramp exercise in healthy subjects. Eur J Appl Physiol 115:57–70
Boone J, Vandekerckhove K, Coomans I, Prieur F, Bourgois JG (2016) An integrated view on the oxygenation responses to incremental exercise at the brain, the locomotor and respiratory muscles. Eur J Appl Physiol 116:2085–2102
Bourdillon N, Mollard P, Letournel M, Beaudry M, Richalet JP (2009) Non-invasive evaluation of the capillary recruitment in the human muscle during exercise in hypoxia. Respir Physiol Neurobiol 165:237–244
Bowtell JL, Cooke K, Turner R, Mileva KN, Sumners DP (2014) Acute physiological and performance responses to repeated sprints in varying degrees of hypoxia. J Sci Med Sport 17:399–403
Cannon DT, Howe FA, Whipp BJ, Ward SA, McIntyre DJ, Ladroue C, Griffiths JR, Kemp GJ, Rossiter HB (2013) Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol 115:839–849
Chin LM, Kowalchuk JM, Barstow TJ, Kondo N, Amano T, Shiojiri T, Koga S (2011) The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J Appl Physiol 111:1259–1265
Collins MH, Pearsall DJ, Zavorsky GS, Bateni H, Turcotte RA, Montgomery DL (2000) Acute effects of intense interval training on running mechanics. J Sports Sci 18:83–90
Day JR, Rossiter HB, Coats EM, Skasick A, Whipp BJ (2003) The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. J Appl Physiol 95:1901–1907
DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH (2004) The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee extension exercise. Exp Physiol 89:293–302
Fontana FY, Keir DA, Bellotti C, De Roia GF, Murias JM, Pogliaghi S (2015) Determination of respiratory compensation point in healthy adults: can non-invasive near-infrared spectroscopy help? J Sci Med Sport 18:590–595
Fujimoto T, Itoh M, Tashiro M, Yamaguchi K, Kubota K, Ohmori H (2000) Glucose uptake by individual skeletal muscles during running using whole-body positron emission tomography. Eur J Appl Physiol 83:297–302
Grassi B, Quaresima V (2016) Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21:091313
Grassi B, Quaresima V, Marconi C, Ferrari M, Cerretelli P (1999) Blood lactate accumulation and muscle deoxygenation during incremental exercise. J Appl Physiol 87:348–355
Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B (2007) Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12:062105
Hansen J, Sander M, Hald CF, Victor RG, Thomas GD (2000) Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol 527:387–396
Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK (2010) Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol 299:R72–R79
Hiroyuki H, Hamaoka T, Sako T, Nishio S, Kime R, Murakami M, Katsumura T (2002) Oxygenation in vastus lateralis and lateral head of gastrocnemius during treadmill walking and running in humans. Eur J Appl Physiol 87:343–349
Hug F, Bendahan D, Le Fur Y, Cozzone PJ, Grelot L (2004) Heterogeneity of muscle recruitment pattern during pedaling in professional road cyclists: a magnetic resonance imaging and electromyography study. Eur J Appl Physiol 92:334–342
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fiber types in thirty-six human muscles: an autopsy study. J Neurol Sci 18:111–129
Jones AM, Doust JH (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14:321–327
Keir DA, Fontana FY, Robertson TC, Murias JM, Paterson DH, Kowalchuk J, Pogliaghi S (2015) Exercise intensity thresholds: identifying the boundaries of sustainable performance. Med Sci Sports Exerc 47:1932–1940
Kime R, Niwayama M, Fujioka M, Shiroishi K, Osawa T, Shimomura K, Osada T, Murase N, Katsumura T (2010) Unchanged muscle deoxygenation heterogeneity during bicycle exercise after 6 weeks of endurance training. Adv Exp Med Biol 662:353–358
Krssak M, Petersen KF, Bergeron R, Price T, Laurent D, Rothman DL, Roden M, Gin Shulman (2000) Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab 85:748–754
McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC (2005) Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563:903–913
Miyachi M, Tabata I (1992) Relationship between arterial oxygen desaturation and ventilation during maximal exercise. J Appl Physiol 73:2588–2591
Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M (1992) Oxygen availability and motor unit activity in humans. Eur J Appl Physiol Occup Physiol 64:552–556
Niwayama M, Sone S, Murata H, Yoshida H, Shinohara S (2007) Errors in muscle oxygenation measurement using spatially-resolved NIRS and its correction (in Japanese). J Jpn Coll Angiol 47:17–20
Novacheck TF (1998) The biomechanics of running. Gait Posture 7:77–95
Ogawa T, Hayashi K, Ichinose M, Nishiyasu T (2007) Relationship between resting ventilatory chemosensitivity and maximal oxygen uptake in moderate hypobaric hypoxia. J Appl Physiol 103:1221–1226
Ogita F, Tabata I (1992) Oxygen uptake during swimming in a hypobaric hypoxic environment. Eur J Appl Physiol Occup Physiol 65:192–196
Osawa T, Kime R, Hamaoka T, Katsumura T, Yamamoto M (2011) Attenuation of muscle deoxygenation precedes EMG threshold in normoxia and hypoxia. Med Sci Sports Exerc 43:1406–1413
Peltonen JE, Rantamäki J, Niittymäki SP, Sweins K, Viitasalo JT, Rusko HK (1995) Effects of oxygen fraction in inspired air on rowing performance. Med Sci Sports Exerc 27:573–579
Quaresima V, Komiyama T, Ferrari M (2002) Differences in oxygen re-saturation of thigh and calf muscles after two treadmill stress tests. Comp Biochem Physiol A Mol Integr Physiol 132:67–73
Rådegran G, Hellsten Y (2000) Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 168:575–591
Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y (1988) Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 65:1548–1552
Simpson KJ, Bates BT (1990) The effects of running speed on lower extremity joint moments generated during the support phase. Int J Sport Biomech 6:309–324
Spencer MD, Murias JM, Grey TM, Paterson DH (2012a) Regulation of VO2 kinetics by O2 delivery: insights from acute hypoxia and heavy-intensity priming exercise in young men. J Appl Physiol 112:1023–1032
Spencer MD, Murias J, Paterson DH (2012b) Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol 112:3349–3360
Subudhi AW, Dimmen AC, Roach RC (2007) Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol 103:177–183
Vogiatzis I, Habazettl H, Louvaris Z, Andrianopoulos V, Wagner H, Zakynthinos S, Wagner PD (2015) A method for assessing heterogeneity of blood flow and metabolism in exercising normal human muscle by near-infrared spectroscopy. J Appl Physiol 118:783–793
Wadley GD, Lee-Young RS, Canny BJ, Wasuntarawat C, Chen ZP, Hargreaves M, Kemp BE, McConell GK (2006) Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab 290:E694–E702
Wang L, Yoshikawa T, Hara T, Nakao H, Suzuki T, Fujimoto S (2006) Which common NIRS variable reflects muscle estimated lactate threshold most closely? Appl Physiol Nutr Metab 31:612–620
Zavorsky GS, Montgomery DL, Pearsall DJ (1998) Effect of intense interval workouts on running economy using three recovery durations. Eur J Appl Physiol Occup Physiol 77:224–230
Acknowledgements
We would like to express our gratitude to the subjects in this study. This study was supported by a Grant-in-Aid for Research Activity Start-up (23800075) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was performed without any conflict of interest, financial or otherwise.
Additional information
Communicated by I. Mark Olfert.
Rights and permissions
About this article
Cite this article
Osawa, T., Arimitsu, T. & Takahashi, H. Hypoxia affects tissue oxygenation differently in the thigh and calf muscles during incremental running. Eur J Appl Physiol 117, 2057–2064 (2017). https://doi.org/10.1007/s00421-017-3696-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3696-8