Abstract
Purpose
The aim of this project was to study the impact of pedal rate on breakpoints in muscle oxygenation (deoxy[Hb + Mb] and total[Hb + Mb]) and activation (iEMG and MPF) at high intensities during ramp exercise.
Methods
Twelve physically active students performed incremental ramp exercises at 60 rpm, starting either at 50 or 80 W (i.e., 60rpm50 and 60rpm80), and at 100 rpm, starting at 50 W (100rpm50). Pulmonary VO2, muscle activation (iEMG and MPF) and oxygenation were recorded with EMG and NIRS, respectively. IEMG, MPF, deoxy[Hb + Mb] and total[Hb + Mb] were expressed as functions of work rate (WR) and pulmonary VO2 (%VO2peak) and analyzed with double-linear models.
Results
The breakpoints (BP) of iEMG, MPF, total[Hb + Mb] and deoxy[Hb + Mb] in %VO2peak did not differ among the pedal rate conditions (P > 0.05), whereas the BPs in WR were significantly lower in 100rpm50 compared to 60rpm50 and 60rpm80 (P < 0.01). Across the pedal rate conditions the BP (in %VO2peak) of total[Hb + Mb] (82.7 ± 1.5 %VO2peak) was significantly lower (P < 0.01) compared to the BP in iEMG (84.3 ± 1.7 %VO2peak) and MPF (84.2 ± 1.6 %VO2peak), whereas the BP in deoxy[Hb + Mb] (87.4 ± 1.4 %VO2peak) and respiratory compensation point (89.9 ± 1.8 %VO2peak) were significantly higher (P < 0.01) compared to the BP in total[Hb + Mb], iEMG and MPF. Additionally, the BPs in iEMG, MPF, total[Hb + Mb] and deoxy[Hb + Mb], and the RCP were highly correlated (r > 0.90; P < 0.001).
Conclusions
The present study showed that muscle activation and oxygenation at high intensities during incremental exercise are related to pulmonary VO2 rather than external WR, with a close interrelationship between that muscle activation, oxygenation and pulmonary VO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A thorough insight into the acute physiological responses to changes in exercise intensity provides valuable information into the functional capacity and exercise (in)tolerance of healthy subjects and patient populations. The integration of the physiological systems (i.e., central and peripheral) determines the relationship between oxygen supply (QO2) to demand (VO2) and allows the individual to adjust to changes in metabolic demand. These adjustments can be visualized through the integration of the Fick equation \(\left( {V{\text{O}}_{2} = Q\;\left( {C_{{({\text{a}} - {\text{v}})}} {\text{O}}_{2} } \right)} \right)\) (VO2 oxygen uptake; Q blood flow; C (a-v)O2 arterio-venous O2 difference), which describes convective delivery of oxygen, with Fick’s law of diffusion \(\left( {V{\text{O}}_{2} = D{\text{O}}_{2} \;\left( {P_{\text{mv}} {\text{O}}_{2} - P_{\text{mito}} {\text{O}}_{2} } \right)} \right)\) (DO2 O2 diffusion capacity; P mvO2 microvascular O2 pressure; P mitoO2 mitochondrial O2 pressure) which quantifies the diffusive determinants of VO2 (Wagner 1995). The development of near-infrared spectroscopy (NIRS) allowed non-invasive assessment of the relationship between muscle QO2 and VO2 by measuring the concentration of oxygenated (oxy[Hb + Mb]) and deoxygenated hemoglobin and myoglobin (deoxy[Hb + Mb]), in this way providing information on the oxygenation status at the muscle level. Deoxy[Hb + Mb] in particular has received much attention since it is believed to be a reflection of microvascular O2 extraction (i.e., proportional to C (a-v)O2) (Delorey et al. 2003; Grassi et al. 2003; Ferreira et al. 2005). Additionally, total[Hb + Mb] (as the sum of oxy[Hb + Mb] and deoxy[Hb + Mb]) provides information on the increase in hematocrit (Hct) of the microcirculation with exercise (Davis and Barstow 2013). Microvascular Hct is thought to be the primary determinant of O2 diffusion capacity (DO2) (Poole et al. 2013).
The deoxy[Hb + Mb] pattern has been studied in the transition between constant work rate bouts and during incremental ramp exercise to address the dynamic QO2/VO2m relationship. Ramp exercise can be especially insightful with regard to this dynamic relationship since it incorporates metabolic demands, which range from rest to peak aerobic power. When the deoxy[Hb + Mb] profile was plotted against % peak work rate (%WRpeak), it was initially believed to be best described by a sigmoid model in several populations and conditions (Ferreira et al. 2007; Boone et al. 2009, 2011; DiMenna et al. 2010; Chin et al. 2011; McNarry et al. 2011), pointing at a non-linear QO2/VO2m relationship (Murias et al. 2013). Recently however, Spencer et al. (2011) showed that a double-linear model was more appropriate to fit the deoxy[Hb + Mb] response, especially from moderate to maximal work rates. In this way, a breakpoint in the deoxy[Hb + Mb] response at high intensities was quantified as the work rate at which deoxy[Hb + Mb] started to level-off, indicating that relative microvascular O2 extraction had reached an upper limit during dynamic rhythmic contractions. The underlying mechanisms for this breakpoint have not yet been established. Osawa et al. (2011) and Racinais et al. (2014) related the breakpoint in deoxy[Hb + Mb] to alterations in neuromuscular activity obtained from electromyography recordings, whereas Belotti et al. (2013) showed that the attenuation in the deoxy[Hb + Mb] coincided with the maximal lactate steady state.
Manipulation of pedal rate has been shown to induce different muscle fiber recruitment patterns (Citterio and Agostini 1984; Beelen et al. 1993; Sargeant 1994; Pringle et al. 2003; Bieuzen et al. 2007). Additionally, some studies suggest that convective O2 supply is limited at high pedal rates (>80 rpm) due to the short relaxation phase, especially at high intensities (Buchler et al. 1985; Walloe and Wesche 1988; Hoelting et al. 2001). Despite these possible differences in neuromuscular activity and convective O2 supply, the pattern of deoxy[Hb + Mb] during ramp exercise and constant work exercise did not differ among pedal rates (Ferreira et al. 2006; Zorgati et al. 2013), suggesting a dissociation between muscle oxygenation and activation [in contrast to Osawa et al. (2011) and Racinais et al. (2014)]. In these studies, however, neither the surface electromyography (EMG) and convective O2 supply were assessed nor was an analysis of any breakpoint in the deoxy[Hb + Mb] response performed.
Additionally, it can be suggested that the breakpoint in deoxy[Hb + Mb] (and the possible coincident breakpoints in neuromuscular activation) during incremental ramp exercise demarcates a similar ‘critical intensity’ as the critical power which has been identified as the point that distinguished the heavy from the severe intensity domain in constant work rate transitions, above which there is no longer a steady state in VO2 and blood lactate concentration (Poole et al. 1988). In this context, Barker et al. (2006) showed that, while the general metabolic demand (as assessed from pulmonary VO2) was higher at higher pedal rates for a given external work rate, the VO2 at critical power was not different across the pedal rates. The unanswered question, therefore, is if the breakpoint in deoxy[Hb + Mb], indicative of the attainment of a maximum relative O2 extraction, is mechanistically linked with the breakpoint in muscle activation as functions of metabolic rate or external work rate. In this way, studying the physiological responses (NIRS, EMG and pulmonary gas exchange) to ramp exercise performed at different pedal rates may clarify the mechanistic determinants of the O2 supply to O2 utilization relationship, especially during severe exercise.
The purpose of the present study, therefore, was to provide insight into the underlying mechanisms of the dynamic QO2/VO2m relationship to incremental ramp cycle exercise using different pedal rates to potentially perturb the relationship between muscle deoxy[Hb + Mb], muscle activation (EMG) and general metabolic responses (as quantified by the Respiratory compensation point, RCP, assessed from pulmonary VO2) during ramp cycle exercise. It was hypothesized that, within each pedal rate condition all response breakpoints (EMG, NIRS, RCP) would occur at the same work rate and metabolic rate (VO2), but that across pedal rate conditions all response breakpoints would be driven by metabolic rate rather than external work rate (similar to that observed with critical power during constant work rate exercise) and would therefore, occur at the same %VO2peak, independent of work rate.
Methods
Subjects
Twelve male physically active students (PA students), mean ± SD age 20.6 ± 2.7 years volunteered to take part in this study. The subjects had a mean body mass and mean height of 72.9 ± 6.3 kg and 1.81 ± 0.07 m, respectively, and were all active in a wide variety of non-competitive sport activities. The subjects were informed about the protocol and the aim of the study and signed an informed consent approved by the ethical committee of the Ghent University hospital. A medical history questionnaire and an examination including rest and exercise ECG were performed prior to the start of the study. All subjects were declared to be in good health and none of them presented medical contraindications for participation in the study.
Experimental procedure and protocol
The experimental protocol consisted of three incremental ramp protocols (25 W min−1) to exhaustion preceded by 3 min of baseline cycling on an electromagnetically braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands). One ramp protocol was executed at a pedal rate of 100 rpm, starting from a baseline of 50 W (100rpm50) and two ramp protocols were executed at 60 rpm, starting from a baseline of either 50 W (60rpm50) or 80 W (60rpm80). This latter protocol was conducted so that the protocol would be initiated from a similar baseline VO2 as for the 100rpm50 protocol (determined experimentally prior to the start of this study). We chose to use these elevated baseline work rates of 50 or 80 W to avoid possible changes in mechanical efficiency at very low work rates (Boone et al. 2008). The three ramp exercise tests were performed in random order and were terminated when the subjects could no longer maintain the instructed pedal rate (i.e., <55 rpm in 60rpm50 and 60rpm80 and <95 rpm in 100rpm50), despite strong verbal encouragement. Ten minutes prior to the start of each ramp exercise the subjects performed three repetitions of maximal voluntary contractions (MVC) while seated upright on a bench with their legs hanging above the floor. The subjects were asked to maximally extend their right leg (with the EMG electrodes) against an immovable bar at a knee angle of 100° to obtain a maximal isometric contraction and associated EMG response. The MVC trials were held for at least 5 s and subjects were allowed to rest for at least 2 min in between trials. The entire experimental procedure was conducted within a period of 3 weeks to avoid effects of changes in physical fitness with at least 72 h in between two tests. The subjects were asked to abstain from strenuous exercise and training for at least 24 h prior to their visit to the laboratory.
Measurements
During the exercise tests, \(\dot{V}{\text{O}}_{2}\) was measured continuously on a breath-by-breath basis by means of a computerized O2–CO2 analyzer–flowmeter combination (Jaeger Oxycon Pro, Germany). Prior to the start of each test, the gas analyzers (an O2 analyzer with functioning based on the differential-paramagnetic principle and an infrared CO2 analyzer) and volume transducer (‘triple V’) were calibrated as per manufacturer’s recommendations.
Myoelectric activity was detected by means of surface electromyography (surface EMG) and recorded with bipolar 34 mm diameter Ag–AgCl electrodes (Blue Sensor) at a sampling frequency of 1,000 Hz. The electrodes were placed longitudinally on the distal part of the M. Vastus Lateralis of the right leg. Each electrode site was prepared by shaving, abrading and swabbing the site with diluted ethanol. The reference electrode was placed over the spiny process of prominent cervical vertebrae. The EMG signal was checked for movement artifacts and the wires connected to the electrodes were taped on the thigh of the subjects. To make sure that the electrodes would be placed at the same location of the muscle on the subsequent visits to the laboratory, the electrode sites were pen-marked. Myoelectric signals were relayed from the bipolar electrodes to a Telemyo device (Noraxon Inc., Scottsdale, USA). The iEMG signal of the MVC was averaged for each trial and the highest averaged iEMG was used as the value for MVC.
Muscle oxygenation (oxy[Hb + Mb] and deoxy[Hb + Mb]) was measured by means of a frequency-domain multidistance near-infrared spectroscopy system (Oxiplex TS, ISS, Champaign, Illinois, USA). The NIRS probe consisted of eight light-emitting diodes, operating at wavelengths of 690 and 830 nm and one detector fiber bundle (source–detector distances = 2.0–3.5 cm). The probe was positioned longitudinally on the distal section of the left M. Vastus Lateralis at the same level as the EMG electrodes, and secured with Velcro® straps around the thigh. Prior to the placement of the NIRS probe, the NIRS system was calibrated using manufacturer’s recommended procedures, and the skin was carefully shaved. Pen marks were made on the skin to indicate the margins of the probe to check for any downward sliding during the cycling exercise and for accurate repositioning of the probe on the subsequent visit to the laboratory. The NIRS signals were recorded at a frequency of 25 Hz and afterwards digitally averaged into 1-s values.
Data analysis
General metabolic responses
The second-by-second VCO2 values were expressed as a function of VO2. The gas exchange threshold (GET) of each ramp exercise test was determined by means of the V-slope method, i.e., the point at which VCO2 increases disproportionate to VO2 (Beaver et al. 1986). The respiratory compensation point (RCP) was determined as the second disproportionate increase in ventilation (V E) relative to work rate. The baseline VO2 was calculated as the mean VO2 of the final 90 s of the baseline cycling at 50 W (60rpm50 and 100rpm50) or 80 W (60rpm80). The VO2peak was determined as the highest 30 s average obtained during the exercise test. The second-by-second \(\dot{V}{\text{O}}_{2}\) values were expressed as a function of work rate (WR) and the slope of the VO2/WR relationship (ΔVO2/ΔWR) below (\(S1_{{V{\text{O}}_{2} }}\)) and above (\(S2_{{V{\text{O}}_{2} }}\)) the GET was calculated by means of linear regression analysis. The first and last minutes of the ramp exercise were not taken into account to ensure that the non-linear increases in VO2 (at the onset of the test and the possible leveling-off at VO2peak) would not influence the calculation of \(S1_{{V{\text{O}}_{2} }}\) and \(S2_{{V{\text{O}}_{2} }}\). Additionally, VO2 was expressed as a function of %WRpeak in 5 % WR increments by calculating the 30 s mean VO2 around the WR corresponding to the 5 % increment, to allow intra-individual comparison among the three protocols.
Muscle activation
The raw EMG signals were rectified, band-pass filtered (1,000 Hz) and integrated using commercially available software (MyoResearch2.10, Noraxon Inc., Scottsdale, USA). The iEMG values of each ramp exercise test were averaged over 1-s intervals and normalized to the maximum iEMG obtained during the MVC trials. The change in Mean Power Frequency (MPF) during the ramp exercises was expressed relative to the MPF during baseline cycling which was set at 100 %. Subsequently, VO2 data, expressed relative to VO2peak (%VO2peak) were left-shifted by 20 s to account for the circulatory transit delay between muscle and lung to align changes in ‘muscle VO2’ with changes in iEMG and MPF (Boone et al. 2009; Spencer et al. 2011). Normalized iEMG, MPF and VO2 (%VO2peak) were reduced into 100 equal bins (Boone et al. 2009; Spencer et al. 2011) and iEMG and MPF were expressed as a function of absolute (W), relative (%WRpeak) WR and VO2 (%VO2peak). The pattern of iEMG and MPF was analyzed by means of a double-linear model (Osawa et al. 2011) (SigmaPlot 11.0, Systat Software, CA, USA), yielding two linear regressions with the lowest error sum of squares:
where m represents the slope and b is the y-intercept value. From this double-linear model, the breakpoint (BP) in linearity of iEMG and MPF as functions of WR and VO2 could be determined. Additionally, iEMG and MPF were expressed as functions of %WRpeak in 5 % increments to allow intra-individual comparison between the three protocols.
Muscle (de)oxygenation
The absolute deoxy[Hb + Mb] and total[Hb + Mb] (i.e., the sum of deoxy[Hb + Mb] and oxy[Hb + Mb]) were calculated for baseline cycling at 50 or 80 W (i.e., final 90 s average, f 0) and at the end of the ramp exercise (highest 15 s average). The amplitude (A) of the deoxy[Hb + Mb] and total[Hb + Mb] responses were calculated as the difference between the highest and baseline deoxy[Hb + Mb] or total[Hb + Mb] response. Since it was observed that baseline and peak total[Hb + Mb] was higher in 100rpm50 compared to 60rpm50 and 60rpm80, absolute total[Hb + Mb] values were used for further analysis (see below).
Subsequently, VO2 data, expressed relative to VO2peak (%VO2peak), were left-shifted by 20 s to account for the circulatory transit delay between muscle and lung to align changes in ‘muscle VO2’ with changes in deoxy[Hb + Mb] and total[Hb + Mb] (Boone et al. 2009; Spencer et al. 2011). In this regard, it should be noted that the circulatory transit delay will decrease as cardiac output and blood flow increase with exercise intensity. The deoxy[Hb + Mb] responses were normalized to the total amplitude of the response with 0 % corresponding to baseline cycling (i.e., final 90 s) and 100 % the highest continuous 15 s average values at the end of the ramp exercise.
These normalized VO2, deoxy[Hb + Mb] and absolute total[Hb + Mb] responses for each individual were reduced into 100 equal bins (Boone et al. 2009; Spencer et al. 2011). The deoxy[Hb + Mb] and total[Hb + Mb] values were expressed as functions of absolute work rate (WR), relative work rate (%WRpeak) and VO2 (%VO2peak) and analyzed by means of a double-linear model (see above) similar to that previously described by Spencer et al. (2011). In short, the data set used in the analysis included the data between the point where deoxy[Hb + Mb] and total[Hb + Mb] began a systematic increase above baseline values (based on visual inspection) and the end of the exercise test and the double-linear model was determined by the lowest error sum of squares. This double-linear model yields a linear function for the middle portion of deoxy[Hb + Mb] and total[Hb + Mb] and a linear function for the plateau in deoxy[Hb + Mb] and total[Hb + Mb], with the breakpoint reflecting the intersection of these two linear functions. Additionally, the normalized deoxy[Hb + Mb] (%) and absolute total[Hb + Mb] (µM) were expressed as functions of %WRpeak in 5 % increments to allow comparison between the three protocols.
Statistical analysis
The descriptive statistics were calculated as mean values ± SD. Repeated measures ANOVA (SPSS 19.0) was used to compare the parameters that quantify the pulmonary \(\dot{V}{\text{O}}_{2}\) response (baseline \(\dot{V}{\text{O}}_{2}\), \(\dot{V}{\text{O}}_{2}\) peak, GET, RCP, \(S1_{{V{\text{O}}_{2} }}\), \(S2_{{V{\text{O}}_{2} }}\)), muscle activation [m, b and BP of the iEMG and MPF response as functions of WR and VO2 (%VO2peak)] and muscle deoxygenation [baseline and amplitude of total[Hb + Mb] and deoxy[Hb + Mb]; m, b and BP of total[Hb + Mb] and deoxy[Hb + Mb] response as functions of WR and VO2 (%VO2peak)] between the three protocols (100rpm50, 60rpm50 and 60rpm80). Furthermore, Pearson product-moment correlation was calculated to quantify the relationships between the WR and VO2 (%VO2peak) at RCP and the BPs of the iEMG, MPF, total[Hb + Mb] and deoxy[Hb + Mb] response. Additionally, VO2, iEMG, MPF, deoxy[Hb + Mb] and total[Hb + Mb] values expressed in 5 % WR increments were compared between the three protocols by means of repeated measures ANOVA. In case of significant differences obtained from the repeated measures ANOVA post hoc Tukey tests were performed. The significance level was set at P < 0.05.
Results
The WRpeak was 385 ± 31 W, 377 ± 29 W and 375 ± 29 W for 100rpm50, 60rpm50 and 60rpm80, respectively, and did not differ significantly among the ramp protocols (P = 0.28).
General metabolic responses
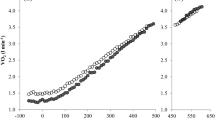
In Fig. 1, the mean VO2 responses to 100rpm50, 60rpm50 and 60rpm80 are presented. In Table 1, baseline VO2, GET, RCP, VO2peak, \(S1_{{V{\text{O}}_{2} }}\) and \(S2_{{V{\text{O}}_{2} }}\) are presented for the three ramp exercise tests.
Baseline VO2 was significantly higher in 100rpm50 and 60rpm80 compared to 60rpm50 (P < 0.001) but did not differ significantly between 100rpm50 and 60rpm80, as intended (P = 0.862). VO2peak did not differ significantly among the protocols (P = 0.749), whereas the work rate at GET and RCP was significantly lower in 100rpm50 compared to 60rpm50 (P = 0.021 and 0.013) and 60rpm80 (P = 0.014 and 0.019). The VO2 at the GET and RCP did not differ significantly among the protocols, both in absolute (P = 0.371 and 0.133, respectively) and relative (%VO2peak) values (P = 0.461 and 0.219, respectively). There was a significant interaction effect (P = 0.021) for the slope of the VO2/WR relationship (\(S1_{{V{\text{O}}_{2} }}\) and \(S2_{{V{\text{O}}_{2} }}\)). The post hoc analysis showed that \(S1_{{V{\text{O}}_{2} }}\) was significantly lower in 100rpm50 compared to 60rpm50 (P < 0.01) and 60rpm80 (P < 0.001), whereas above the GET, no difference was observed in \(S2_{{V{\text{O}}_{2} }}\) among the protocols (P = 0.259). \(S2_{{V{\text{O}}_{2} }}\) was significantly higher than \(S1_{{V{\text{O}}_{2} }}\) for the 100rpm50 protocol only (P < 0.001). Furthermore, VO2 was significantly higher in 100rpm50 compared to 60rpm50 and 60rpm80 from 15 to 70 % WRpeak (Fig. 1).
Muscle activation
The mean iEMG value elicited for MVC was 73 ± 14, 75 ± 12 and 72 ± 12 mV for 100rpm50, 60rpm50 and 60rpm80, respectively (P = 0.859). The mean-normalized iEMG and MPF responses for 100rpm50, 60rpm50 and 60rpm80 as functions of absolute (W) and relative (%WRpeak) WR (in 5 % WR increments) are shown in Fig. 2. In Table 2, the parameters of the double-linear model fit to the iEMG and MPF responses as functions of WR and VO2 (%VO2peak) are presented.
For the iEMG and MPF as functions of WR and %WRpeak the breakpoints (BP) were significantly lower in 100rpm50 compared to 60rpm50 (P < 0.01 for both iEMG and MPF) and 60rpm80 (P = 0.013 and P < 0.01 for iEMG and MPF, respectively). However, for the iEMG and MPF as a function of VO2, the BP did not differ significantly among the protocols (P = 0.098 and P = 0.121 for iEMG and MPF, respectively). The BP in the iEMG/WR and MPF/WR relationships was significantly correlated around the identity line in the three protocols (r = 0.99; P < 0.001 in 100rpm50, 60rpm50 and 60rpm80, respectively).
Muscle oxygenation
The absolute deoxy[Hb + Mb] and total[Hb + Mb] are presented in Table 3. Baseline deoxy[Hb + Mb] was significantly higher in 100rpm50 compared to 60rpm50 (P = 0.024) but not compared to 60rpm80 (P = 0.114). At WRpeak, deoxy[Hb + Mb] was significantly higher in 100rpm50 compared to 60rpm50 and 60rpm80 (P = 0.042 and P = 0.038, respectively). Baseline total[Hb + Mb] was significantly higher in 100rpm50 compared to 60rpm50 and 60rpm80 (P = 0.018 and P = 0.034). At WRpeak total[Hb + Mb] for 100rpm50 was higher compared to 60rpm50 (P = 0.046) but not compared to 60rpm80 (P = 0.093).
The mean deoxy[Hb + Mb] and total[Hb + Mb] responses as functions of WR, VO2 (%VO2peak) and %WRpeak (in 5 % WR increments) are presented in Fig. 3. The parameters of the ‘double linear’ fit to the normalized deoxy[Hb + Mb] and total[Hb + Mb] responses as functions of absolute and relative (%WRpeak) WR, and VO2 (%VO2peak) to 100rpm50, 60rpm50 and 60rpm80 are presented in Table 4. For the deoxy[Hb + Mb] as functions of WR and %WRpeak, the breakpoint (BP) was significantly lower in 100rpm50 compared to 60rpm50 (P < 0.001) and 60rpm80 (P < 0.01). For the deoxy[Hb + Mb] as a function of %VO2peak the BP did not differ significantly among the three protocols (P = 0.142). Statistical analysis of deoxy[Hb + Mb] over 5 %WR increments revealed that deoxy[Hb + Mb] was significantly (P < 0.05) higher in 100rpm50 compared to 60rpm50 and 60rpm80 for 15–80 % WRpeak.
For the total[Hb + Mb] as functions of WR and %WRpeak, the BP was significantly lower in 100rpm50 compared to 60rpm50 (P < 0.001) and 60rpm80 (P < 0.01). For the total[Hb + Mb] as a function of %VO2, the BP did not differ significantly among the three protocols (P = 0.201). Statistical analysis of total[Hb + Mb] over 5 %WR increments revealed that total[Hb + Mb] was significantly (P < 0.05) higher in 100rpm50 compared to 60rpm50 and 60rpm80 for 15–100 % WRpeak.
Pulmonary VO2 vs. muscle oxygenation vs. muscle activation
Statistical analysis revealed that the BP in total[Hb + Mb] (expressed as W, %WRpeak and %VO2peak) was significantly lower (9 ± 5 W) compared to the BP in iEMG (P < 0.01). Also, the BP in iEMG was significantly lower (10 ± 4 W) compared to the BP in deoxy[Hb + Mb] (P < 0.001). Finally, the BP in deoxy[Hb + Mb] was significantly lower (8 ± 5 W) compared to the RCP (P < 0.001). In Fig. 4, the relationships between the breakpoints (in absolute WR, left panels; in %VO2peak, right panels) in total[Hb + Mb], iEMG and deoxy[Hb + Mb], as well as the relationship with RCP are shown.
The BP in deoxy[Hb + Mb] (as a function of absolute WR) was significantly correlated to the BP in total[Hb + Mb] (r = 0.97, r = 0.90, r = 0.93 for 100rpm50, 60rpm50 and 60rpm80, respectively; P < 0.001) and iEMG (r = 0.98, r = 0.99, r = 0.98 for 100rpm50, 60rpm50 and 60rpm80, respectively; P < 0.001) and the RCP (r = 0.99, r = 0.99, r = 0.99 for 100rpm50, 60rpm50 and 60rpm80, respectively; P < 0.001). Although the BPs in VO2 (%VO2peak), in total[Hb + Mb], iEMG and deoxy[Hb + Mb] did not differ among the protocols (see above), the BP in deoxy[Hb + Mb] (expressed as %VO2peak) was significantly correlated to the BP in total[Hb + Mb] (r = 0.63, P = 0.014; r = 0.58, P = 0.027; r = 0.82, P < 0.01 for 100rpm50, 60rpm50 and 60rpm80, respectively) and iEMG (r = 0.68, P = 0.011; r = 0.62, P = 0.022; r = 0.64, P = 0.18 for 100rpm50, 60rpm50 and 60rpm80, respectively), and the RCP (r = 0.78, P < 0.01; r = 0.73, P = 0.016; r = 0.62, P = 0.26 for 100rpm50, 60rpm50 and 60rpm80, respectively).
Discussion
The present study examined the impact of pedal rate on the muscle oxygenation and neuromuscular responses to ramp exercise to provide insight into the leveling-off in deoxy[Hb + Mb] at near-maximal intensity during ramp exercise. The main findings were first, that across the pedal rate conditions the BP in deoxy[Hb + Mb], total[Hb + Mb], iEMG and MPF occurred at a similar %VO2peak, independent of WR. Second, it was observed within each pedal rate condition that the breakpoint in the deoxy[Hb + Mb] pattern (as %VO2peak) was preceded by, but strongly related to the breakpoints in iEMG and MPF, which were in turn preceded by the breakpoint in the total[Hb + Mb] response.
Impact of pedal rate
In the present study, it was observed that the breakpoints in muscle oxygenation (deoxy[Hb + Mb] and total[Hb + Mb]) and muscle activation (iEMG and MPF) occurred at a similar metabolic rate, independent of external work rate, across the pedal rate conditions. The observation that a higher pedal rate induces a greater metabolic rate (as assessed from a higher pulmonary VO2) for a given external work rate, especially at low to moderate intensities, is similar to earlier studies (Jones et al. 2004; Barker et al. 2006). Even though the lower slope of the VO2/W relationship at 100 rpm indicates that the impact of pedal rate decreases at higher intensities, the lower WR with a similar VO2 at the breakpoints in deoxy[Hb + Mb], total[Hb + Mb], iEMG and MPF, and RCP indicates that the whole-body VO2 is still affected by the pedal rate condition at high, but submaximal work rates during ramp exercise, despite no difference in WRpeak and VO2peak (Barker et al. 2006).
The higher general metabolic demand at 100 rpm compared to 60 rpm can likely be attributed to an increased amount of internal work, more pronounced ancillary muscle activation for stabilization and/or to an altered muscle fiber activation at higher pedal rates. It has been suggested that a less-efficient energy transfer from the legs to the cranks at higher pedal rates (Kautz and Neptune 2002) alone or in combination with an insufficient adaptation in the muscle activation and deactivation dynamics (Neptune and Kautz 2001), especially at very low intensities, can affect internal work, even though a baseline work rate of 50 W was used to minimize the effect of changes in internal work (Boone et al. 2008). It is possible that the O2 cost for additional internal work, especially in subjects not used to cycling-specific exercise, at high pedal rates gradually decreases as work rate increases and this might explain the lower \(S1_{{V{\text{O}}_{2} }}\) at 100 rpm compared to 60 rpm. Jones et al. (2004) also suggest that a higher pedal rate could induce an increased proportional contribution of type II fibers early in the ramp exercise to meet the external work rate (Beelen et al. 1993; Sargeant 1994), whereas at lower pedal rates fast-twitch fibers are recruited more progressively as work rate increases. Since metabolic efficiency is lower in fast-twitch compared to slow-twitch muscle fibers (Crow and Kushmerick 1982), this possible disturbance in the muscle fiber recruitment pattern at high pedal rate might contribute to the lower \(S1_{{V{\text{O}}_{2} }}\) at 100 rpm. It is traditionally presumed, however, that exercise below the GET requires such low forces that few fast-twitch fibers are recruited.
Muscle fiber characteristics in combination with the sequential recruitment of muscle fibers types (i.e., progressively increasing recruitment of fast-twitch fibers: the size principle) have been suggested to underpin the sigmoid deoxy[Hb + Mb] response to incremental ramp exercise (Boone et al. 2008; Chin et al. 2011; Murias et al. 2013). In this context, it was shown that the balance between muscle O2 delivery and consumption differs at rest and during exercise (McDonough et al. 2005) and that different patterns of C (a-v)O2 emerge (Ferreira et al. 2006) in rat slow-twitch and fast-twitch muscles. These results indicate that fast-twitch muscles have lower resting and submaximal exercise Q m values (y-intercept closer to zero) and rely to a greater extent on fractional O2 extraction and thus a more pronounced muscle O2 diffusion to adjust to changes in metabolic demand. Hence, if cycling at a high pedal rate (i.e., 100 rpm) disturbs the sequential recruitment of muscle fibers, with a more pronounced contribution of fast-twitch fibers earlier into the incremental exercise (Beelen et al. 1993; Sargeant 1994), this might impact the deoxy[Hb + Mb] and total[Hb + Mb] response to ramp exercise. More specifically, as seen in the present study, deoxy[Hb + Mb] and total[Hb + Mb] would be shifted to the left at higher pedal rates following the fiber type-specific responses in convective O2 supply and O2 utilization. It should be noted that this is the first time that total[Hb + Mb] was fit by a double-linear model yielding a breakpoint in the linearity at a similar (but slightly lower; see below) work rate compared to the breakpoint in deoxy[Hb + Mb].
Muscle oxygenation and activation during ramp exercise
As discussed above, VO2 can be described by the interaction of the Fick equations for convective and diffusive determinants of the delivery of O2 to the myocyte mitochondria (Wagner 1995; Poole et al. 2013). According to Fick’s Law of Diffusion, muscle oxygen uptake is determined by both the O2 diffusion capacity (DO2) and O2 pressure gradient between capillaries and myocyte (PO2cap − PO2mito), where VO2m = DO2 × (PO2cap − PO2mito). The O2 diffusion capacity depends on the surface area for diffusion and is predominantly related to red blood cell (RBC) flux and velocity through the capillaries and thus to microvascular hematocrit (Hct) (for review see Poole et al. 2013). At rest, most capillaries support RBC flux but microvascular Hct is less than that of systemic circulation (Klitzman and Duling 1979; Kindig and Poole 1998; Kindig et al. 2002; Russell et al. 2003). Recently, Davis and Barstow (2013) suggested that changes in total[Hb + Mb], measured with NIRS during exercise, reflect an increase in microvascular [Hb] and thus Hct, since muscle [Mb] presumably remains unchanged throughout exercise. Therefore, the increase in total[Hb + Mb] observed in the present study during the ramp protocols at different pedal rates points at an increase in microvascular Hct. According to the calculations of Davis and Barstow (2013), the current increase in total[Hb + Mb] of 19.9 % (6.1–35.3 %) would reflect a 50 % increase in [Hb] and an increase in capillary Hct from 22 to 33 %. At ~83 %VO2peak total[Hb + Mb] started to level-off, indicating that microvascular Hct, and thus DO2 reached a ceiling. In addition, intramuscular pressure at higher intensity contractions (near-end exercise) probably limits a further increase in blood flow through the capillaries (Kagaya and Ogita 1992).
Following the leveling-off in total[Hb + Mb] and thus DO2, the only mechanism by which VO2m can increase is by increasing the O2 pressure gradient (or microvascular O2 extraction), which is reflected by the deoxy[Hb + Mb] response. However, deoxy[Hb + Mb] also reaches a ceiling at ~88 %VO2peak in the ramp exercises at 60 rpm [i.e., at a slightly higher work rate (20 ± 6 W) compared to total[Hb + Mb]]. This indicates that relative microvascular O2 extraction also has an upper limit during dynamic rhythmic contractions. Since both DO2 (as total[Hb + Mb]) and relative O2 extraction (as deoxy[Hb + Mb]) level-off at 83–90 %VO2peak, it seems likely that a further increase in pulmonary (i.e., whole-body) VO2 may reflect an increased aerobic energy turnover in other leg muscles or muscle groups not observed by the NIRS.
From the present study, it appears that the muscle oxygenation responses are closely related to the neuromuscular activation pattern. The EMG threshold is highly correlated with the leveling-off in total[Hb + Mb] and deoxy[Hb + Mb], occurring at a work rate between the breakpoints of total[Hb + Mb] and deoxy[Hb + Mb] (BPtotal[Hb + Mb] < BPiEMG < BPdeoxy[Hb + Mb] < RCP). The BP in iEMG reflects a change in intramuscular action potentials due to the recruitment of new motor units, predominantly consisting of fast-twitch muscle fibers, and to increased rate coding of already recruited motor units, to compensate for the loss in contractility of fatiguing motor units, which can be assessed from the sudden drop in MPF (Takaishi et al. 1992). Osawa et al. (2011) and Racinais et al. (2014) observed a similar correlation between the EMG threshold and the BP in deoxy[Hb + Mb], although in that study the BP in deoxy[Hb + Mb] preceded the EMG threshold, which is in contrast to the present study. It is possible that a plateau in DO2 might be indicative of muscle O2 insufficiency as work rate continues to increase. The subsequent increase in anaerobic energy production might affect group III and IV afferents and central motor output, altering the recruitment and firing rate of motor units and intracellular action potentials (Light et al. 2008; Arabadhziev et al. 2010). This will induce a non-linear burst of iEMG as force and power output continue to increase. Additionally, it appears from the coincident decrease in MPF that the newly recruited fibers quickly fatigue.
The precipitating event-plateauing of DO2 (as total[Hb + Mb]) and relative O2 extraction (as deoxy[Hb + Mb]) versus recruitment of easily fatiguable fibers (as assessed from iEMG and MPF) is currently unclear. However, the cascade of events occurring in response to ramp exercise described above clearly suggests a close relationship between the mechanisms underpinning the muscle oxygen uptake and neuromuscular activity pattern (in combination with the specific characteristics of the muscle fiber types). The work rate domain between 80–90 % WRpeak (83–91 % VO2peak) provides a crucial intensity that might be determinant for the exercise tolerance, especially since the RCP was closely related to, but slightly higher than, the breakpoint in deoxy[Hb + Mb], similar to the study of Oussaidene et al. (2013). When accounting for the circulatory lag time (~6 s at near-maximal exercise) between muscle and lung, the RCP would closely approximate the BP in deoxy[Hb + Mb]. The observation that the breakpoints in iEMG, total[Hb + Mb] and deoxy[Hb + Mb], and RCP occur at a lower work rate but a similar metabolic demand (VO2) is in line with the study of Barker et al. (2006), in which it was shown that the VO2 at the critical power was similar, despite a lower external work rate, at 100 rpm compared to 60 rpm. It is possible that critical power could be related to the cascade of events reported in the present study especially since critical power has been shown to correspond to 80–90 % VO2peak (Poole et al. 1988; Smith et al. 1999; Hill et al. 2002) and critical torque has recently been associated with change in neuromuscular activity (Burnley et al. 2012). In this context, it will be important to study muscle oxygenation responses above and below the critical power. While the current observations relate to incremental ramp exercise, it remains to be confirmed if these observations and relationships are also valid for constant work rate exercise. Specifically, do the changes in NIRS, EMG and gas exchange that take place during incremental exercise in the vicinity of the critical power work rate and/or metabolic rate (~80–90 % VO2max) also describe the changes in muscle oxygenation and recruitment when constant work rate exercise is performed above vs below critical power. Previous studies that found correlations between responses during incremental exercise (e.g., breakpoints in RCP, EMG, or NIRS) and those observed during constant work rate exercise (e.g., critical power and/or maximum lactate steady state) (Belotti et al. 2013) suggest that this is the case. However, further focused research is necessary to confirm the potential mechanistic similarities between incremental and constant work rate responses.
Conclusions
In summary, the present study showed that there is a close relationship between the pulmonary ‘whole-body’ VO2, muscle (de)oxygenation response and neuromuscular activity during incremental exercise. A leveling-off in total[Hb + Mb] (as a reflection of DO2) preceded the sudden increase in iEMG and decrease in MPF (as reflections of additional recruitment of highly fatiguable fibers) which in turn preceded the leveling-off in deoxy[Hb + Mb] (as a reflection of microvascular O2 extraction). Additionally, it was shown that RCP was closely matched to the breakpoint in deoxy[Hb + Mb]. Furthermore, it was shown that changing pedal rate can induce a change in the external work rate to metabolic work rate relationship but not the metabolic rate at which the breakpoints in neuromuscular activity and muscle deoxygenation occur, confirming the close interrelationship between muscle activity, muscle oxygenation and whole-body VO2 during incremental ramp exercise. The observation that the breakpoints are associated with metabolic rate rather than external work rate suggests that the mechanisms underpinning the breakpoint in deoxy[Hb + Mb] might be closely related to the critical power concept demarcating the heavy from the severe intensity domain during constant work rate exercise.
Abbreviations
- 100rpm50:
-
100 rpm starting from baseline 50 W
- 60rpm50:
-
60 rpm starting from baseline 50 W
- 60rpm80:
-
60 rpm starting from baseline 80 W
- C (a-v)O2 :
-
Arterio-venous O2 difference
- Deoxy[Hb + Mb]:
-
Concentration of deoxygenated hemo- and myoglobin
- DO2 :
-
O2 diffusion capacity
- iEMG:
-
Integrated electromyography
- GET:
-
Gas exchange threshold
- Hct:
-
Hematocrit
- MPF:
-
Mean power frequency
- PO2 :
-
O2 pressure
- QO2 :
-
Oxygen delivery
- RCP:
-
Respiratory compensation point
- \(S1_{{V{\text{O}}_{2} }}\) :
-
Slope of the VO2/WR relationship below the GET
- \(S2_{{V{\text{O}}_{2} }}\) :
-
Slope of the VO2/WR relationship above the GET
- Total[Hb + Mb]:
-
Summation of concentration of deoxy[Hb + Mb] and oxy[Hb + Mb]
- VO2m :
-
Muscle oxygen uptake
References
Arabadhziev TI, Dimitrov VG, Dimitrova NA, Dimitrov GV (2010) Interpretation of EMG integral or RMS and estimates of ‘neuromuscular efficiency’ can be misleading in fatiguing contraction. J Electromyogr Kinesiol 20:223–232
Barker T, Poole DC, Noble LM, Barstow TJ (2006) Human critical power-oxygen uptake relationship at different pedal rates. Exp Physiol 91:621–632
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Beelen A, Sargeant AJ, Lind A, De Haan A, Kernell D, Van Mechelen W (1993) Effect of contraction velocity on the pattern of glycogen depletion in human muscle fiber types. In: Sargeant AJ, Kernell D (eds) Neuromuscular fatigue, North Holland, Amsterdam, pp 93–95
Belotti C, Calabria E, Capelli C, Pogliaghi S (2013) Determination of maximal lactate steady state in healthy adults: can NIRS help? Med Sci Sports Exerc 45:1208–1216
Bieuzen F, Lepers R, Vercruyssen F, Hausswirth C, Brisswalter J (2007) Muscle activation during cycling at different cadences: effect of maximal strength capacity. J Electromyogr Kinesiol 17:731–738
Boone J, Koppo K, Bouckaert J (2008) The VO2 response to submaximal ramp cycle exercise: influence of ramp slope and training status. Respir Physiol Neurobiol 161:291–297
Boone J, Koppo K, Barstow TJ, Bouckaert J (2009) Pattern of deoxy[Hb + Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol 105:851–859
Boone J, Koppo K, Barstow TJ, Bouckaert J (2011) Effect of exercise protocol on deoxy[Hb + Mb]: incremental step versus ramp exercise. Med Sci Sports Exerc 42:935–942
Buchler B, Magder S, Roussos C (1985) Effects of contraction frequency and duty cycle on diaphragmatic blood flow. J Appl Physiol 58:265–273
Burnley M, Vanhatalo A, Jones AM (2012) Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol 113:215–223
Chin LMK, Kowalchuk JM, Barstow TJ, Kondo N, Amano T, Shiojiri T, Koga S (2011) The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J Appl Physiol 111:1259–1265
Citterio G, Agostini E (1984) Selective activation of quadriceps muscle fibers according to bicycling rate. J Appl Physiol 57:371–379
Crow MT, Kushmerick MJ (1982) Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79:147–166
Davis ML, Barstow TJ (2013) Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. Respir Physiol Neurobiol 186:180–187
Delorey DS, Kowalchuk JM, Paterson DH (2003) Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95:113–120
DiMenna FJ, Bailey SJ, Jones AM (2010) Influence of body position on muscle deoxy[Hb + Mb] during ramp cycle exercise. Respir Physiol Neurobiol 173:138–145
Ferreira LF, Townsend DK, Lutjemeijer BJ, Barstow TJ (2005) Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol 98:1820–1828
Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ (2006) Effects of pedal frequency on estimated muscle microvascular O2 extraction. Eur J Appl Physiol 96:558–563
Ferreira LF, Koga S, Barstow TJ (2007) Dynamics of non-invasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 103:1999–2004
Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P (2003) Muscle oxygenation and pulmonary gas exchange kinetics during cycle exercise on-transitions in humans. J Appl Physiol 95:149–158
Hill DW, Poole DC, Smith JC (2002) The relationship between power and the time to achieve VO2max. Med Sci Sports Exerc 34:709–714
Hoelting BD, Scheuermann BW, Barstow TJ (2001) Effect of contraction frequency on leg blood flow during knee extension exercise in humans. J Appl Physiol 91:671–679
Jones AM, Campbell IT, Pringle JSM (2004) Influence of muscle fibre type and pedal rate on the VO2-work rate slope during ramp exercise. Eur J Appl Physiol 91:238–245
Kagaya A, Ogita F (1992) Blood flow during muscle contraction and relaxation in rhythmic exercise at different intensities. Ann Physiol Anthrop 11:251–256
Kautz SA, Neptune RR (2002) Biomechanical determinants of pedaling energetics: internal and external work are not independent. Exerc Sport Sci Rev 30:159–165
Kindig CA, Poole DC (1998) A comparison of the microcirculation in the rat spinotrapezius and diaphragm muscles. Microvasc Res 55:249–259
Kindig CA, Richardson TE, Poole DC (2002) Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol 92:2513–2520
Klitzman B, Duling BR (1979) Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol Heart Circ Physiol 237:H481–H490
Light AR, Hughen RW, Zhang K, Rainier J, Liu Z, Lee J (2008) Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100:1184–1201
McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC (2005) Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563:903–913
McNarry MA, Welsman JR, Jones AM (2011) Influence of training and maturity status on the cardiopulmonary responses to ramp incremental cycle and upper body exercise in girls. J Appl Physiol 110:375–381
Murias JM, Spencer MD, Keir DA, Paterson DH (2013) Systemic and vastus lateralis muscle blood flow and O2 extraction during ramp incremental cycle exercise. Am J Physiol Regul Integr Comp Physiol 304:R720–R725
Neptune RR, Kautz SA (2001) Muscle activation and deactivation dynamics: the governing properties in fast cyclical human movement performance. Exerc Sports Sci Rev 29:76–81
Osawa T, Kime R, Hamaoka T, Katsumura T, Yamamoto M (2011) Attenuation of muscle deoxygenation precedes EMG threshold in normoxia and hypoxia. Med Sci Sports Exerc 43:1406–1413
Oussaidene K, Prieur F, Bougault V, Borel B, Matran R, Mucci P (2013) Cerebral oxygenation during hyperoxia-induced increase in exercise tolerance for untrained men. Eur J Appl Physiol 113:2047–2056
Poole DC, Ward SA, Gardner GW, Whipp BJ (1988) Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31:1265–1279
Poole DC, Copp SW, Ferguson SK, Musch TI (2013) Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98:1645–1658
Pringle JSM, Doust JH, Carter H, Tolfrey K, Jones AM (2003) Effect of pedal rate on primary and slow-component oxygen uptake responses during heavy-cycle exercise. J Appl Physiol 94:1501–1507
Racinais S, Buchheit M, Girard O (2014) Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front Physiol 5:142
Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI (2003) Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 285:H251–H258
Sargeant AJ (1994) Human power output and muscle fatigue. Int J Sports Med 15:116–121
Smith JC, Dangelmaier BS, Hill DW (1999) Critical power is related to cycling time trial performance. Int J Sports Med 20:374–378
Spencer MD, Murias JM, Paterson DH (2011) Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol 112:3349–3360
Takaishi T, Ono T, Yasuda Y (1992) Relationship between muscle fatigue and oxygen uptake during cycle ergometer exercise with different ramp slope increments. Eur J Appl Physiol 65:335–339
Wagner PD (1995) Muscle O2 transport and O2 dependent control of metabolism. Med Sci Sport Exerc 27:47–53
Walloe L, Wesche J (1988) Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol 405:257–273
Zorgati H, Collomp K, Amiot V, Prieur F (2013) Effect of pedal cadence on the heterogeneity of muscle deoxygenation during moderate exercise. Appl Physiol Nutr Metab 38:1206–1210
Conflict of interest
This study was performed without any conflict of interest, financial or otherwise.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jean-René Lacour.
Rights and permissions
About this article
Cite this article
Boone, J., Barstow, T.J., Celie, B. et al. The impact of pedal rate on muscle oxygenation, muscle activation and whole-body VO2 during ramp exercise in healthy subjects. Eur J Appl Physiol 115, 57–70 (2015). https://doi.org/10.1007/s00421-014-2991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-2991-x