Abstract
Purpose
The purpose of the present study was to characterize hypothesized relationships among fatigability and cardiorespiratory fitness in individuals with chronic motor-incomplete SCI (iSCI) during treadmill walking. The theoretical framework was that exacerbated fatigability would occur concomitantly with diminished cardiorespiratory fitness in people with iSCI.
Methods
Subjects with iSCI (n = 8) and an able-bodied reference group (REF) (n = 8) completed a 6-min walking bout followed by a walking bout of 30-min or until volitional exhaustion, both at a self-selected walking speed. Fatigability was assessed using both perceived fatigability and performance fatigability measures. Pulmonary oxygen uptake kinetics (VO2 on-kinetics) was measured breath-by-breath and changes in deoxygenated hemoglobin/myoglobin concentration (∆[HHb]) of the lateral gastrocnemius was measured by near-infrared spectroscopy. Adjustment of VO2 and ∆[HHb] on-kinetics were modeled using a mono-exponential equation.
Results
Perceived fatigability and performance fatigability were 52% and 44% greater in the iSCI group compared to the REF group (p = 0.003 and p = 0.004). Phase II time constant (τp) of VO2 on-kinetics and ∆[HHb] ½ time during resting arterial occlusion were 55.4% and 16.3% slower in iSCI vs REF (p < 0.01 and p = 0.047, respectively).
Conclusions
The results of the present study may suggest that compromised O2 delivery and/or utilization may have contributed to the severity of fatigability in these individuals with iSCI. The understanding of the extent to which fatigability and VO2 and Δ[HHb] on-kinetics impacts locomotion after iSCI will assist in the future development of targeted interventions to enhance function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 282,000 people are living with spinal cord injuries (SCI) in the United States (SCI Facts and Figures 2016). The majority of the reported injuries are classified as incomplete SCI (iSCI) in which partial sensory and/or motor function is preserved below the level of the lesion (Kirshblum and Waring 2014). Partial preservation of nervous system function increases the potential for recovering locomotor capabilities in this subset of the SCI community (Burns et al. 1997). Despite the possibility for regaining ambulatory function, the metabolic cost of walking is often elevated in people with iSCI (Waters and Lunsford 1985; Waters and Mulroy 1999; Kuo and Donelan 2010; Gollie et al. 2017). Therefore, people with iSCI may be more susceptible to activity-induced fatigue that further contributes to their physical activity limitations.
Fatigability is defined as a determinant characteristic of fatigue comprised of both performance fatigability and perceived fatigability (Enoka and Duchateau 2016). Performance fatigability describes a decline in force, endurance, power, speed, reactivity, or accuracy of performance of a given activity or task. Perceived fatigability on the other hand describes an individual’s self-reported feeling of tiredness as a function of the duration and intensity of the task or activity. Several studies have demonstrated greater skeletal muscle fatigability following SCI (Shields 1995; Papaiordanidou et al. 2014), which is a potential contributor to whole-body performance fatigability. Conversely, given the potential disassociation between physiological and psychological responses to exercise (Chaudhuri and Behan 2004; Lewis et al. 2007) it is not clear if perceived fatigability accurately reflects physiological changes in this population. Yet, when measurement endpoints are volitional, perceptions of tiredness or weariness may influence performance and test outcomes.
Adequate cardiorespiratory function and capacity is essential for sustaining physical activity at any level. Given the reported declines in cardiorespiratory fitness (Haas et al. 1986), autonomic nervous system dysfunction (West et al. 2013), and skeletal muscle alterations (Shields 1995; Biering-Sørensen et al. 2009) following SCI, compromised oxygen (O2) delivery and/or utilization may restrict physical performance and physical activity tolerance beyond the limits imposed by physical limitations resulting from the level of the spinal lesion. Furthermore, slowed pulmonary oxygen uptake kinetics (VO2 on-kinetics) during moderate-intensity exercise (i.e., below anaerobic threshold) has been proposed as a marker for fatigability (Grassi et al. 2011).
The purpose of the present study was to assess fatigability and VO2 and muscle deoxygenation on-kinetics in individuals with iSCI during treadmill walking. The theoretical framework was that exacerbated fatigability would occur concomitantly with diminished VO2 and muscle deoxygenation on-kinetics in people with iSCI.
Methods
Subjects
Subjects in with iSCI C and D (n = 8) according to the American Spinal Association Impairment Scale (AIS) (Kirshblum and Waring 2014) and a reference (REF) group (n = 8) were included in this study. Inclusion criteria for the iSCI group included subjects: (1) 18 years of age or older, (2) able to stand with minimal assistance from one other person and initiate and complete at least one step independently with or without assistive walking aids, and (3) able to walk safely on a treadmill. REF group included subjects without iSCI and otherwise healthy who had not engaged in structured exercise for at least the previous 6 months prior to enrollment. All subjects were free of any significant orthopedic complications, spasms or contractures preventing safe ambulation on the treadmill, known cardiovascular, pulmonary, or metabolic diseases, HIV infection or use of antiretroviral therapy, and severe psychiatric disease. Subjects were recruited from the greater Washington D.C. metropolitan area and enrolled on a convenience basis. The protocol and procedures were approved by the Institutional Review Board of George Mason University (#618911-6). Verbal and written explanations of the experimental protocol and risks related to testing procedures were presented to all subjects and written informed consent was obtained from all individual subjects prior to voluntary participation in this study.
Pre-experimental procedures
The study used a cross-sectional design comparing two groups; one group with iSCI and a non-injured REF group. All subjects performed the following assessments in the stated order, after informed consent and formal enrollment into the study. Each subject completed a resting arterial occlusion test to determine muscle deoxygenation capacity of the left lateral gastrocnemius as assessed by near-infrared spectroscopy (NIRS) (Erickson et al. 2013). This procedure was followed by a constant work-rate (CWR) treadmill test to estimate fatigability and pulmonary VO2 and muscle deoxygenation on-kinetics. All subjects completed a familiarization period to identify their preferred walking speed on the treadmill. Blood pressure and heart rate were obtained in the seated position prior to initiation of the CWR treadmill test following ≈2 min of rest.
Resting arterial occlusion
Subjects were placed in a seated position and fitted with a blood pressure cuff (Hokanson, Bellevue, WA, USA) at the level of the thigh just above the knee and on the same leg as the NIRS optode. The NIRS optode was placed on the belly of the left lateral gastrocnemius muscle. The cuff was rapidly inflated to ≥220 mmHg to completely occlude venous and arterial blood flow and desaturate the muscle hemoglobin/myoglobin. Complete hemoglobin/myoglobin desaturation was operationally defined as the observance of a sustained plateau (at least 30 s) in change in oxygenated (Δ[O2Hb]) and deoxygenated (Δ[HHb]) hemoglobin/myoglobin concentrations with minimal change in total (∆[tHb]) hemoglobin/myoglobin concentration. Skeletal muscle deoxygenation capacity (∆[HHb]capacity), was determined by assessing the amplitude change in the Δ[HHb] variable from the onset of cuff inflation to the end of the occlusion test. The time to reach 50% of the plateau of Δ[HHb]capacity (Δ[HHb] ½ time) was calculated to index the rate of ∆[HHb] during resting arterial occlusion (Wilson et al. 1989).
Exercise protocol
The CWR treadmill protocol included two bouts of walking separated by 6 min of rest. Bout 1 consisted of 6 min of treadmill walking at the subject’s self-selected walking speed. Bout 2 included treadmill walking until volitional exhaustion (defined as the point the subject was no longer able to sustain the self-selected walking speed) or attainment of a sustained walking interval of 30 min and was performed at the same speed as Bout 1. The cut-off of 30 min was selected based on previous observations and the assumption that none of the subjects in the iSCI group would be able to walk on the treadmill for 30 min. Subjects were in a standing rest position prior to the onset of Bouts 1 and 2, however, were allowed to rest in a seated position for 4 min following the completion of Bout 1. CWR tests were performed on a standard motorized treadmill (Trackmaster TMX22). All subjects were allowed to use the handrails for balance during the CWR treadmill test but were asked not to unload the lower extremity during the test. All walking bouts were performed with complete weight-bearing by the lower extremity and under voluntary control without the use of adjuvant therapies (i.e., body-weight support, neuromuscular electrical stimulation, robotics, manual assistance).
Materials
Pulmonary VO2 was measured continuously during the CWR test using breath-by-breath open circuit spirometry (Ultima™ CardiO ®2 , Medical Graphics Corp, St. Paul, MN, USA). Heart rate (HR) was measured at rest and during the CWR test by 12-lead electrocardiography (ECG) (Mortora Instrument Inc., Milwaukee, WI, USA). After standard calibration and subject preparation, pulmonary gas analysis was performed over the entire CWR test. VO2 measurements were made via breath-by-breath and the data points were 8 number averages in which no breath was counted in two bins.
NIRS is used to study skeletal muscle oxygenation and oxidative metabolism (Hamaoka et al. 2007). Muscle oxygenation indices were obtained using a continuous-wave NIRS (Oxymon MK-III, Artinis Medical Systems, The Netherlands) with spatial resolution. The NIRS device consists of emitting continuous-wave light into the tissue detecting changes in ∆[O2Hb], ∆[HHb], and ∆[tHb] (Hamaoka et al. 2007). The three transmitters each emit two wavelengths (760 and 850 nm) of light at three separate transmitter–receiver distances (30, 35, and 40 mm). As a default setup to maximize penetration, all data analysis was conducted on a 40-mm transmitter–receiver channel using a sampling rate of 1000 Hz per second. The NIRS system was calibrated prior to each data collection using the calibration bin supplied by the manufacturer. To account for potential differences in adipose tissue thickness between groups, which has been shown to influence the accuracy of the NIRS recordings (McCully and Hamaoka 2000), a skinfold measurement was obtained at the site of the NIRS optode placement.
Data analysis
Fatigability was determined by both perceived and performance fatigability measures. Perceived fatigability was assessed at the completion of walking Bout 2 of the CWR treadmill test using the method by Schnelle et al. (2012). Immediately following the completion of walking Bout 2, subjects were asked if they felt tired, energetic, or the same compared to before completing the bout (Table 1). If the subject reported feeling tired, he/she were asked if they felt a little more tired (score of 5), somewhat more tired (score of 6), or extremely more tired (score of 7). The same procedure was followed if they reported feeling energetic. Therefore, higher scores reflect greater perceived fatigability at the completion of walking. Performance fatigability was calculated during Bout 2 of the CWR treadmill test as the total time walked in seconds. The test was terminated at 30 min if the subject was able to walk for this entire duration.
During low-to-moderate exercise intensities VO2 on-kinetics has shown potential prognostic value for populations such as congestive heart failure (Schalcher et al. 2003) as well as a marker of fatigability (Grassi et al. 2011). VO2 on-kinetics was calculated from the initiation of phase II of VO2 to the end of walking Bout 1 in the absence of a slow component. If a slow component was present, VO2 on-kinetics were calculated from the onset of phase II and ending at the 3rd minute of walking Bout 1 to exclude the slow component from the analysis. VO2 data were analyzed using a curvilinear least squares fitting procedure (OriginLab, 2016, Northampton, MA, USA) and a mono-exponential model as described in Eq. (1) (Ozyener et al. 2001; Jones and Poole 2005).
where ∆VO2(t) is VO2 at any point in time throughout the exercise transient; BSL is the baseline steady-state VO2 during standing rest prior to the start of walking; AMP is the amplitude of the increase in VO2 above BSL; τp is the time constant (i.e., time taken to reach 63% of the steady-state response); and TDp is the time-delay. Breaths determined to be errant such as coughing, swallowing and sighing were excluded by omitting falling breaths outside a 99% prediction band (Lamarra et al. 1987; Rossiter et al. 2000). The individual breath-by-breath responses for walking Bout 1 were then time-aligned to correspond to the start of the bout (i.e., time point zero), linearly interpolated on a second-by-second basis, and time-averaged in 5-s bins to reduce the “noise” and increase the confidence of the parameter estimation (Ozyener et al. 2001).
Muscle ∆[HHb] on-kinetics were determined during Bout 1 of the CWR test as this variable is considered an estimate of fractional O2 extraction (Grassi and Quaresima 2016). ∆[HHb] on-kinetics were calculated using the process as described previously by Chin and associates (Chin et al. 2010). Briefly, ∆[HHb] was time-aligned to the onset of walking Bout 1 (time = 0) and second-by-second ∆[HHb] was generated by averaging 10 data points per second, then further averaged into 10-s time bins. The time-delay for the ∆[HHb] response (TD-∆[HHb]) was determined using second-by-second data and defined as the first increasing point that consistently remained above the nadir of the signal. ∆[HHb] was modeled using an exponential function as described in Eq. (1). The model was fit to 90 s immediately following TD-∆[HHb] to determine ∆[HHb] time constant (τ-∆[HHb]). Mean response time (∆[HHb]-MRT) was calculated as the sum of the TD∆-[HHb] and τ-∆[HHb], which is suggested to reflect adjustments in muscle O2 utilization and microvascular blood flow (Chin et al. 2010). The ∆[HHb] amplitude (∆[HHb]AMP) was determined as the difference between the TD-∆[HHb] to end of the 90-s model fit.
Statistical analyses
Fatigability, on-kinetic estimates of VO2 and ∆[HHb], and resting arterial occlusion measures were analyzed using one-way analysis of variance (ANOVA) for two independent groups. Pearson product moment correlation coefficients (r) was used to assess relationships amongst walking speed, τp and ∆[HHb]-MRT. Statistical significance was set at p < 0.05 for two-tailed hypotheses. All values are expressed as mean ± SD. Statistical analyses were performed using SPSS version 19 (IBM, Inc., Armonk, NY, USA).
Results
Over the course of a 2-year period, 12 individuals with SCI were screened for participation in the study. One individual was excluded based on the preliminary phone screening and three individuals were excluded based on in-person evaluations for eligibility. Eight individuals completed the in-person screening, signed the informed consent form, and were enrolled into the study (Table 2).
Resting arterial occlusion test
One subject from the REF group declined to complete the arterial occlusion, and therefore, was excluded from the analysis. ∆[HHb] during resting arterial occlusion are presented in Table 3. ∆[HHb] ½ time was 16.3% slower in the iSCI group compared to the REF group (185.9 ± 28.7 vs 157.9 ± 18.9 s, p = 0.047). The iSCI group had a ∆[HHb]capacity 18.4% less than the REF group (8.9 ± 5.8 vs 10.7 ± 5.0 a.u, p = 0.521).
Fatigability
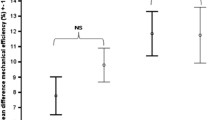
The iSCI group walked at a significantly slower self-selected walking speed compared to the REF group (0.4 ± 0.2 vs 1.3 ± 0.2 m/s; p < 0.01). After Bout 2 of CWR test, subjects with iSCI reported a perceived fatigability score 52% greater than the REF group (5.8 ± 0.7 vs 3.4 ± 1.8; p = 0.003) (Fig. 1). All 8 subjects with iSCI reported feeling more tired after completing walking Bout 2 compared to only three participants in the REF group. The remaining subjects of the REF group reported feeling “neither tired nor energetic” or “more energetic”. The performance fatigability score was 44% higher in subjects with iSCI compared to REF subjects (1153.4 ± 529.5 vs 1800 ± 0.00 s; p = 0.004). Only two subjects with iSCI were able to complete the entire 30 min (i.e. 1800 s) of Bout 2 of CWR test compared to all 8 REF subjects.
Perceived fatigability (a) and performance fatigability (b) for incomplete spinal cord injury (iSCI) (black bars) and reference (REF) (white bars) subjects following walking Bout 2 of the constant work-rate (CWR) treadmill test. Perceived fatigability scores are reported as indicated by Table 1 and performance fatigability is reported in seconds (s). Bars represent group means and error bars represent standard deviations. *Significant difference (p ≤ 0.05) between groups
VO2 on-kinetic estimates
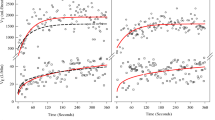
The VO2 on-kinetic profiles of a representative subject from the iSCI and REF groups are displayed in Fig. 2. Analysis of VO2 on-kinetics is presented in Table 4. The intensity of walking Bout 1 was within the moderate-intensity domain in seven of the eight iSCI subjects and all eight of the REF subjects as determined by the absence of a slow component. The iSCI group had a τp 55.4% slower than the REF group (41.2 ± 7.7 vs 23.3 ± 6.5 s; p < 0.01). AMP was 19.4% lower in the iSCI compared to the REF group (612.7 ± 127.9 vs 744.6 ± 173.9 ml/min; p = 0.106). τp was correlated with walking speed (r = −0.77; p < 0.01).
a Depicts VO2 on-kinetic response of a representative incomplete spinal cord injured (iSCI) subject during Bout 1 of the constant work-rate (CWR) test walking at 0.31 m/s. b Depicts VO2 on-kinetic response of a representative subjects from the reference group (REF) during Bout 1 of the CWR test walking at 1.34 m/s. VO2 on-kinetic profiles were modeled according to Eq. (1). The variances of the breath-by-breath response from the model fit are shown as a residual plot. BSL baseline VO2, AMP amplitude, τp phase II time constant, TDp time-delay, m/s meters per second, ml/min milliliters per minute, s seconds
∆[HHb] on-kinetic estimates
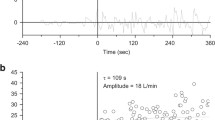
The same REF subject that declined to complete the arterial occlusion assessment did not complete NIRS measurements during the CWR test, and therefore, was not included in the analysis of ∆[HHb] on-kinetics. Two subjects from the iSCI group were excluded from the analysis of ∆[HHb] on-kinetics due to the absence of a mono-exponential rise in ∆[HHb] following the onset of walking Bout 1 of the CWR test. The ∆[HHb] on-kinetic profiles of a representative subject from the iSCI and REF group are presented in Fig. 3.
a Depicts ∆[HHb] on-kinetic response of a representative incomplete spinal cord injured (iSCI) subject during 6 min of constant work-rate (CWR) treadmill walking at 0.31 m/s. b Depicts ∆[HHb] on-kinetic response of a representative subject from the reference group (REF) during 6 min of CWR treadmill walking at 1.34 m/s. ∆[HHb] profiles were modeled using the exponential function described in Eq. (1). BSL baseline [HHb], ∆[HHb] amp amplitude, τ-∆[HHb] time constant, TD-∆[HHb] time-delay, ∆[HHb]-MRT mean response time, m/s meters per second, a.u. arbitrary units, s seconds
∆[HHb] on-kinetics are presented in Table 5. TD-∆[HHb] of the iSCI group was 46.4% slower than the REF group (30.5 ± 13.8 vs 19.0 ± 8.6 s, p = 0.095). ∆[HHb]-MRT of the iSCI group was 34.3% slower than that observed in the REF group (44.7 ± 12.3 vs 31.6 ± 13.6 s, p = 0.097). ∆[HHb]AMP was 29.3% lower in the iSCI compared to the REF group (3.5 ± 1.9 vs 4.7 ± 2.6 a.u., p = 0.359). Walking speed was not significantly correlated to∆[HHb]-MRT (r = −0.49; p = 0.092).
Discussion
The findings of the present study supported the hypothesis that individuals with iSCI are more fatigable than those without iSCI as a result of both greater perceived fatigability and performance fatigability. Compared to the REF group, the iSCI group also demonstrated prolonged τp and resting ∆[HHb] ½ time. Collectively, these results suggest limitations in O2 delivery and/or utilization could have contributed to the fatigability observed during treadmill walking in these subjects with iSCI.
The current results are in agreement with earlier reports of elevated levels of fatigue and fatigability in individuals with SCI (Shields 1995; Jensen et al. 2007; Fawkes-Kirby et al. 2008). Several factors are suggested to contribute to the greater fatigue reported in those with SCI such as age, level of injury (i.e., paraplegic vs tetraplegic), extent of injury (i.e., complete vs incomplete), time since injury, as well as physiological and psychological factors (Shields 1995; Jensen et al. 2007; Fawkes-Kirby et al. 2008; Hammell et al. 2009; Biering-Sørensen et al. 2009; Pelletier and Hicks 2011; Craig et al. 2012; Petrie et al. 2014; Papaiordanidou et al. 2014; Nooijen et al. 2015). For example, in those seeking outpatient rehabilitation fatigue was greater in people with incomplete lesions (Fawkes-Kirby et al. 2008). One conjecture proposed by the authors is that individuals with iSCI engage in more physically demanding activities compared to those with complete SCI (Fawkes-Kirby et al. 2008). Similar findings were reported in individuals with subacute SCI completing inpatient rehabilitation (Nooijen et al. 2015). Peak oxygen uptake (VO2peak) tended to relate with severity of fatigue, with individuals having lower VO2peak reporting higher levels of fatigue (Nooijen et al. 2015). Furthermore, the presence of fatigue in people with SCI seems to be especially problematic when performing tasks over extended periods of time (Craig et al. 2012). Our findings further support the notion that individuals with iSCI are more susceptible to fatigue as compared to able-bodied individuals when performing prolonged whole-body volitional activity and that this difference seems to be in part influenced by cardiorespiratory limitations.
The ability to maintain or re-establish a homeostatic state is essential for sustaining physical activity. Afferent sensory feedback is an important regulator of sensations associated with disturbances to homeostasis experienced during activity (Smirmaul 2012). The insult to the nervous system following SCI increases the potential for compromised afferent feedback and thus altering one’s ability to accurately respond to homeostatic disturbances. For example, Lewis et al. (2007) found inconsistent associations between physiological responses and ratings of perceived exertion during peak graded arm ergometry in those with SCI. In the present study, all iSCI subjects reported feeling “more tired” at the completion of Bout 2 of the CWR treadmill test as compared to before the test. This finding, in combination with the reported greater performance fatigability, may suggest that the interaction between these two fatigability constructs was preserved in the current study.
The selection of preferred walking speed is suggested, at least in part, to be based on the minimization of energy expenditure (Inman 1967; Zarrugh et al. 1974; Cavagna et al. 1977; Waters et al. 1988). SCI results in an increased energetic cost of walking as compared to neurologically intact individuals, placing greater stress on the cardiorespiratory system for a given walking intensity (Waters and Lunsford 1985; Waters and Mulroy 1999; Kuo and Donelan 2010; Gollie et al. 2017). In the present study, there were no differences observed in metabolic cost of walking (i.e., AMP) at each subject’s self-selected speed despite the mean AMP of the REF being 19.4% greater than that seen in the iSCI group. The iSCI group experienced 52% greater perceived fatigability and 44% greater performance fatigability when compared to the REF group while walking at significantly slower speeds irrespective of the lack of differences observed in AMP. Therefore, additional factors other than metabolic cost likely contribute to the elevated fatigability experienced during volitional walking by those with iSCI.
Phase II VO2 on-kinetics during self-selected treadmill walking was prolonged in those with iSCI compared to REF subjects despite a significantly slower walking speed. In accordance with the hypothesis that slowed VO2 on-kinetics can be used as a marker of fatigability during moderate-intensity exercise (Grassi et al. 2011), the iSCI subjects experienced greater fatigability and prolonged VO2 on-kinetics. The REF group had VO2 on-kinetics that were similar to patterns previously reported in healthy young individuals during moderate-intensity activity (Brittain et al. 2001; Gurd et al. 2009). A similar prolongation of VO2 on-kinetics observed in SCI was suggested to be the result of alterations in skeletal muscle when assessed during leg cycling with functional electric stimulation (FES) (Barstow et al. 1995). Skeletal muscle atrophy (Shah et al. 2006), reductions in skeletal muscle oxidative capacity (Shields 1995; Erickson et al. 2013), and changes in skeletal muscle fiber type characteristics (Biering-Sørensen et al. 2009; Petrie et al. 2014, 2015) as previously demonstrated following iSCI could potentially prolong VO2 on-kinetics and exacerbate fatigability.
The combination of resting and dynamic measures of skeletal muscle oxygenation as measured by NIRS may provide critical information about the severity of fatigability during whole-body exercise given the potential mismatch between O2 delivery and/or utilization at the onset of activity after SCI. At the onset of walking Bout 1, both the iSCI and REF groups experienced a sudden decrease in the ∆[HHb] signal suggesting greater muscle O2 availability compared to utilization. The decrease in the ∆[HHb] signal was then followed by a rapid increase towards steady-state (i.e., phase II) demonstrating increased muscle O2 utilization in relation to O2 delivery. The non-significant findings between groups in TD-∆[HHb] and ∆[HHb]-MRT may in part be due to the heterogeneity of the iSCI subjects. For example, half of the iSCI subjects were ambulatory within the community at enrollment, either with the use of walking aids or independently. Compared to those who relied on a wheelchair for community locomotion, those with ambulatory capabilities reported faster TD-∆[HHb] (20.7 vs 40.3 s) and ∆[HHb]-MRT (34.3 vs 55.1 s) (data not reported). Mechanisms contributing to impaired skeletal muscle oxygen extraction, specifically in those with iSCI who are reliant on a wheelchair for community locomotion, may potentially be explained by delayed muscle oxidative metabolism (Grassi et al. 1996), reduced muscle activation/fiber type recruitment patterns (Chin et al. 2011), and/or differences in walking speeds/work intensity (Sheriff 2003).
Reduced skeletal muscle oxidative capacity has been identified as a contributing factor to skeletal muscle fatigability after SCI (Shields 1995; McCully et al. 2011; Kent-Braun et al. 2012; Petrie et al. 2014, 2015). Erikson et al. (2013) observed reduced mitochondrial capacity in individuals with SCI AIS A and B compared to non-injured individuals. Despite similarities in the ∆[HHb]capacity, the current study found prolonged resting ∆[HHb] ½ time during complete arterial occlusion in the iSCI group compared to the REF group. These findings suggest that the capacity of the lateral gastrocnemius for O2 extraction was preserved in the SCI group while the rate of skeletal muscle deoxygenation (i.e., ∆[HHb] ½ time) was compromised. These data provide an indirect index of changes in skeletal muscle deoxygenation while controlling for changes in blood flow and volume. The delay in ∆[HHb] ½ time during resting arterial occlusion suggests a reduction in skeletal muscle oxidative metabolism in the iSCI group.
Clinical implications
There has been extensive work done in the area of restoring walking function after SCI (Mehrholz et al. 2012; Morawietz and Moffat 2013; Mehrholz et al. 2017). This includes research of novel therapies aiming to promote neurological recovery (Edgerton et al. 2008). While several therapeutic approaches have shown promise for re-establishing walking capabilities, especially in those with iSCI AIS C and D, limited evidence is available describing potential limitations to walking once achieved after injury. The results of the current study provide initial evidence of both perceived and performance fatigability as barriers to walking performance following iSCI. Moreover, the reported impairments to oxygen transport and/or utilization offers one possible avenue for the development of targeted interventions to improve walking function in this population.
Limitations
Our sample size was small and not representative of the total population of people who have iSCI. Interpretation of the findings of this study must be delimited to these subjects and cannot be generalized to the SCI population or the iSCI subset. In addition, we were not able to control for exercise intensity because of the challenges associated with maximal exercise treadmill testing in people with SCI. The relationships found between walking speed and τp preclude the ability to rule out walking speed as a contributing factor to differences in τp. Our subjective and objective data support that the intensity fell within the moderate level given the presence of a plateau and absence of further rise in VO2 over the 6-min treadmill walking bout (Poole and Jones 2012) in all but one subject. Moreover, the walking speed was faster in the REF group. Fast walking speeds are expected to be associated with prolonged VO2 on-kinetics. Thus, the difference in speed represented a conservative bias and could have contributed to type II bias. The lack of multiple square-wave exercise bouts and thus the inability to develop ensemble averages of the breath-by-breath data may have influenced the analysis (Lamarra et al. 1987). While repeated square-wave bouts are preferred experimentally, this was not practical when working with these patients with iSCI as it would have required additional days of testing and subject involvement. While a second bout of exercise was completed during the treadmill tests in this study, prior exercise priming of VO2 on-kinetic mechanisms may have occurred during the second bout. Accordingly, we adopted a single stage protocol used by others in clinical populations (Arena et al. 2001; Keyser et al. 2010) to facilitate data analysis with the certainty that priming effects would not be inconsistently present in our dataset.
Conclusion
This study demonstrated that individuals with iSCI have both increased perceived fatigability and performance fatigability compared to non-injured individuals during volitional treadmill walking. Furthermore, prolonged phase II VO2 on-kinetics during the rest-to-work transition and resting ∆[HHb] ½ time were observed in those with iSCI. Taken together, these results suggest that compromised delivery and/or O2 utilization may have contributed to the severity of fatigability in these individuals with iSCI. The understanding of the extent to which fatigability and VO2 and Δ[HHb] on-kinetics impacts locomotion after iSCI will assist in the future development of targeted interventions to enhance function.
Abbreviations
- AIS:
-
American Spinal Injury Association Impairment Scale
- AMP:
-
VO2 amplitude
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- BSL:
-
Baseline VO2
- cm:
-
Centimeter
- CWR:
-
Constant work-rate
- ECG:
-
Electrocardiography
- HR:
-
Heart rate
- ∆[HHb]:
-
Change in deoxygenated myoglobin/hemoglobin concentration
- ∆[HHb]AMP :
-
∆[HHb] amplitude
- ∆[HHb]capacity :
-
∆[HHb] capacity
- ∆[HHb] ½ time:
-
∆[HHb] half-time
- ∆[HHb]-MRT:
-
∆[HHb] mean response time
- iSCI:
-
Incomplete spinal cord injury
- kg:
-
Kilogram
- ml/min:
-
Milliliter per minute
- m/s:
-
Meters per second
- NIRS:
-
Near-infrared spectroscopy
- O2 :
-
Oxygen
- ∆[O2Hb]:
-
Change in oxygenated myoglobin/hemoglobin concentration
- REF:
-
Non-injured reference group
- s:
-
Seconds
- SCI:
-
Spinal cord injury
- SD:
-
Standard deviation
- TD-∆[HHb]:
-
∆[HHb] time-delay
- TDp:
-
VO2 time-delay
- τ-∆[HHb]:
-
∆[HHb] time constant
- τp:
-
VO2 time constant
- TSI:
-
Tissue saturation index
- ∆[tHb]:
-
Change in total myoglobin/hemoglobin concentration
- VO2 on-kinetics:
-
Pulmonary oxygen uptake kinetics
- VO2peak :
-
Peak oxygen uptake
- ∆VO2(t):
-
VO2 as a function of time
References
Arena R, Humphrey R, Peberdy MA (2001) Measurement of oxygen consumption on-kinetics during exercise: implications for patients with heart failure. J Card Fail 7:302–310. doi:10.1054/jcaf.2001.27666
Barstow TJ, Scremin AM, Mutton DL et al (1995) Gas exchange kinetics during functional electrical stimulation in subjects with spinal cord injury. Med Sci Sports Exerc 27:1284–1291
Biering-Sørensen B, Kristensen IB, Kjaer M, Biering-Sørensen F (2009) Muscle after spinal cord injury. Muscle Nerve 40:499–519. doi:10.1002/mus.21391
Brittain CJ, Rossiter HB, Kowalchuk JM, Whipp BJ (2001) Effect of prior metabolic rate on the kinetics of oxygen uptake during moderate-intensity exercise. Eur J Appl Physiol 86:125–134. doi:10.1007/s004210100514
Burns SP, Golding DG, Rolle WA et al (1997) Recovery of ambulation in motor-incomplete tetraplegia. Arch Phys Med Rehabil 78:1169–1172
Cavagna GA, Heglund NC, Taylor CR (1977) Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol 233:R243–R261
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. Lancet Lond Engl 363:978–988. doi:10.1016/S0140-6736(04)15794-2
Chin LMK, Heigenhauser GJF, Paterson DH, Kowalchuk JM (2010) Effect of hyperventilation and prior heavy exercise on O2 uptake and muscle deoxygenation kinetics during transitions to moderate exercise. Eur J Appl Physiol 108:913–925. doi:10.1007/s00421-009-1293-1
Chin LMK, Kowalchuk JM, Barstow TJ et al (2011) The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J Appl Physiol Bethesda Md 1985(111):1259–1265. doi:10.1152/japplphysiol.01216.2010
Craig A, Tran Y, Wijesuriya N, Middleton J (2012) Fatigue and tiredness in people with spinal cord injury. J Psychosom Res 73:205–210. doi:10.1016/j.jpsychores.2012.07.005
de Smirmaul B de PC (2012) Sense of effort and other unpleasant sensations during exercise: clarifying concepts and mechanisms. Br J Sports Med 46:308–311. doi:10.1136/bjsm.2010.071407
Edgerton VR, Courtine G, Gerasimenko YP et al (2008) Training locomotor networks. Brain Res Rev 57:241–254. doi:10.1016/j.brainresrev.2007.09.002
Enoka RM, Duchateau J (2016) Translating fatigue to human performance. Med Sci Sports Exerc 48:2228–2238. doi:10.1249/MSS.0000000000000929
Erickson ML, Ryan TE, Young H-J, McCully KK (2013) Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol 113:2275–2283. doi:10.1007/s00421-013-2657-0
Fawkes-Kirby TM, Wheeler MA, Anton HA et al (2008) Clinical correlates of fatigue in spinal cord injury. Spinal Cord 46:21–25. doi:10.1038/sj.sc.3102053
Gollie JM, Guccione AA, Panza GS et al (2017) Effects of overground locomotor training on walking performance in chronic cervical motor incomplete spinal cord injury: a pilot study. Arch Phys Med Rehabil 98:1119–1125. doi:10.1016/j.apmr.2016.10.022
Grassi B, Quaresima V (2016) Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21:091313. doi:10.1117/1.JBO.21.9.091313
Grassi B, Poole DC, Richardson RS et al (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol Bethesda Md 1985(80):988–998
Grassi B, Porcelli S, Salvadego D, Zoladz JA (2011) Slow VO2 kinetics during moderate-intensity exercise as markers of lower metabolic stability and lower exercise tolerance. Eur J Appl Physiol 111:345–355. doi:10.1007/s00421-010-1609-1
Gurd BJ, Peters SJ, Heigenhauser GJF et al (2009) Prior heavy exercise elevates pyruvate dehydrogenase activity and muscle oxygenation and speeds O2 uptake kinetics during moderate exercise in older adults. Am J Physiol Regul Integr Comp Physiol 297:R877–R884. doi:10.1152/ajpregu.90848.2008
Haas F, Axen K, Pineda H (1986) Aerobic capacity in spinal cord injured people. Cent Nerv Syst Trauma J Am Paralys Assoc 3:77–91
Hamaoka T, McCully KK, Quaresima V et al (2007) Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12:062105. doi:10.1117/1.2805437
Hammell KW, Miller WC, Forwell SJ et al (2009) Fatigue and spinal cord injury: a qualitative analysis. Spinal Cord 47:44–49. doi:10.1038/sc.2008.68
Inman VT (1967) Conservation of energy in ambulation. Arch Phys Med Rehabil 48:484–488
Jensen MP, Kuehn CM, Amtmann D, Cardenas DD (2007) Symptom burden in persons with spinal cord injury. Arch Phys Med Rehabil 88:638–645. doi:10.1016/j.apmr.2007.02.002
Jones AM, Poole DC (eds) (2005) Oxygen uptake kinetics in sport, exercise and medicine. Routledge, London
Kent-Braun JA, Fitts RH, Christie A (2012) Skeletal muscle fatigue. In: Terjung R (ed) Comprehensive physiology. Wiley, Hoboken
Keyser RE, Rus V, Mikdashi JA, Handwerger BS (2010) Exploratory study on oxygen consumption on-kinetics during treadmill walking in women with systemic lupus erythematosus. Arch Phys Med Rehabil 91:1402–1409. doi:10.1016/j.apmr.2010.06.003
Kirshblum S, Waring W (2014) Updates for the international standards for neurological classification of spinal cord injury. Phys Med Rehabil Clin N Am 25:505–517. doi:10.1016/j.pmr.2014.04.001
Kuo AD, Donelan JM (2010) Dynamic principles of gait and their clinical implications. Phys Ther 90:157–174. doi:10.2522/ptj.20090125
Lamarra N, Whipp BJ, Ward SA, Wasserman K (1987) Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol Bethesda Md 1985(62):2003–2012
Lewis JE, Nash MS, Hamm LF et al (2007) The relationship between perceived exertion and physiologic indicators of stress during graded arm exercise in persons with spinal cord injuries. Arch Phys Med Rehabil 88:1205–1211. doi:10.1016/j.apmr.2007.05.016
McCully KK, Hamaoka T (2000) Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev 28:123–127
McCully KK, Mulcahy TK, Ryan TE, Zhao Q (2011) Skeletal muscle metabolism in individuals with spinal cord injury. J Appl Physiol 111:143–148. doi:10.1152/japplphysiol.00094.2011
Mehrholz J, Kugler J, Pohl M (2012) Locomotor training for walking after spinal cord injury. In: The Cochrane Collaboration (ed) Cochrane database of systematic reviews. Wiley, Chichester
Mehrholz J, Harvey LA, Thomas S, Elsner B (2017) Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord. doi:10.1038/sc.2017.31
Morawietz C, Moffat F (2013) Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 94:2297–2308. doi:10.1016/j.apmr.2013.06.023
Nooijen CFJ, Vogels S, Bongers-Janssen HMH et al (2015) Fatigue in persons with subacute spinal cord injury who are dependent on a manual wheelchair. Spinal Cord 53:758–762. doi:10.1038/sc.2015.66
Ozyener F, Rossiter HB, Ward SA, Whipp BJ (2001) Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 533:891–902
Papaiordanidou M, Varray A, Fattal C, Guiraud D (2014) Neural and muscular mechanisms of electrically induced fatigue in patients with spinal cord injury. Spinal Cord 52:246–250. doi:10.1038/sc.2013.172
Pelletier CA, Hicks AL (2011) Muscle fatigue characteristics in paralyzed muscle after spinal cord injury. Spinal Cord 49:125–130. doi:10.1038/sc.2010.62
Petrie MA, Suneja M, Faidley E, Shields RK (2014) Low force contractions induce fatigue consistent with muscle mRNA expression in people with spinal cord injury. Physiol Rep. doi:10.1002/phy2.248
Petrie M, Suneja M, Shields RK (2015) Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J Appl Physiol 118:723–731. doi:10.1152/japplphysiol.00628.2014
Poole DC, Jones AM (2012) Oxygen uptake kinetics. In: Terjung R (ed) Comprehensive physiology. Wiley, Hoboken
Rossiter HB, Howe FA, Ward SA et al (2000) Intersample fluctuations in phosphocreatine concentration determined by 31P-magnetic resonance spectroscopy and parameter estimation of metabolic responses to exercise in humans. J Physiol 528:359–369. doi:10.1111/j.1469-7793.2000.t01-1-00359.x
Schalcher C, Rickli H, Brehm M et al (2003) Prolonged oxygen uptake kinetics during low-intensity exercise are related to poor prognosis in patients with mild-to-moderate congestive heart failure. Chest 124:580–586
Schnelle JF, Buchowski MS, Ikizler TA et al (2012) Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc 60:1527–1533. doi:10.1111/j.1532-5415.2012.04062.x
Shah PK, Stevens JE, Gregory CM et al (2006) Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 87:772–778. doi:10.1016/j.apmr.2006.02.028
Sheriff DD (2003) Muscle pump function during locomotion: mechanical coupling of stride frequency and muscle blood flow. Am J Physiol Heart Circ Physiol 284:H2185–H2191. doi:10.1152/ajpheart.01133.2002
Shields RK (1995) Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol 73:2195–2206
Spinal Cord Injury (SCI) (2016) Facts and figures at a glance. J Spinal Cord Med 39:493–494. doi:10.1080/10790268.2016.1210925
Waters RL, Lunsford BR (1985) Energy cost of paraplegic locomotion. J Bone Jt Surg Am 67:1245–1250
Waters RL, Mulroy S (1999) The energy expenditure of normal and pathologic gait. Gait Posture 9:207–231
Waters RL, Lunsford BR, Perry J, Byrd R (1988) Energy-speed relationship of walking: standard tables. J Orthop Res 6:215–222. doi:10.1002/jor.1100060208
West CR, Bellantoni A, Krassioukov AV (2013) Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 19:267–278. doi:10.1310/sci1904-267
Wilson JR, Mancini DM, McCully K et al (1989) Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation 80:1668–1674
Zarrugh MY, Todd FN, Ralston HJ (1974) Optimization of energy expenditure during level walking. Eur J Appl Physiol 33:293–306
Acknowledgements
The authors would like to express their appreciation to the doctoral students of the Department of Rehabilitation Science at George Mason University who assisted with the completion of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Communicated by I. Mark Olfert.
Rights and permissions
About this article
Cite this article
Gollie, J.M., Herrick, J.E., Keyser, R.E. et al. Fatigability, oxygen uptake kinetics and muscle deoxygenation in incomplete spinal cord injury during treadmill walking. Eur J Appl Physiol 117, 1989–2000 (2017). https://doi.org/10.1007/s00421-017-3685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3685-y