Abstract
Purpose
β2-Agonists have been proposed as weight-loss treatment, because they elevate energy expenditure. However, it is unknown what effect β2-agonists have on energy expenditure in overweight individuals. Furthermore, the influence of β2-agonist R- and S-enantiomer ratio for the increased energy expenditure is insufficiently explored.
Methods
Nineteen males were included in the study of which 14 completed. Subjects were 31.6 (±3.5) years [mean (±95% CI)] and had a fat percentage of 22.7 (±2.1)%. On separate days, subjects received either placebo or inhaled racemic (rac-) formoterol (2 × 27 µg). After an overnight fast, energy expenditure and substrate oxidation were estimated by indirect calorimetry at rest and during submaximal exercise. Plasma (R,R)- and (S,S)-formoterol enantiomer levels were measured by ultra-performance liquid chromatograph–mass spectrometry.
Results
At rest, energy expenditure and fat oxidation were 12% (P ≤ 0.001) and 38% (P = 0.006) higher for rac-formoterol than placebo. Systemic (R,R):(S,S) formoterol ratio was correlated with change in energy expenditure at rest in response to rac-formoterol (r = 0.63, P = 0.028), whereas no association was observed between fat percentage and rac-formoterol-induced change in energy expenditure. During exercise, energy expenditure was not different between treatments, although carbohydrate oxidation was 15% higher (P = 0.021) for rac-formoterol than placebo. Rac-formoterol-induced shift in substrate choice from rest to exercise was related to plasma ln-rac-formoterol concentrations (r = 0.75, P = 0.005).
Conclusion
Selective β2-adrenoceptor agonism effectively increases metabolic rate and fat oxidation in overweight individuals. The potential for weight loss induced by β2-agonists may be greater for R-enantiopure formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity is increasing worldwide accompanied by several diseases such as diabetes and hypertension (Ng et al. 2014). One potential approach to combat obesity is to target β2-adrenoceptors with selective agonists. While the main clinical application of β2-adrenoceptor agonists (β2-agonists) is to relieve the bronchoconstriction associated with respiratory disease, animal studies have provided evidence for β2-agonists as repartitioning agents, since chronic treatment elicits muscle hypertrophy and reduces fat mass (Emery et al. 1984; Hulot et al. 1996; Ryall et al. 2006). Likewise, Hostrup et al. (2015) demonstrated equivalent action of chronic β2-adrenergic stimulation in humans observing 1.4 kg of fat loss after 4 weeks of daily treatment with oral terbutaline complemented by an elevation of lean body mass by 1.7 kg. However, while the potential of β2-adrenergic stimulation in the fight against obesity is palpable, chronic use of β2-agonists may be associated with adverse effects. Chronic high-dose treatment with older generation β2-agonists such as terbutaline and clenbuterol demonstrate functionally impairing hypertrophy and collagenous infiltrations in heart muscle of animals due to unwanted activation of β1-adrenoceptors (Jeppsson et al. 1986; Gregorevic et al. 2005; Lynch and Ryall 2008). Nevertheless, with the introduction of the newer generation β2-agonists with superior affinity and selectivity for the β2-adrenoceptor as compared to the common short-acting β2-agonists, salbutamol and terbutaline (Baker 2010), this adverse effect is less pronounced.
The new generation long acting β2-agonist formoterol has shown some potential as a body weight-reducing agent. Lee et al. (2013) observed a 13% increase in energy expenditure following oral ingestion of 96 µg formoterol in lean young men. However, it is unknown what effect formoterol elicits in overweight subjects. It has been reported that the increase in plasma glycerol, a marker of adipose tissue lipolysis, following treatment with salbutamol is lower in obese compared to lean subjects (Blaak et al. 2004). Furthermore, Schiffelers et al. (2001) observed a dampened increase in energy expenditure upon non-selective β-adrenergic stimulation with isoprenaline at rest in obese compared to lean subjects. β2-adrenergic stimulation has been demonstrated to lower the respiratory exchange ratio (RER) at rest in lean subjects, indicating an elevated fat oxidation (Lee et al. 2013). On the other hand, Kalsen et al. (2014) observed a higher RER in lean subjects upon β2-adrenergic stimulation with terbutaline during exercise at an intensity corresponding to 70% of maximal oxygen consumption (\({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\)). These findings indicate a transition from increased fat oxidation at rest to an increased carbohydrate oxidation during exercise upon β2-adrenergic stimulation. However, this warrants further investigation.
β2-agonists are normally administered as (1:1) racemic (rac) mixtures of R- and S-enantiomers with stereoselective behavior (Jacobson et al. 2015, 2016). The R-enantiomers of β2-agonists salbutamol and formoterol exhibit activity, while the S-enantiomers generally are considered pharmacologically inert at the β2-adrenoceptor (Källström et al. 1996; Schmidt et al. 2000). Accordingly, R-enantiopure formulations of β2-agonists may be superior agents for weight loss than racemic. However, data are scant regarding the influence of the systemic R:S ratios on energy expenditure changes induced by β2-agonists in humans.
The purpose of the present study was, therefore, to examine the effect of β2-adrenergic stimulation on energy expenditure and substrate oxidation mediated by the selective agonist rac-formoterol in inhaled therapeutic doses at rest and during exercise in active overweight men. A secondary purpose was to investigate the relation between (R,R):(S,S)-formoterol ratio and change in energy expenditure induced by rac-formoterol.
Materials and methods
Subjects and ethics approval
Nineteen recreationally active overweight [body mass index (BMI) of 25–36 kg × (m2)−1] men (21–43 years of age) volunteered to participate in the study. Subjects were non-smokers, had no chronic disease or known allergy towards medication, and did not use β2-agonist or other prescription medication. Before inclusion in the study, subjects met for a screening. The screening was conducted in the morning after an overnight fast and consisted of a medical examination with measurements of resting blood pressures and heart rate, electrocardiography, and body composition in a dual-energy X-ray absorptiometry (DXA) scanner. Furthermore, \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) and performance were measured during an incremental exercise test to exhaustion on a bike ergometer. Subjects were informed about risks and discomforts related to the different tests and procedures of the study. Each subject gave written and oral informed consent prior to inclusion in the study. The study was approved by the ethics committee of Copenhagen (H-1-2012-090) and performed in accordance with the Helsinki II declaration.
Of the 19 subjects that were screened, 14 completed the study. Five subjects dropped out of the study either because of discomfort with blood sampling (n = 2), personal reasons (n = 2), or schedule problems (n = 1). Characteristics of the 14 subjects who completed the study are presented in Table 1.
Experimental intervention
The intervention consisted of two experimental trials in randomized order. At each of the trials, subjects met in the morning at the clinic after an overnight fast. Upon arrival, subjects inhaled either three puffs of 9 µg formoterol (27 µg) (Oxis Turbohaler®, AstraZeneca, London, UK) or placebo (three puffs from identically looking placebo Turbohaler®, AstraZeneca, London, UK) during supervision and a catheter was inserted in the antecubital vein for blood sampling. Subjects then rested on a bed for 45 min. Thirty minutes into the resting period, after which inhaled formoterol had reached its peak systemic concentrations (Derks et al. 1997), indirect calorimetry was measured breath-by-breath with a gas analyzing system (JAEGER MasterScreen CPX; Viasys Healthcare GmbH, Hoechberg, DE) for 15 min for determination of energy expenditure and oxidation of fat and carbohydrates. Furthermore, a blood sample (9 mL) was drawn and resting heart rate and blood pressure were determined (Omron M7, Omron Healthcare Europe, The Netherlands). Hereafter, subjects inhaled another three puffs of 9 µg formoterol or placebo. Thirty min after administration of the second dose, subjects performed steady-state submaximal cycling at an intensity corresponding to 30% of \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) (55 ± 7 W) (mean ± 95% CI) for 8 min and at 50% (105 ± 9 W) of \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) for 4 min. Indirect calorimetry was measured breath-by-breath during the exercise for determination of energy expenditure and oxidation of fat and carbohydrates. At the end of exercise, a blood sample (9 mL) was drawn.
The two experimental trials were separated by at least 3 days to ensure complete wash-out of formoterol (elimination half-life of formoterol ~10 h) (Lecaillon et al. 1999). Throughout the study, subjects were instructed to maintain their regular level of physical activity and to abstain from caffeine, alcohol, and strenuous exercise 48 h prior to each experimental trial.
Stimulation of β2-adrenoceptors
β2-Adrenoceptors were stimulated with the highly selective β2-agonist formoterol, which has a long duration of action (Baker 2010; Löfdahl and Svedmyr 1989). We followed the guidelines of inhalation of formoterol, which advises to administer maximally 27 µg at one time. As such, we administered 2 × 27 µg formoterol by inhalation separated by an hour. Formoterol or placebo was inhaled from identically looking Turbohalers. Drug administration was administered in a double-blinded manner. Inhalation technique was thoroughly practiced at the screening visit and all subjects were familiar with the side effects of formoterol.
Dual-energy X-ray absorptiometry
Subjects’ whole-body lean mass and fat percentage were determined by dual-energy X-ray absorptiometry (Lunar DPX-IQ, Version 4.7 E, Lunar Corporation, Madison, WI, USA). Subjects were placed in the scanner in supine position undressed and euhydrated for 20 min before the scan. To reduce variation, two scans at medium speed were performed according to the manufacturer’s guidelines using the medium research analysis software. The scanner was calibrated before scan, using daily calibration procedures (Lunar “System Quality Assurance”). All scans were conducted by the same hospital technician.
Maximal oxygen consumption
\({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) was measured breath-by-breath with a gas analyzing system during an incremental bike ergometer test to exhaustion starting at 150 W and increasing 30 W every min until pedaling frequency fell below 70 rpm for more than 5 s (Monark 839E, Vansbro, SE). Criteria for achievement of \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) were a respiratory exchange ratio above 1.10 or no further increase in oxygen uptake despite an increased power output. \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) was defined as the highest value averaged in any 30 s period. The gas analyzing system was calibrated with a 3-L syringe and with gasses of known O2 and CO2 concentrations.
Indirect calorimetry
Energy expenditure of fat and carbohydrates was quantified using indirect calorimetry as previously described (Lee et al. 2013). \({\dot{\text{V}}\text{O}}_{2}\) and \({\dot{\text{V}}\text{CO}}_{2}\) were measured with a gas analyzing system. Energy expenditure and oxidation of carbohydrates and fat were calculated using the following equations deduced by Ferrannini (1988) as done in Lee et al. (2013): \({\text{EE}} = (3.91 \times {\dot{\text{V}}\text{O}}_{2} + 1.1 \times {\dot{\text{V}}\text{CO}}_{2} )/1000{-}3.34 \times 0.14 \times {\text{BW}}/1440\), \({\text{carbohydrate oxidation}} = ((4.55 \times {\dot{\text{V}}\text{CO}}_{2} - 3.21 \times {\dot{\text{V}}\text{O}}_{2} )/1000 \, - 2.87 \times 0.14 \times {\text{BW}}/1440) \times 1000\), \({\text{fat oxidation}} = (( 1. 6 7 \times {\dot{\text{V}}\text{O}}_{2} - 1. 6 7 \times {\dot{\text{V}}\text{CO}}_{2} )/ 1000{-} 1. 9 2 \times 0. 1 4 \times {\text{BW}}/ 1 4 40) \times 1000\). where EE is energy expenditure in kcal min−1, carbohydrate oxidation is in mg min−1, fat oxidation is in mg min−1, and BW is body weight in kg.
Analysis of plasma K+, glucose, and lactate
Venous blood samples were collected in heparinized syringes and analyzed immediately for plasma K+, glucose, and lactate in a blood gas analyzer ABL 800 Flex (Radiometer, Copenhagen, Denmark). Plasma K+, glucose, and lactate were determined for 12 subjects as two subjects refused to have a catheter inserted into their antecubital vein on the second study visit.
Analysis of plasma concentrations of formoterol
Enantioselective formoterol analyses were based on our previous work with salbutamol and salmeterol (Jacobson et al. 2014, 2015, 2016) and undertaken using UPLC–MS/MS (ultra-performance liquid chromatography–mass spectrometer; Waters Acquity® H-class UPLC system coupled to a Waters Xevo® triple quadrupole mass spectrometer; Waters Corporation, Milford, MA) with chromatography performed using an Astec® CHIROBIOTIC™ T2 chiral column (Sigma-Aldrich). In brief, calibration samples were prepared by spiking drug-free human plasma with unlabeled racemic formoterol (rac-formoterol fumarate dihydrate; Carbosynth, Compton, UK). Internal standard (rac-formoterol-D6; Toronto Research Chemicals, Toronto, Canada) equivalent to 5 ng mL−1 was added to each calibration and study sample aliquot (400 μL), and then, dilute ammonia solution (150 μL) was added to each and vortex mixed before the addition of 850 μL of HPLC grade ethyl acetate. This was vortex mixed for 1 min, then centrifuged at 15,000g for 5 mins. The organic supernatant was then collected, and the ethyl acetate extraction repeated and the two residues combined; solvent was then evaporated under nitrogen at 40 °C and reconstituted using 80 μL of methanol and vortex mixed prior to analysis via UPLC–MS/MS. Rac-formoterol levels were calculated by combining levels for both (R,R)-formoterol and (S,S)-formoterol enantiomers. The assay met acceptance criteria demonstrating linearity (r 2 = 0.9998), precision less than 15% relative standard deviation (%RSD) over the calibration range, and method detection limit of (MDL) of 30 pg mL−1 calculated using the S/N ratio method with MDL defined as S/N = 10. Because of destruction of one sample, formoterol concentrations were determined for 12 subjects.
Statistics
Statistical analysis was performed in SPSS version 24 (IBM, Armonk, NY, USA). Data were tested for normality using the Shapiro Wilks test and Q–Q plots. Data were normally distributed and are presented as means with the 95% confidence interval (95% CI), apart from plasma concentration of rac-formoterol, which was not normally distributed and is presented as median with interquartile range. To estimate differences between treatments, linear mixed modelling was used for each dependent variable with treatment as a fixed factor, and age and weight as confounding time-invariant covariates (White et al. 1994; Cheymol 2000) as well as random intercept for subjects. In case of repeated measures, time was included in the model as a fixed factor with a random slope for subjects. Pearson’s product moment analysis was used to estimate correlation patterns. Magnitudes of the main outcome statistics are presented with the standardized mean difference (SD) to represent clinical significance as described by Cohen (1988) and P values to represent probability.
Results
Plasma concentrations of rac-formoterol
The median (±interquartile range) plasma concentration of rac-formoterol at the end of the last exercise bout during the formoterol-trial was 68 (±62) pg mL−1 with inter-individual variation ranging from 38 to 275 pg mL−1.
Systemic effects
Five subjects experienced tachycardia and tremor after inhalation of rac-formoterol. Resting heart rate and systolic blood pressure were 10 (±5) bpm and 7 (±2) mmHg higher (P ≤ 0.001) for formoterol than placebo, respectively, while diastolic blood pressure was 3 (±3) mmHg lower (P = 0.03) for rac-formoterol than placebo and with no difference in mean arterial pressure between treatments (Table 2). Plasma concentration of K+ at rest was lower (P ≤ 0.001) for rac-formoterol than placebo, being 3.5 (±0.2) mM for formoterol and 4.0 (±0.1) mM for placebo, whereas plasma glucose and lactate were higher (P ≤ 0.001) for rac-formoterol than placebo, being 5.4 (±0.2) and 5.0 (±0.2) mM, respectively, and 1.4 (±0.3) and 0.8 (±0.1) mM, respectively.
Energy expenditure and oxidation of fat and carbohydrates at rest
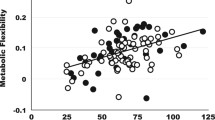
At rest, energy expenditure and fat oxidation were 12% (Cohen’s SD 1.35, P < 0.001) (Fig. 1a) and 38% (Cohen’s SD 1.01, P = 0.006) (Fig. 1c) higher for rac-formoterol than placebo, respectively, whereas no statistical difference was observed between treatments in oxidation of carbohydrates (Cohen’s SD 0.47, P = 0.179) (Fig. 1d). Resting ventilation was higher (P = 0.0002) for formoterol [9.8 (±0.7)] L min−1 than placebo [8.5 (±0.6)] L min−1. The systemic (R,R):(S,S) formoterol enantiomer ratio was significantly correlated with the relative rac-formoterol-induced change in energy expenditure at rest compared to placebo [r = 0.63 (±0.33), P = 0.028] (Fig. 1b), whereas neither fat percentage (r = 0.11, P > 0.50) nor \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) (r = 0.04, P > 0.50) predicted any relevant change.
a Mean (±95% CI) (n = 14) values of energy expenditure at rest for rac-formoterol (black bars) and placebo (white bars). b Bivariate correlation between relative rac-formoterol-induced % change in energy expenditure compared with placebo at rest versus the systemic (R,R):(S,S) formoterol enantiomer ratio. Individual values (n = 12). Dotted grey lines show the 95% CI of the dashed black regression line. c, d Mean (±95% CI) (n = 14) values of fat and carbohydrate oxidation at rest for formoterol and placebo. **Different from placebo (P ≤ 0.01). ***Different from placebo (P ≤ 0.001)
Energy expenditure and oxidation of fat and carbohydrates during exercise
During exercise, energy expenditure was not different between treatments (Cohen’s SD 0.34, P = 0.215) (Fig. 2a). However, oxidation of fat tended to be lower for rac-formoterol than placebo (−13%) (Cohen’s SD 0.40, P = 0.139) (Fig. 2b), whereas oxidation of carbohydrates was 15% higher for rac-formoterol compared to placebo (Cohen’s SD 0.64, P = 0.021) (Fig. 2c). Ventilation during exercise was higher (P < 0.0001) for formoterol [43.2 (±2.9)] L min−1 than placebo [38.6 (±3.0)] L min−1. Increase in RER from rest to exercise was higher for rac-formoterol than placebo (treatment by time interaction for RER, P = 0.006) (Fig. 2d). Rac-formoterol-induced change in RER compared with placebo was significantly related to plasma ln-rac-formoterol during exercise [r = 0.75 (±0.19), P = 0.005] (Fig. 2e).
a–c Mean (±95% CI) (n = 14) values of energy expenditure (a) and oxidation of fat (b) and carbohydrates (c) during exercise for rac-formoterol (black bars) and placebo (white bars). d Mean (±95% CI) (n = 14) respiratory exchange ratio (RER) for rac-formoterol and placebo at rest and during exercise. e Bivariate correlation between relative rac-formoterol-induced % change in RER versus the natural log (ln) plasma concentration of rac-formoterol during exercise. Individual values (n = 12). Dotted grey lines indicate the 95% CI of the dashed black regression line. *Different from placebo (P ≤ 0.05). #Significant (P ≤ 0.01) interaction (treatment by time)
Discussion
The novel findings of the present study were that β2-adrenergic stimulation mediated by the selective agonist rac-formoterol in inhaled therapeutic doses increased resting energy expenditure in active overweight men. During exercise, on the other hand, rac-formoterol did not increase energy expenditure or oxidation of fat, but increased oxidation of carbohydrates. Furthermore, the present study indicates that neither fat percentage nor \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) is predictive of the rac-formoterol-induced increase in energy expenditure at rest, but is rather related to the systemic (R,R):(S,S) formoterol enantiomer ratio.
The application of selective β2-adrenoceptor agonists as weight-loss treatment has been suggested by several authors (Lund and Gillum 2016; Astrup 1995), since chronic treatment with β2-adrenoceptor agonists effectively increases thermogenesis and reduces fat mass in a variety of mammalian species, including humans (Hostrup et al. 2015; Lynch and Ryall 2008; Lee et al. 2013). However, the doses shown to reduce fat mass in previous studies were supratherapeutic and may, therefore, given side effects associated with long-term high-dose treatment (Burniston et al. 2006), not be feasible in a clinical setting. In the present study, we observed that β2-adrenergic stimulation mediated by low-dose and therapeutic inhalation of rac-formoterol (27 µg) elevated resting energy expenditure by 12% active overweight men, which is on par with that observed by Lee et al. (2013) after oral administration of rac-formoterol in markedly higher doses (160 µg) in healthy lean subjects. The similarity in response between the markedly lower doses of rac-formoterol administered in the present study as compared to Lee et al. (2013) is most likely related to the higher systemic bioavailability of the inhaled route of administration compared to the oral route. The systemic bioavailability ratio between inhaled and oral administration of β2-agonists has been shown to be approximately 4:1 (Elers et al. 2012; Dyreborg et al. 2016). Although we observed no significant correlation between the ln-plasma concentration of rac-formoterol and energy expenditure (r = 0.39, P = 0.21, data not shown), we found that the (R,R):(S,S) formoterol enantiomer ratio could predict 40% of the response (Fig. 1b). This finding is consistent with the observation that the activity of rac-formoterol resides with the (R,R)-enantiomer, which has been shown to be 1000 times more potent than the (S,S)-enantiomer (Källström et al. 1996; Schmidt et al. 2000). While it has been speculated that the β2-adrenergic-induced increase in energy expenditure is lowered in obese individuals (Lee et al. 2013), we observed no association between rac-formoterol-induced change in energy expenditure at rest and subjects’ percent body fat or fitness level (\({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\)). However, the current study is limited by subjects’ fat percentage being located within a narrow interval in the lower end of the overweight spectrum. The association in mention might have emerged with a greater number of subjects with a more diverse fat percentage distribution, but more studies are needed to elucidate that.
The mechanisms underlying the β2-adrenergic-induced increase in energy expenditure at rest are possibly multifactorial. β2-adrenoceptors are highly abundant in skeletal muscle and β2-adrenergic stimulation augments activity of muscle Na+/K+ ATPase and Ca2+ ATPase (Clausen and Flatman 1980; Hostrup et al. 2014), thereby increasing ATP consumption. In support of the former, we observed that plasma K+ was lowered by formoterol at rest. β2-adrenergic stimulation has also been shown to increase leakage of Ca2+ from the sarcoplasmic reticulum (Suko et al. 1993), causing involuntary activation of the myosin ATPase. In addition, β2-adrenergic stimulation induces chronotropy and inotropy of the heart, thus increasing energy consumption of cardiomyocytes. However, although we observed that rac-formoterol increased heart rate by 10 beats × min−1, which is consistent with several reports (Lee et al. 2013; Gross et al. 2008; Whale et al. 2008), such increase would only increase myocardial oxygen consumption by approximately 2.5 mLO2 × min−1 (Simonsen and Kjekshus 1978), why the cardiac contribution to the formoterol-induced increase in energy expenditure at rest possibly only constitutes a neglectable fraction. Finally, β2-adrenergic stimulation may activate brown adipose tissue and induce thermogenesis (Cao et al. 2001). As such, Ernande et al. (2016) demonstrated an increased activation of brown adipose tissue upon β2-adrenergic stimulation in mice, which was mediated through β2-adrenergically induced increases in perfusion rather than direct activation of brown adipose tissue β2-adrenoceptors. However, in contrast, Vosselman et al. (2012) found no effect of unselective beta-adrenergic stimulation with isoprenaline on brown adipose tissue activation measured through flourodeoxyglucose uptake, leaving the effect of beta2-adrenergic stimulation on brown adipose tissue unresolved.
Unlike the increased energy expenditure observed at rest upon β2-adrenergic stimulation, we observed no differences between rac-formoterol and placebo during exercise. This might be attributable to exercise-induced release of adrenaline and noradrenaline (Kalsen et al. 2016), thus acting as competitors for formoterol on the β2-adrenoceptors. In addition, some of the abovementioned β2-adrenergically activated processes, such as heart muscle and skeletal muscle activity, are also increased with exercise, thus reducing the difference in energy expenditure between placebo and β2-adrenergic stimulation as the intensity of the exercise increases.
The rac-formoterol-induced increase in resting energy expenditure observed in the present study was paralleled by a concurrent increase in oxidation of fat by 38%. The reason for this has still not been determined with certainty, but presented in the following are two possible mechanisms that could contribute to this increase. First, β2-adrenergic stimulation has been shown to mobilize free fatty acids from white adipose tissue (Hoeks et al. 2003) and activate hormone-sensitive lipase in skeletal muscle (Jocken et al. 2008). The Randle cycle suggests that glucose oxidation is suppressed with increasing levels of plasma free fatty acids via inhibition of pyruvate dehydrogenase and phosphofructokinase activity (Randle et al. 1963; Randle 1964, 1995; Hue et al. 1988). Unfortunately, we did not measure free fatty acids in plasma, which is a limitation of the study. Second, brown adipose tissue is mainly fueled by fat oxidation (Haman et al. 2002; Bakker et al. 2014), why a potential rac-formoterol-induced activation of brown adipose tissue thermogenesis also may contribute to the lowered RER observed at rest upon β2-adrenergic stimulation in the present study. However, more studies are warranted on this matter before anything can be concluded about the role of brown fat in β2-adrenergically induced increase in energy expenditure.
Notably, we observed that rac-formoterol increased fat oxidation at rest, while carbohydrate oxidation was elevated during exercise compared to placebo. The observation that the relative rac-formoterol-induced change in RER during exercise was related to the systemic concentrations of rac-formoterol may imply that the β2-adrenergic mediated response in substrate oxidation is dose-dependent. However, it cannot be excluded that the rac-formoterol-induced transition towards a higher carbohydrate oxidation during exercise could be attributed to exercise-induced changes in the pharmacokinetics of formoterol. Lee et al. (2013) observed no increase in carbohydrate oxidation at progressively greater dosages of rac-formoterol, why it seems unlikely that the higher carbohydrate oxidation observed during exercise with rac-formoterol was caused by an increased amount of inhaled formoterol or an exercise-induced change in the pharmacokinetics of formoterol. It has been demonstrated that β2-adrenoceptors play a role in regulation of glycogenolysis during exercise via cAMP/PKA-mediated phosphorylation of glycogenolytic and glycolytic enzymes. For instance, stimulation of β2-adrenoceptors with selective agonist terbutaline was shown to increase rate of glycogenolysis during exercise and carbohydrate oxidation (Kalsen et al. 2014; Hostrup et al. 2014), which was accompanied by an increased RER the first 20 min of the exercise bout (Kalsen et al. 2014). In addition, adrenergic stimulation with adrenaline has been shown to increase the activity of glycogen phosphorylase in rodents (Jensen and Dahl 1995), which provides a possible explanation behind the increased glucose oxidation during exercise upon β2-adrenergic stimulation observed in the current study.
Conclusions
In conclusion, β2-adrenergic stimulation with the selective agonist rac-formoterol in therapeutic inhaled doses increases resting energy expenditure and fat oxidation in active overweight individuals irrespective of fat percentage and fitness level. The formoterol-induced increase in energy expenditure was partially related to the systemic (R,R):(S,S) enantiomer formoterol ratio, which implies that the potential of β2-agonists, such as formoterol, as weight-loss agents may be greater for R-enantiopure formulations, especially in patients with lower R,R:S,S ratios. As such, there would be a strong rationale to provide a chiral switch enantiopure formulations rather than racemic mixtures in future intervention trials. Notably, when subjects performed exercise at submaximal intensity, the rac-formoterol-induced increase in energy expenditure was blunted and rac-formoterol increased fat oxidation at rest, while carbohydrate oxidation was elevated during exercise.
Abbreviations
- BW:
-
Body weight
- β2-agonist:
-
Beta2-adrenoceptor agonist
- CI:
-
Confidence interval
- DXA:
-
Dual-energy X-ray absorptiometry
- EE:
-
Energy expenditure
- \({\dot{\text{V}}\text{O}}_{{2{ \hbox{max} }}}\) :
-
Maximal oxygen uptake
- RER:
-
Respiratory exchange ratio
- MDL:
-
Method detection limit
- RSD:
-
Relative standard deviation
- SD:
-
Standardized mean difference
- UPLC–MS/MS:
-
Ultra-performance liquid chromatography–mass spectrometer
References
Astrup A (1995) The sympathetic nervous system as a target for intervention in obesity. Int J Obes Rat Metab Disord 19:S24–S28
Baker JG (2010) The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol 160:1048–1061
Bakker LE, Boon MR, van der Linden RA, Arias-Bouda LP, van Klinken JB, Smit F, Verberne HJ, Jukema JW, Tamsma JT, Havekes LM, van Marken Lichtenbelt WD, Jazet IM, Rensen PC (2014) Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2:210–217
Blaak EE, Schiffelers SL, Saris WH, Mensink M, Kooi ME (2004) Impaired beta-adrenergically mediated lipolysis in skeletal muscle of obese subjects. Diabetologia 47:1462–1468
Burniston JG, Tan LB, Goldspink DF (2006) Relative myotoxic and haemodynamic effects of the beta-agonists fenoterol and clenbuterol measured in conscious unrestrained rats. Exp Physiol 91:1041–1049
Cao W, Medvedev AV, Daniel KW, Collins S (2001) β-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem 276:27077–27082
Cheymol G (2000) Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 39:215–231
Clausen T, Flatman JA (1980) Beta 2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K-transport in rat soleus muscle. Br J Pharmacol 68:749–755
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, Hillsdale
Derks MG, van den Berg BT, van der Zee JS, Braat MC, van Boxtel CJ (1997) Biphasic effect-time courses in man after formoterol inhalation: eosinopenic and hypokalemic effects and inhibition of allergic skin reactions. J Pharmacol Exp Ther 283:824–832
Dyreborg A, Krogh N, Backer V, Rzeppa S, Hemmersbach P, Hostrup M (2016) Pharmacokinetics of oral and inhaled terbutaline after exercise in trained men. Front Pharmacol 7:150
Elers J, Hostrup M, Pedersen L, Henninge J, Hemmersbach P, Dalhoff K, Backer V (2012) Urine and serum concentrations of inhaled and oral terbutaline. Int J Sports Med 33:1026–1033
Emery PW, Rothwell NJ, Stock MJ, Winter PD (1984) Chronic effects of beta 2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci Rep 4:83–91
Ernande L, Stanford KI, Thoonen R, Zhang H, Clerte M, Hirshman MF, Goodyear LJ, Bloch KD, Buys ES, Scherrer-Crosbie M (2016) Relationship of brown adipose tissue perfusion and function: a study through β2-adrenoreceptor stimulation. J Appl Physiol 120:825–832
Ferrannini E (1988) The theoretical bases of indirect calorimetry: a review. Metabolism 37:287–301
Gregorevic P, Ryall JG, Plant DR, Sillence MN, Lynch GS (2005) Chronic beta-agonist administration affects cardiac function of adult but not old rats, independent of beta-adrenoceptor density. Am J Physiol Heart Circ Physiol 289:344–349
Gross NJ, Kerwin E, Levine B, Kim KT, Denis-Mize K, Hamzavi M, Carpenter M, Rinehart M (2008) Nebulized formoterol fumarate: dose selection and pharmacokinetics. Pulm Pharmacol Ther 21:818–823
Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber JM (2002) Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol 93:77–84
Hoeks J, van Baak MA, Hesselink MK, Hul GB, Vidal H, Saris WH, Schrauwen P (2003) Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am J Physiol Endocrinol Metab 285:775–782
Hostrup M, Kalsen A, Ortenblad N, Juel C, Mørch K, Rzeppa S, Karlsson S, Backer V, Bangsbo J (2014) β2-Adrenergic stimulation enhances Ca2+ release and contractile properties of skeletal muscles, and counteracts exercise-induced reductions in Na+–K+–ATPase Vmax in trained men. J Physiol 592:5445–5459
Hostrup M, Kalsen A, Onslev J, Jessen S, Haase C, Habib S, Ørtenblad N, Backer V, Bangsbo J (2015) Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men. J Appl Physiol 119:475–486
Hue L, Maisin L, Rider MH (1988) Palmitate inhibits liver glycolysis. Involvement of fructose 2,6-bisphosphate in the glucose/fatty acid cycle. Biochem J 251:541–545
Hulot F, Ouhayoun J, Manoucheri M (1996) Effect of clenbuterol on productive performance, body composition and muscle biochemistry in the rabbit. Meat Sci 42:457–464
Jacobson GA, Yee KC, Premilovac D, Rattigan S (2014) Enantioselective disposition of (R/S)-albuterol in skeletal and cardiac muscle. Drug Test Anal 6:563–567
Jacobson GA, Yee KC, Wood-Baker R, Walters EH (2015) SULT 1A3 single-nucleotide polymorphism and the single dose pharmacokinetics of inhaled salbutamol enantiomers: are some athletes at risk of higher urine levels? Drug Test Anal 7:109–113
Jacobson GA, Hostrup M, Narkowicz CK, Nichols DS, Haydn Walters E (2016) Enantioselective disposition of (R)-salmeterol and (S)-salmeterol in urine following inhaled dosing and application to doping control. Drug Test Anal. doi:10.1002/dta.2131 (Epub ahead of print)
Jensen J, Dahl HA (1995) Adrenaline stimulated glycogen breakdown in rat epitrochlearis muscles: fibre type specificity and relation to phosphorylase transformation. Biochem Mol Biol Int 35:145–154
Jeppsson AB, Waldeck B, Widmark E (1986) Further studies on the cardiomegaly induced by beta-adrenoceptor agonists. Acta Pharmacol Toxicol (Copenh) 58:121–125
Jocken JW, Roepstorff C, Goossens GH, van der Baan P, van Baak M, Saris WH, Kiens B, Blaak EE (2008) Hormone-sensitive lipase serine phosphorylation and glycerol exchange across skeletal muscle in lean and obese subjects: effect of beta-adrenergic stimulation. Diabetes 57:1834–1841
Källström BL, Sjöberg J, Waldeck B (1996) Steric aspects of formoterol and terbutaline: is there an adverse effect of the distomer on airway smooth muscle function? Chirality 8:567–573
Kalsen A, Hostrup M, Karlsson S, Hemmersbach P, Bangsbo J, Backer V (2014) Effect of inhaled terbutaline on substrate utilization and 300-kcal time trial performance. J Appl Physiol 117:1180–1187
Kalsen A, Hostrup M, Backer V, Bangsbo J (2016) Effect of formoterol, a long-acting β2-adrenergic agonist, on muscle strength and power output, metabolism, and fatigue during maximal sprinting in men. Am J Physiol Regul Integr Comp Physiol 310:1312–1321
Lecaillon JB, Kaiser G, Palmisano M, Morgan J, Della Cioppa G (1999) Pharmacokinetics and tolerability of formoterol in healthy volunteers after a single high dose of Foradil dry powder inhalation via aerolizer. Eur J Clin Pharmacol 55:131–138
Lee P, Day RO, Greenfield JR, Ho KK (2013) Formoterol, a highly β2-selective agonist, increases energy expenditure and fat utilisation in men. Int J Obes (Lond) 37:593–597
Löfdahl CG, Svedmyr N (1989) Formoterol fumarate, a new beta 2-adrenoceptor agonist. Acute studies of selectivity and duration of effect after inhaled and oral administration. Allergy 44:264–271
Lund J, Gillum MP (2016) Towards leanness by ‘Feeding’ a novel thermogenic pathway? Trends Endocrinol Metab 27:529–530
Lynch GS, Ryall JG (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88:729–767
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:766–781
Randle PJ (1964) The interrelationships of hormones, fatty acid and glucose in the provision of energy. Postgrad Med J 40:457–463
Randle PJ (1995) Metabolic fuel selection: general integration at the whole-body level. Proc Nutr Soc 54:317–327
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Ryall JG, Sillence MN, Lynch GS (2006) Systemic administration of beta2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. Br J Pharmacol 147:587–595
Schiffelers SL, Saris WH, Boomsma F, van Baak MA (2001) Beta(1)- and beta(2)-adrenoceptor-mediated thermogenesis and lipid utilization in obese and lean men. J Clin Endocrinol Metab 86:2191–2199
Schmidt D, Källström BL, Waldeck B, Branscheid D, Magnussen H, Rabe KF (2000) The effect of the enantiomers of formoterol on inherent and induced tone in guinea-pig trachea and human bronchus. Naunyn Schmiedebergs Arch Pharmacol 361:405–409
Simonsen S, Kjekshus JK (1978) The effect of free fatty acids on myocardial oxygen consumption during atrial pacing and catecholamine infusion in man. Circulation 58:484–491
Suko J, Maurer-Fogy I, Plank B, Bertel O, Wyskovsky W, Hohenegger M, Hellmann G (1993) Phosphorylation of serine 2843 in ryanodine receptor-calcium release channel of skeletal muscle by cAMP-, cGMP- and CaM-dependent protein kinase. Biochim Biophys Acta 1175:193–206
Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD (2012) Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 61:3106–3113
Whale CI, Sovani MP, Mortimer KJ, Harrison TW, Tattersfield AE (2008) Systemic and bronchodilator effects of inhaled rac-formoterol in subjects with chronic obstructive pulmonary disease: a dose–response study. Br J Clin Pharmacol 65:841–847
White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM et al (1994) Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 90:1225–1238
Acknowledgements
The authors would like to thank Jens Lund for many fruitful scientific discussions during the writing process and Dr. David Nichols (Central Science Laboratory, University of Tasmania) for conducting the UPLC–MS/MS instrumental analyses.
Author information
Authors and Affiliations
Contributions
JO collected, analyzed, and interpreted the data, and drafted the manuscript. GJ developed and supervised the enantioselective UPLC–MS/MS analytical component of the study, including assay performance, analysis and reporting of results, as well as contributing to the final manuscript. CN undertook the UPLC–MS/MS sample preparation and method UPLC–MS/MS optimization, and reviewed the manuscript. VB was the responsible medical doctor of the study and performed medical examination of the subjects, and contributed to interpretation and review of the manuscript. AK, MK, and SJ conducted the human experiments and contributed to interpretation of the data and in drafting of the manuscript. JB and MH designed the study and contributed to data collection, analysis, interpretation, and in drafting of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Funding
The study was supported by the Danish Ministry of Culture. The funder had no role in the design of the study, the collection, analysis, and interpretation of the data or the writing of the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Communicated by Jean-René Lacour.
Rights and permissions
About this article
Cite this article
Onslev, J., Jacobson, G., Narkowicz, C. et al. Beta2-adrenergic stimulation increases energy expenditure at rest, but not during submaximal exercise in active overweight men. Eur J Appl Physiol 117, 1907–1915 (2017). https://doi.org/10.1007/s00421-017-3679-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3679-9