Abstract
Purpose
This study compared maximal eccentric (ECC) and concentric (CON) contractions of the elbow flexors for changes in transverse relaxation time (T2) and indirect markers of muscle damage.
Methods
Twelve young men performed five sets of six maximal isokinetic (30°/s) ECC with one arm followed by CON with the other arm. Magnetic resonance images to assess T2 and cross-sectional area (CSA) of biceps brachii, brachialis, and brachioradialis, and measurements of maximal voluntary isometric contraction (MVC) torque, range of motion (ROM), and muscle soreness were taken before, immediately after, and 1, 3, and 5 days after each exercise.
Results
MVC torque and ROM decreased greater after ECC than CON (p < 0.05), and muscle soreness developed only after ECC. Biceps brachii and brachialis CSA increased immediately after CON, but delayed increases in brachialis CSA were found only after ECC (p < 0.05). T2 of the muscles increased greater after CON (27–34 %) than ECC (16–18 %) immediately post-exercise (p < 0.05), but returned to baseline by 1 day after CON. The biceps brachii and brachialis T2 increased by 9–29 % at 1–5 days after ECC (p < 0.05). The post-ECC T2 changes showed no significant correlations with the changes in MVC torque, muscle soreness, and CSA, but the T2 increase immediately post-ECC was correlated with the peak T2 in 1–5-day post-ECC (r = 0.63, p < 0.05).
Conclusion
These results suggest that muscle activity during exercise was lower in ECC than CON, and the T2 changes after ECC do not necessarily relate to the changes in other indirect markers of muscle damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eccentric contractions, in which a contracting muscle is repeatedly lengthened by greater external force than muscle force, can induce greater muscle damage than concentric or isometric contractions (Clarkson and Sayers 1999; Lavender and Nosaka 2006; Proske and Morgan 2001). Muscle damage induced by eccentric contractions is characterised by prolonged decreases in maximal muscle strength, and the range of motion (ROM), development of delayed onset muscle soreness (DOMS), muscle swelling, increases in creatine kinase (CK) and/or myoglobin in the blood (Chen et al. 2011). Several studies have also shown that prolonged increases in transverse relaxation time (T2) of magnetic resonance imaging (MRI) indicate muscle damage (Foley et al. 1999; Larsen et al. 2007; Nosaka and Clarkson 1996).
Nosaka and Clarkson (1996) reported that T2 of biceps brachii and brachialis increased after the maximal eccentric exercise of the unilateral elbow flexors, peaked at 3–6 days and remained greater than baseline for 23 days after the exercise. They also showed that the magnitude of the increase in T2 reflected the magnitude of muscle damage (Nosaka and Clarkson 1996). Since concentric exercise does not induce muscle damage (Lavender and Nosaka 2006), it is reasonable to assume that T2 changes after concentric exercise are minimal, especially after 1-day post-exercise. In fact, Larsen et al. (2007) showed that T2 in adductor magnus did not increase immediately post-exercise for both legs, but significantly increased only for the eccentrically exercised leg at 9 days after 30-min step exercise in which eccentric contractions were performed by one leg and concentric contractions by the other leg.
Several studies used MRI to evaluate muscle activation in exercise, and showed that T2 increased greater with increasing in the exercise intensity for elbow flexors (Adams et al. 1992) and ankle dorsiflexors (Jenner et al. 1994). It has been shown that the magnitude of T2 increase immediately post-exercise was smaller for eccentric than concentric exercise (Adams et al. 1992). Adams et al. (1992) reported that the extent of T2 increase immediately after eccentric exercise was approximately 15–25 % smaller than that after concentric exercise consisting of 50 elbow flexions using a dumbbell that was set at 40, 60, 80, and 100 % of ten repetition maximum (RM) of concentric curls. This indicates that fewer muscle fibres were recruited in eccentric than concentric contractions at the same absolute intensity (Adams et al. 1992). However, it is not known whether this is also the case for maximal voluntary contractions, in which force production is potentially greater for eccentric than concentric contractions (Komi et al. 2000).
Regarding the relationship between the change in T2 immediately after eccentric exercise from baseline (acute T2 change) and that of several days after the exercise (delayed T2 change), Prior et al. (2001) reported no significant correlation between the two. They also showed that the change in T2 immediately after eccentric exercise from baseline did not relate to the magnitude of muscle damage represented by decreases in maximal voluntary contraction (MVC) strength, DOMS, and increases in muscle volume after eccentric exercise of the knee extensors. However, no previous study has investigated the relationship between the acute T2 change and the delayed T2 change for elbow flexor eccentric exercise. Since it has been shown that elbow flexor eccentric exercise induces greater muscle damage than leg muscle eccentric exercises (Chen et al. 2011), a clearer relationship between the acute T2 change and the delayed T2 change might be demonstrated, if any. Providing a smaller acute T2 change indicates that fewer muscle fibres are recruited as mentioned above, it might be that a smaller increase in T2 immediately post-exercise results in a greater increase in the T2 at 1–5-day post-exercise. It was also assumed that the smaller the acute T2 change, the greater the changes in indirect markers of muscle damage after eccentric exercise, since mechanical strain to the recruited muscle fibres would be greater when the acute T2 increase is smaller.
The present study, therefore, compared eccentric and concentric maximal exercises of the elbow flexors for changes in T2 before, immediately and for 5 days after exercise in relation to other indirect markers of muscle damage. This study also compared between eccentric and concentric elbow flexor exercises for the relationship between the acute T2 change and the delayed T2 change at 1–5-day post-exercise. The specific hypotheses tested in the present study were that (1) the acute T2 change would be smaller after maximum eccentric than maximum concentric exercise and (2) the acute T2 change after eccentric exercise would be negatively correlated with the delayed T2 change, and with the magnitude of decrease in muscle function, DOMS, and muscle swelling indicated by increases in muscle cross-sectional area (CSA).
Methods
Participants
The sample size was determined by a power analysis (G*power, version 3.0.10, Heinrich-Heine University, Dusseldorf, Germany) by setting the effect size as 1, α level of 0.05 and power (1−β) of 0.80 for the possible difference in criterion measures between eccentric and concentric exercises. It showed that at least ten participants were necessary, and thus, 12 men (age 28.1 ± 5.3 years, height 172.3 ± 6.4 cm, and body mass 68.8 ± 10.5 kg) who had not participated in any regular resistance training for at least 1 year prior to this study were recruited. They were requested to avoid any interventions that could affect the outcome measures shown below, such as massage and stretching during the experimental period. They were given detailed explanations of the study protocol before participation and signed an informed consent form in conformity with the Declaration of Helsinki. This study was approved by the local ethics committee for human experiments at the Juntendo University.
Experimental protocols
All participants performed maximal concentric (CON) and eccentric (ECC) exercises of the elbow flexors using one arm for CON and the other arm for ECC. The choice of the non-dominant or dominant arm for CON and ECC was randomised among the participants, such that ECC was performed by the non-dominant arm of six participants and by the dominant arm of other six participants. The two exercises were performed on the same day, and ECC was performed first followed by CON based on a previous study (Moore et al. 2005). The dependent variables included MVC torque, ROM, perceived muscle soreness by a visual analog scale (VAS), T2 of MRI, CSA of each muscle of the elbow flexors (i.e., biceps brachii, brachialis, and brachioradialis), and blood lactate. Blood lactate was measured immediately before and after exercise. Other measures were taken immediately before, immediately after (within 30 min) and 1, 3, and 5 days after each exercise. The order of the measurements performed immediately after each exercise was MRI, VAS, ROM, and MVC torque, and the MRI was performed within 3 min after each exercise. The same order was maintained for the measurements taken at 1, 3, and 5-day post-exercise. These measurements were taken in a room maintained at 24–26 °C, which was similar to that of the previous studies (Kouzaki et al. 2016; Tsuchiya et al. 2016).
Exercises
After a warm-up exercise consisting of 3–5 elbow flexions and extensions with a 2-kg dumbbell, each participant was seated on the chair of an isokinetic dynamometer (Biodex Multi-Joint System 3, NY, USA), and the arm was set at a shoulder joint angle of 45° flexion and the elbow joint was aligned with the rotation axis of the isokinetic dynamometer, while the lever arm of the isokinetic dynamometer was secured to the subject’s wrist in a supinated position. The ECC consisted of five sets of six maximal voluntary isokinetic (30°/s) eccentric contractions of the elbow flexors with a ROM from 90° flexion to 0° (full extension). The participants were verbally encouraged to maximally resist throughout the ROM for 3 s, and after each contraction, the isokinetic dynamometer returned the arm to the 90° flexed position at a constant velocity of 30°/s, creating a 3 s passive recovery between contractions. The CON consisted of five sets of six maximal voluntary isokinetic concentric contractions of the elbow flexors at 30°/s with a range of motion from 0° flexion to 90°. The contraction time of CON was the same as ECC. The rest period between sets was 90 s in both ECC and CON. The peak torque during concentric and eccentric contractions was recorded at a sampling rate of 100 Hz in a computer connected to the isokinetic dynamometer, and the elbow joint angle at the peak torque (peak torque angle) was also determined. The total work output during CON and ECC was calculated by the summation of 30 contractions using a software program (Biodex System 3 application, NY, USA).
Blood sample for lactate was collected from a finger using a refrigerated capillary tube (20 μl) before and 3 min after the exercise, and each sample was immediately placed to a tube with a solution. These samples were analysed using a Biosen C-line (EKF-Diagnostics, Cardiff, United Kingdom).
Muscle damage markers
Maximal voluntary isometric contraction (MVC) torque
MVC torque was measured on the same apparatus and positioning as those described for the eccentric exercise. Participants performed two 5-s MVCs at 90° elbow joint angle with a 15 s rest between contractions. A higher peak torque value of the two contractions was used as the MVC torque according to a previous study (Tsuchiya et al. 2014).
Range of motion (ROM)
To quantify the elbow joint ROM, two elbow joint angles (extended and flexed joint angles) were measured using a goniometer (Takase Medical, Tokyo, Japan). The extended joint angle was recorded when participants attempted to fully extend the elbow joint, with the elbow held by their side and the hand in supination. The flexed joint angle was determined when participants attempted to fully flex the elbow joint with the hand in supinated position. ROM was obtained by subtracting the flexed joint angle from the extended joint angle (Chen et al. 2012).
Muscle soreness
Muscle soreness was assessed using a 100-mm VAS in which 0 indicates “no pain” and 100 is “worst pain imaginable” (Chen et al. 2009). Each participant was asked to indicate the pain level on the scale when the investigator palpated the biceps brachii, brachialis, and brachioradialis, respectively, using a thumb, while the participant relaxed and let the arm down at the side (natural position). All tests were conducted by the same investigator who had practiced to apply the same pressure over time and among participants.
Magnetic resonance imaging (MRI)
MRI was used to assess T2 and CSA of biceps brachii, brachialis, and brachioradialis by a 0.3-T AIRIS-imaging system (Hitachi Medical, Tokyo, Japan). A T2-weighted spin-echo sequence (repetition time 2500 ms, echo time 30 and 90 ms, 256 × 256 matrix, and scan time 286 s) was applied to each arm using an extremity body coil, while each participant laid supine on the bed of the system. The images of biceps brachii and brachialis were obtained at two-thirds of the distance from the acromion to the lateral epicondyle of the humerus (Kulig et al. 2001). The image of brachioradialis was obtained at 6 cm below the lateral epicondyle of the humerus. Scanned images of MRI were transferred to a personal computer, and CSA was calculated manually by tracing each muscle using an image analysis software (Image J, Maryland, USA), and T2 was analysed with a software provided by the Aquarius Net Station (TeraRecon, California, USA). Three circular regions of interests (ROIs) in each image were set in the transverse section of the biceps brachii, brachialis, and brachioradialis, respectively, and care was taken not to include an area other than muscle, such as subcutaneous fat, tendon, and aponeuroses. The average T2 value of the three ROIs (each area = 200 mm2) for each muscle was calculated (Nosaka and Clarkson 1996). The T2 change between the pre-exercise and immediately post-exercise values was calculated using the average value of biceps brachii and brachialis, and was refereed to as acute T2 change. The delayed T2 change was defined as the difference between the baseline value and the highest value (the average of biceps brachii and brachialis) found at either 1, 3, or 5 days after exercise. For these analyses, the brachioradialis was excluded, since the changes in T2 of this muscle were minimum.
Statistical analyses
Changes in the dependent variables (peak torque, work, MVC torque, ROM, VAS, T2, CSA, and blood lactate) over time were compared between CON and ECC by a two-way repeated measure analysis of variance (ANOVA). When a significant interaction or time effect was found, a Bonferroni’s multiple comparison was followed as a post hoc test. Student’s t test was used to compare the CON and ECC in the total work output and peak torque angle. Pearson product–moment correlation coefficient was used to assess the relationships between selected variables, such as T2 change immediately post-exercise from baseline, peak T2 change in 1–5-day post-exercise from baseline, and the largest changes in MVC torque (peak MVC), ROM (peak ROM), CSA (peak CSA), and muscle soreness (peak DOMS) from baseline found in 1–5 days after exercise. For these variables except peak DOMS (absolute value was used), the magnitude of change from the baseline value was used for the correlation analyses. A significance level was set at p < 0.05. All variables are expressed as means ± standard deviations (SD).
Results
Concentric and eccentric exercises
The angle at peak torque was smaller (p < 0.05) for ECC (70.0 ± 7.7°) than CON (85.5 ± 10.4°). Peak torque during ECC was greater (p < 0.05) than that of CON at the first (ECC 63.0 ± 13.8 Nm and CON 48.9 ± 10.3 Nm) and second sets (ECC 56.1 ± 14.4 Nm and CON 44.1 ± 9.9 Nm), but no significant differences were observed in 3–5 sets (Fig. 1a). The work output of ECC (392.2 ± 85.9 J) was greater (p < 0.05) than that of CON (323.3 ± 81.6 J) for the first set only (Fig. 1b), and the total work did not differ between ECC (1475.9 ± 629.7 J) and CON (1325.8 ± 508.9 J). Blood lactate increased (p < 0.05) from the baseline (ECC 2.5 ± 0.8 mm/L and CON 2.5 ± 0.8 mm/L) to immediately after exercise (ECC 4.9 ± 1.2 mm/L and CON 5.4 ± 1.4 mm/L), without a significant difference between ECC and CON.
MVC torque and ROM
After CON, MVC torque decreased immediately (22.7 ± 13.0 %, p < 0.05) and 1 day (11.3 ± 7.6 %, p < 0.05) after exercise, but returned to baseline at 3-day post-exercise (Fig. 2a). After ECC, MVC torque decreased immediately after (32.7 ± 13.9 %, p < 0.05) and remained lower than the baseline at 1 (33.3 ± 12.6 %, p < 0.05) and 3 days (16.5 ± 9.7 %, p < 0.05) after exercise. MVC torque was greater for CON than ECC at 1, 3, and 5 days after exercise (p < 0.05). As shown in Fig. 2b, ROM decreased immediately post-exercise for both ECC and CON (p < 0.05), but decreases from the baseline at 1–5-day post-exercise were found only for ECC (p < 0.05). ROM was smaller for ECC than CON immediately, 1, 3, and 5 days after exercise (p < 0.05).
Changes (mean ± SD) in maximal voluntary isometric contraction (MVC) torque (a) and range of motion (ROM) (b) before (pre), immediately after (0), and 1, 3, and 5 days after concentric (CON) and eccentric (ECC) exercises. *Denotes a significant (p < 0.05) difference between CON and ECC. # Denotes a significant (p < 0.05) difference from pre-exercise value
Muscle soreness
Muscle soreness did not develop at all time points after CON, but developed after ECC for biceps brachii, brachialis, and brachioradialis (Fig. 3). The biceps brachii soreness developed at 1 day, and remained higher than the baseline at 3 and 5-day post-exercise (p < 0.05). The brachialis soreness developed at 1-day post-exercise, and remained higher than the baseline at 3-day post-exercise, but the brachioradialis soreness was evident at only 1-day post-exercise.
Changes (mean ± SD) in muscle soreness assessed by visual analog scale (VAS) for the palpation of biceps brachii (a), brachialis (b), and brachioradialis (c) before (pre), immediately after (0), and 1, 3, and 5 days after concentric (CON) and eccentric (ECC) exercises. *Denotes a significant (p < 0.05) difference between CON and ECC. #Denotes a significant (p < 0.05) difference from pre-exercise value
Cross-sectional area (CSA)
Changes in CSA of biceps brachii, brachialis, and brachioradialis are shown in Fig. 4. No significant difference between ECC and CON was found for brachioradialis that did not show significant changes, but a significant interaction effect was evident for biceps brachii and brachialis. CSA of biceps brachii increased immediately post-exercise (21.3 ± 17.9 %, p < 0.05) for CON, but no such increase was seen after ECC. An increase in CSA of brachialis was observed immediately post-exercise for CON (21.2 ± 19.0 %, p < 0.05), but it returned to baseline by 1-day post-exercise. In contrast, CSA of brachialis increased (p < 0.05) at 1 (24.9 ± 29.8 %), 3 (41.1 ± 47.9 %), and 5 days (36.2 ± 36.9 %) after ECC.
Changes (mean ± SD) in cross-sectional area (CSA) of biceps brachii (a), brachialis (b), and brachioradialis (c) before (pre), immediately after (0), and 1, 3, and 5 days after concentric (CON) and eccentric (ECC) exercises. *Denotes a significant (p < 0.05) difference between CON and ECC, and n.s. shows no significant difference between CON and ECC. #Denotes a significant (p < 0.05) difference from pre-exercise value
T2
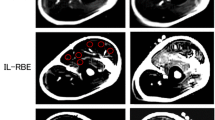
MR images taken from the upper arm of one participant are shown in Fig. 5a. A distinct difference between CON and ECC is seen, such that an increased T2 was found only immediately after CON, but increased T2 was observed for 5 days after ECC. The increase in T2 after ECC was greater for brachialis than biceps brachii and brachioradialis. This is also shown for the averaged results of all participants, as shown in Fig. 6. No significant difference in T2 was found for the baseline between ECC and CON, and between biceps brachii, brachialis, and brachioradialis. A significant interaction effect was found for all muscles, and T2 changed significantly for biceps brachii and brachialis, but not brachioradialis after ECC. T2 of biceps brachii and brachialis increased immediately post-exercise for CON and ECC, but the magnitude of the increase was greater for CON (biceps brachii 34 ± 8 %, brachialis 27 ± 9 %, p < 0.05) than ECC (biceps brachii 18 ± 10 %, brachialis 16 ± 10 %, p < 0.05). T2 returned to baseline by 1-day post-exercise for CON, but was still significantly elevated above baseline for biceps brachii (9–15 %, p < 0.05) and brachialis (17–29 %, p < 0.05) at 1–5 days after ECC.
Changes (mean ± SD) in transverse relaxation time (T2) of biceps brachii (a), brachialis (b), and brachioradialis (c) before (pre), immediate after (0), and 1, 3, and 5 days after concentric (CON) and eccentric (ECC) exercises. *Denotes a significant (p < 0.05) difference between CON and ECC. #Denotes a significant (p < 0.05) difference from pre-exercise value
Correlations between T2 and other measures
The change in T2 immediately after ECC or CON (i.e., acute T2 change) was not significantly correlated with the absolute peak torque or the total work generated both in the first two sets and in the five sets in ECC or CON. The acute T2 change was not significantly correlated with any muscle damage markers for CON. A significant (p < 0.05) correlation was found between the acute T2 change and the delayed maximal T2 change after ECC (r = 0.63, Fig. 7a). The relationships between the magnitude of the T2 changes (acute and delayed) and muscle damage markers after ECC are shown in Table 1. A significant (p < 0.05) correlation was evident only for the delayed T2 and peak ROM (r = −0.83; Fig. 7b).
Relationship between the magnitude of change in T2 (average of biceps brachii and brachialis) immediately after eccentric exercise (acute T2 change) and the magnitude of maximal T2 change found at either 1, 3, or 5 days after eccentric exercise (delayed T2 change) (a), and the magnitude of the delayed T2 change and the magnitude of the largest change in the range of motion around the elbow joint found at either 1, 3, or 5 days after eccentric exercise (peak ROM change) (b)
Discussion
The present study tested the two hypotheses: (1) the acute T2 change would be smaller after eccentric than concentric exercise and (2) the acute T2 change would be negatively correlated with delayed T2 changes, and the magnitude of changes in muscle damage markers at 1–5 days after eccentric exercise. The results showed that T2 increased greater after CON than ECC immediately after exercise, but returned to baseline by 1-day post-exercise for CON, while T2 was still elevated at 1–5 days after ECC (Figs. 5, 6). The acute T2 change after ECC and the peak T2 change found at 1–5 days after ECC were positively correlated (r = 0.63, Fig. 7a). The magnitude of acute T2 change after ECC showed no correlation with the peak MVC torque, peak CSA, and peak DOMS (Table 1). These results support the first hypothesis, but not the second one.
As shown in Fig. 1, peak torque and work output were greater for the first and second sets during ECC compared to CON. It has been reported that the elbow flexors could produce approximately 30 % greater force during isokinetic eccentric than concentric contractions (Komi et al. 2000). The present study also showed that peak torque in ECC in the first two sets was approximately 30 % greater than that in CON. It is possible that the greater decrease in peak torque during ECC than CON was due to muscle damage induced by eccentric contractions. The peak torque angle during ECC (70.7 ± 7.7°) was similar to that of the previous study (Komi et al. 2000). The peak torque angle during CON (85.5 ± 10.4°) in the present study was also similar to that of the previous study (Chen et al. 2012).
In spite of the greater peak torque, the T2 increase of the biceps brachii, brachialis, and brachioradialis immediately after ECC was significantly smaller than that after CON (Fig. 6). It has been shown that T2 reflects the recruitment of motor units (Adams et al. 1992; Jenner et al. 1994). Akima (2012) reported that the muscle fibre recruitment was strongly correlated with the T2 increase immediately post-exercise for triceps surae muscles. Using electromyography (EMG), Qi et al. (2011) showed that biceps brachii muscle activity was approximately 30 % lower during isokinetic (30°/s) eccentric than concentric contractions at 80 % of maximal voluntary concentric contraction torque. Komi et al. (2000) compared maximal isokinetic eccentric and concentric contractions of the elbow flexors for four different velocities, and showed that EMG activity during eccentric contractions was approximately 15–30 % lower when compared with concentric contractions. In the present study, the acute T2 increase was 63–93 % greater for CON than ECC. These values were greater than those reported in the study by Qi et al. (2011), in which submaximal contractions were performed, and also those reported in the study by Komi et al. (2000), in which maximal contractions were performed. It was stated that MRI T2 relaxation time change immediately after exercise showed cumulative muscle activity during exercise, whereas EMG was representative of muscle activity of the time of muscle contraction (Kulig et al. 2001). Thus, the smaller T2 increase immediately after ECC than CON suggests that fewer muscle fibres were recruited during ECC, in spite of the greater peak torque during first two sets in ECC than CON. Because of this, it seems reasonable to assume that greater mechanical strain was imposed to the muscle fibres in ECC than CON, resulting in greater muscle damage after ECC than CON.
Changes in MVC torque (Fig. 2a), ROM (Fig. 2b), and DOMS (Fig. 3) after ECC were comparable to those reported in the previous studies in which a similar eccentric exercise of the elbow flexors to that of the present study was performed (Chapman et al. 2006; Chen et al. 2009). The present study showed that brachialis CSA significantly increased from 1 to 5 days (25–35 %) after ECC, but this was not the case for CON, and no significant CSA increases were evident for biceps brachii and brachioradialis even after ECC. Nosaka et al. (2001) reported that brachialis was more damaged by eccentric exercise of the elbow flexors than biceps brachii. Kawakami et al. (1994) reported that brachialis contributed more (47 %) than biceps brachii (34 %) and brachioradialis (19 %) for the force production of the elbow flexors during isometric, concentric, and eccentric contractions. Therefore, the increase in brachialis CSA probably indicates that brachialis was more involved in ECC than the other two agonist muscles. This may explain why the increases in T2 at 1–5 days after ECC were greater for brachialis than biceps brachii.
The increases in T2 of biceps brachii and brachialis in the present study after eccentric contractions were smaller and shorter-lasting than those reported in the previous studies (Nosaka et al. 2001; Nosaka and Clarkson 1996). For example, Nosaka and Clarkson (1996) showed that the T2 of biceps brachii and brachialis peaked 3–6 days (approximately 70 % increase) after 24 maximal eccentric contractions of elbow flexors and remained elevated for 23 days after exercise (approximately 25 % increase). It should be noted that muscle strength decreased 45 % immediately post-exercise and remained 30 % lower than the baseline at 5-day post-exercise in the study of Nosaka and Clarkson (1996), which were much greater than those found in the present study. It seems possible that the smaller changes in T2 in the present study after ECC were due to less muscle damage when compared with the previous study (Nosaka and Clarkson 1996).
As shown in Fig. 7a, the magnitude of the increase in T2 immediately after eccentric exercise was positively correlated with the magnitude of maximal increase in T2 found between 1 and 5 days post-exercise. This was against the hypothesis that the acute T2 change would be negatively correlated with the delayed T2 change, based on the assumption that a smaller acute T2 change would reflect less muscle fibres that were recruited during ECC. It was thought that if less muscle fibres were recruited during maximal eccentric contractions, mechanical strain to the recruited muscle fibres would be greater, possibly resulting in greater muscle damage reflected in greater increases in T2 in 1–5-day post-exercise. However, Fig. 7a shows that the greater the increase in T2 immediately post-ECC, the greater the increase in T2 at 1–5-day post-exercise. It should be noted that the acute T2 change was not correlated with total work generated during ECC. As discussed above, the magnitude of increase in T2 immediately after exercise appears to represent the overall use of a muscle, and thus, it seems unlikely that the T2 increase immediately post-exercise represents how motor units were recruited during the exercise. It may be that the greater T2 increase immediately after ECC simply represents greater exercise effort. If so, it is possible that the participants whose T2 increase immediately post-exercise was large produced greater force for the whole range of motion induced greater muscle damage than those whose T2 increase immediately post-exercise was small.
As shown in Table 1, no significant correlations were evident between the magnitude of the T2 change immediately after ECC and the magnitude of change in the muscle damage markers. This suggests that the acute T2 change does not predict the magnitude of muscle damage. Regarding the relationships between the peak T2 change from baseline found in 1–5-day post-ECC and the indirect muscle damage markers, a significant correlation was only found for ROM (Table 1). Nosaka and Clarkson (1996) reported large changes in T2 and indirect muscle damage markers after the eccentric exercise of the elbow flexors, but did not report the correlations between the T2 and the muscle damage markers. Prior et al. (2001) reported no significant correlations between the acute T2 change and MVC, CSA, and muscle soreness. This was in line with the findings of the present study. It is interesting that the delayed T2 change was significantly correlated with the peak ROM change (Fig. 7b). This may suggest that the cause of the delayed increase in T2 and the decrease in ROM is associated. It is possible that the decrease in ROM is partially associated with muscle swelling (Howell et al. 1993). It is stated that the increased T2 after eccentric exercise is due to oedema (Foley et al. 1999; Nosaka and Sakamoto 2001), probably associated with increased free water in extracellular matrix (Rodenburg et al. 1994). However, the present study found no correlation between the magnitude of T2 change (acute and delayed) and change in CSA. This may be due to the relatively small changes in T2 and CSA. Further research is necessary to investigate the association between T2 and ROM changes and if T2 and CSA changes are related when both show larger increases after eccentric exercise as reported in the previous studies (Foley et al. 1999; Larsen et al. 2007; Nosaka and Clarkson 1996).
In summary, muscle activation was lower during maximal eccentric than maximal concentric contractions of the elbow flexors, which was indicated by the smaller increase in T2 immediately after eccentric than concentric contractions. However, increases in T2 from baseline at 1–5-day post-exercise were evident only after eccentric exercise, and the magnitude of increase in T2 immediately after eccentric exercise was positively correlated with the magnitude of increase in T2 at 1–5 days after the exercise. These results suggest that the cause of T2 increase is not the same between immediately and days after exercise. More studies are required to investigate the mechanisms underpinning the delayed T2 increase after eccentric exercise.
Abbreviations
- ANOVA:
-
Analysis of variance
- CK:
-
Creatine kinase
- CON:
-
Concentric contraction
- CSA:
-
Cross-sectional area
- DOMS:
-
Delayed onset muscle soreness
- ECC:
-
Eccentric contraction
- EMG:
-
Electromyography
- MRI:
-
Magnetic resonance imaging
- MVC:
-
Maximal voluntary isometric contraction
- ROI:
-
Region of interest
- ROM:
-
Range of motion
- T2:
-
Transverse relaxation time
- VAS:
-
Visual analog scale
References
Adams GR, Duvoisin MR, Dudley GA (1992) Magnetic resonance imaging and electromyography as indexes of muscle function. J Appl Physiol 73:1578–1583
Akima H (2012) Evaluation of functional properties of skeletal muscle using functional magnetic resonance imaging (fMRI). J Phys Fitness Sports Med 1:621–630
Chapman DW, Newton M, Sacco P, Nosaka K (2006) Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med 27:591–598
Chen TC, Chen HL, Lin MJ, Wu CJ, Nosaka K (2009) Muscle damage responses of the elbow flexors to four maximal eccentric exercise bouts performed every 4 weeks. Eur J Appl Physiol 106:267–275
Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K (2011) Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol 111:211–223
Chen HL, Nosaka K, Chen TC (2012) Muscle damage protection by low-intensity eccentric contractions remains for 2 weeks but not 3 weeks. Eur J Appl Physiol 112:555–565
Clarkson PM, Sayers SP (1999) Etiology of exercise-induced muscle damage. Can J Appl Physiol 24:234–248
Del Valle A, Thomas CK (2005) Firing rates of motor units during strong dynamic contractions. Muscle Nerve 32:316–325
Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA (1999) MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol 87:2311–2318
Howell JN, Chleboun G, Conatser R (1993) Muscle stiffness, strength loss, swelling and soreness following exercise-induced injury in humans. J Physiol 464:183–196
Jenner G, Foley JM, Cooper TG, Potchen EJ, Meyer RA (1994) Changes in magnetic resonance images of muscle depend on exercise intensity and duration, not work. J Appl Physiol 76:2119–2124
Kawakami Y, Nakazawa K, Fujimoto T, Nozaki D, Miyashita M, Fukunaga T (1994) Specific tension of elbow flexor and extensor muscles based on MRI. Eur J Appl Physiol 68:139–147
Komi PV, Linnamo V, Silventoinen P, Sillanpää M (2000) Force and EMG power spectrum during eccentric and concentric actions. Med Sci Sports Exerc 32:1757–1762
Kouzaki K, Nosaka K, Ochi E, Nakazato K (2016) Increases in M-wave latency of biceps brachii after elbow flexor eccentric contractions in women. Eur J Appl Physiol 116:939–946
Kulig K, Powers CM, Shellock FG, Terk M (2001) The effects of eccentric velocity on activation of elbow flexors: evaluation by magnetic resonance imaging. Med Sci Sports Exerc 33:196–200
Larsen RG, Ringgaard S, Overgaard K (2007) Localization and quantification of muscle damage by magnetic resonance imaging following step exercise in young women. Scand J Med Sci Sports 17:76–83
Lavender AP, Nosaka K (2006) Changes in fluctuation of isometric force following eccentric and concentric exercise of the elbow flexors. Eur J Appl Physiol 96:235–240
Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ (2005) Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288:E1153–E1159
Nosaka K, Clarkson PM (1996) Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 28:953–961
Nosaka K, Sakamoto K (2001) Effect of elbow joint angle on the magnitude of muscle damage to the elbow flexors. Med Sci Sports Exerc 33:22–29
Nosaka K, Sakamoto K, Newton M, Sacco P (2001) How long does the protective effect on eccentric exercise-induced muscle damage last? Med Sci Sports Exerc 33:1490–1495
Prior BM, Jayaraman RC, Reid RW, Cooper TG, Foley JM, Dudley GA, Meyer RA (2001) Biarticular and monoarticular muscle activation and injury in human quadriceps muscle. Eur J Appl Physiol 85:185–190
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537:333–345
Qi L, Wakeling JM, Ferguson-Pell M (2011) Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. J Electromyogr Kinesiol 21:1056–1063
Rodenburg JB, de Boer RW, Schiereck P, van Echteld CJ, Bär PR (1994) Changes in phosphorus compounds and water content in skeletal muscle due to eccentric exercise. Eur J Appl Physiol Occup Physiol 68:205–213
Tsuchiya Y, Sakuraba K, Ochi E (2014) High force eccentric exercise enhances serum tartrate-resistant acid phosphatase-5b and osteocalcin. J Musculoskelet Neuronal Interact 14:50–57
Tsuchiya Y, Yanagimoto K, Nakazato K, Hayamizu K, Ochi E (2016) Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: a randomized, double-blind, placebo-controlled, parallel-group trial. Eur J Appl Physiol 116:1179–1188
Acknowledgments
This study was supported by the Grant-in-Aid for Young Scientists (B; 20614473).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Ochi, E., Tsuchiya, Y. & Nosaka, K. Differences in post-exercise T2 relaxation time changes between eccentric and concentric contractions of the elbow flexors. Eur J Appl Physiol 116, 2145–2154 (2016). https://doi.org/10.1007/s00421-016-3462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3462-3