Abstract
Purpose
We investigated the muscle activation patterns and the center of pressure (COP) displacement in stepping behavior to determine the relations between anticipatory synergy adjustments (ASAs) and anticipatory postural adjustments (APAs) during support surface translation.
Methods
Surface muscle activity of eleven leg and trunk muscles was analyzed to identify sets of four muscle modes (M-modes). Linear combination of M-modes and their relationship to changes in the COP shift in the anterior–posterior (AP) direction were then determined. Uncontrolled manifold (UCM) analysis was performed to determine variance components in the M-mode space and indices of M-mode synergy stabilizing the COP shift.
Results
Prior to the step initiation, synergies stabilizing COP were seen in both conditions. The synergy index started to drop before a change in the averaged activation levels across trials in postural muscles. The magnitude of synergy index was significantly larger under the perturbation condition.
Conclusions
Results of the study have revealed that the central nervous system is able to prepare multi-muscle synergies when a step is performed during support surface translation. Prior to APAs, ASAs reduce stability of COPAP coordinate that is to be adjusted during the APAs. These findings may help get closer to understanding of physiological mechanism of postural preparation to external perturbation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stepping is one of the most important control strategies to adjust posture and avoid falling after a perturbation. The ability to take a step to the constraints imposed by the environment requires controlling the relative motion between the whole-body center of mass and its base of support, with large inertial forces that could potentially threaten stability. There are reproducible mechanical events preceding the take-off of the stepping foot. These early adjustments have been assumed to allow unloading the stepping leg and to create a moment of the vertical force rotating the body about the ankle joints (Ito et al. 2003). The mechanisms of balance maintenance in volitional stepping with preferred spatial–temporal dynamics provide insight into the neural control of posture and movement.
Since Bernstein (1967) used the notion of synergies as the means of solving the notorious problem of motor redundancy, postural control has been viewed as the problem of coordinated changes in the activation of numerous muscles of the lower extremities and the trunk. Muscle synergies have been shown to provide for flexible control of a variety of postural tasks and require good multi-muscle coordination. Some researchers defined synergies to be muscle groups with parallel scaling of activation levels (d’Avella et al. 2003; Ivanenko et al. 2004, 2005; Torres-Oviedo et al. 2006; Ting 2007), while others defined them as elemental variable forming a basis on which synergies are built (Krishnamoorthy et al. 2004; Wang et al. 2005; Robert et al. 2008; Klous et al. 2011; Krishnan et al. 2012). In particular, the analysis of multi-muscle synergies based on the uncontrolled manifold (UCM) hypothesis has provided a useful tool for quantitative assessment (Schöner 1995; Scholz and Schöner 1999; Latash 2010). Within this analysis, synergies are associated with co-varied adjustments of independent elemental variables (for example, electromyographic signals, EMGs) that stabilize values or time profiles of a specific performance variable (for example, COP trajectory), to which all the elemental variables contribute (Latash 2010).
Previous studies have shown anticipatory synergy adjustments (ASAs) in preparation to multi-muscle postural tasks (Klous et al. 2011; Krishnan et al. 2012). During steady-state standings, postural muscles show high indices of co-variation stabilizing such variables as COP coordinate and shear force magnitude (Robert et al. 2008; Wang and Asaka 2008; Klous et al. 2011; Krishnan et al. 2011, 2012). If a subject is required to produce a quick action from a steady-state, the indices of co-variation drop are delayed until the action initiation. ASAs have been shown to shift toward the time of action initiation under the simple reaction-time instruction (Wang et al. 2006a, b), and be delayed and reduced in magnitude in the elderly (Wang et al. 2013, 2015a, b).
Several recent studies have provided evidence that ASAs are associated with postural adjustments to minimize the impact of the expected perturbation without producing major COP shifts. These adjustments represent changes in the activation levels of trunk and leg muscles seen about 150 ms prior to action initiation (Klous et al. 2011; Bertucco et al. 2013). As such, ASAs differ from anticipatory postural adjustments (APAs) observed about 100 ms prior to an expected perturbation, which counteract the effects of perturbation on posture. It is considered that ASAs and APAs represent changes in muscle activation patterns that are produced by the central nervous system in anticipation of an action (Klous et al. 2012).
Note that early postural adjustments (EPAs) and APAs have been known for a long time (Belen’kii et al. 1967; Elble et al. 1994). Despite the evident differences in their characteristic timing, both postural adjustments have frequently been addressed as APAs. When a person prepares to make a whole-body action, for example to take a step, postural adjustments are seen several hundred ms prior to the stepping foot take-off (Couillandre et al. 2002; Elble et al. 1994; Lepers and Breniere 1995; Halliday et al. 1998), which is much earlier than typical APAs. Indeed, EPAs and APAs are two different phenomena with variable timing: EPAs are seen 400–500 ms and APAs, 100 ms or so prior to an action (Krishnan et al. 2012). In the current study, we used APAs for postural adjustments before taking a step, the timing of about 100 ms prior to toe off.
To explore the relations between ASAs and APAs in postural preparation to action, we investigated the muscle activation patterns and the early shifts of COP in stepping behavior using a support surface translation paradigm. In this paradigm, postural responses to support surface translations were induced in standing position through a custom-designed movable platform with a force plate. Indeed, perturbations of a support surface translation are widely used to study postural control (Horak and Nashner 1986; Torres-Oviedo and Ting 2007; Wang et al. 2010). In the current study, support surface perturbations were used to investigate multi-muscle synergies involved in feed-forward postural adjustments using the uncontrolled manifold (UCM) approach. When externally perturbed, understanding the manner in which muscle synergies are used might actually be more relevant for studying the mechanisms underlying falls in daily life. We compared the organization of leg and trunk muscles into groups (M-modes) and co-variation of M-mode involvement (M-mode synergies) between the stable and perturbed conditions. Based on the previous study, our prediction was that both the stable and perturbed conditions would be characterized by ASAs seen prior to APAs during preparation to stepping. Because the nervous system takes advantage of available abundance of the neuromuscular system, we expected ASAs to be stronger in condition when the support surface was translated as compared to when it was stable. If supported, the hypotheses will have important implications for developing effective fall prevention interventions of healthy elderly.
Methods
Subjects
Eight healthy young subjects (four males and four females) aged 21–25 participated in the experiment. The mean age of the subjects was 23.4 ± 1.5 years; mean body mass 59.0 ± 3.6 kg; and mean height 166.3 ± 4.9 cm. All participants were right foot-dominant according to preferred foot usage when kicking a ball, stepping up on a chair, and leaping off in the long jump (Nachshon et al. 1983). They gave their informed consent to take part in this study, which is consistent with the 1964 Declaration of Helsinki.
Experimental setup and procedures
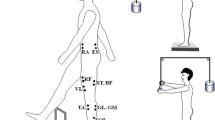
Backward perturbations were induced in standing position through a custom-designed movable platform with a force plate. The subjects were instructed to stand with weight equally distributed between the two feet, and their arms hanging loosely by their sides. Sagittal plane balance was perturbed at random intervals by the movable platform in the backward direction. The platform’s displacement was 5 cm, peak velocity was 12 cm/s, and peak acceleration was 0.1 g.
The experimental condition was taking a step forward with the right leg in response to perturbations from an original posture of side-by-side quiet stance. The task goal was to take a single step from a stationary position and subsequently stand as still as possible without further adjustments to the foot placement. In the initial position, subjects maintained a comfortable posture with their feet placed shoulder width apart while standing on the force plate installed on the movable platform. This foot position was marked on the top of the force plate to retain consistent foot placement across all the trials. The subjects were instructed to look straight ahead throughout the trial, and react naturally to backward perturbation of the support surface. In the normal stepping task (STNS), the subjects were free to initiate the step following a ‘get ready’ cue. In the perturbation stepping task (STPS), they were instructed that at any given time instance; the plat form would suddenly translate backward. The subjects were asked to respond in a natural way to take a step with preferred spatial–temporal dynamics.
Before the start of data collection, 1–3 practice trials, in each experimental condition, were given to all subjects for familiarization with the task. The subjects were free to choose their preferred pace and distance when taking a step. The total of 20 trials for each condition was performed. Four blocks of normal stepping trials (STNS) were alternated with four blocks of perturbation stepping trials (STPS). Two different conditions were thus presented in blocks of five trials. There were at least a 6-s interval between trials and a 5-min interval after four blocks (20 trials) to reduce the effects of muscular fatigue. For safety precautions, an assistant stood in reaching distance behind the subject to prevent a fall in each perturbation trial.
Data collection
Electromyographic (EMG) activity was recorded from eleven lower limb and trunk muscles of the subject’s right side. After the skin was shaved and cleaned with alcohol, bipolar electrodes were placed on the muscle bellies and oriented in the direction of the muscle fibers. The surface EMG (Noraxon Telemyo 2400t V2, Scottsdale, AZ, USA) was recorded from the following muscles: tibialis anterior (TA), lateral head of gastrocnemius (GL), medial head of gastrocnemius (GM), soleus (SOL), rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), biceps femoris (BF), semitendinosus (ST), rectus abdominis (RA), and erector spinae (ES). The electrodes were placed in pairs with the center-to-center distance of 25 mm; the ground electrode was placed over the epicondyle of the tibia. The Telemyo has an internal band-pass filter of 10–500 Hz to remove known non-muscle frequencies. Raw EMG data were digitized at the sampling frequency of 1,500 Hz. Kinetic data were collected at 1500 Hz from a force platform under the feet (Kistler, 9281B, Winterthur, Switzerland). A foot switch was attached under the heads of the metatarsal bones of right foot to measure the timing of toe off, and thin socks were used to secure the sensor in place.
Data processing
The data were processed offline using MATLAB 8.0 software (MathWorks, Natick, MA). Raw EMG data were rectified and filtered with a fourth-order, zero-lag Butterworth low-pass filter with a cut-off frequency at 50 Hz. Signals from the force plate were low-pass filtered at 20 Hz. The ‘time zero’ (t 0) was defined by the toe off time using the signal from the foot switch. Then, all trials were aligned to t 0 and integrals of EMGs were calculated. In order to do this, the data for further analysis were selected in the range from −1000 ms (before t 0) to t 0, out of which −1000 ms to −900 ms of the data in the STNS condition were taken for the baseline activity.
To identify the initiation of APAs for EMG data, we defined t EMG as the instant in time when the average muscle activation across trials for each condition differed by more than ±2 standard deviations from the baseline activity for at least 25 ms continuously. Each muscle was assigned one tEMG value that corresponded to either a burst or an inhibition.
Rectified EMG signals for each muscle for each subject were integrated over 10-ms intervals in a time window from −250 ms to t 0. These EMG integrals for each of 10 ms were corrected by subtracting the EMG integrals of the averaged 10 ms baseline activity. The outcome of the adjusted EMG integral will be denoted as IEMG. ∆IEMG indices were further normalized (∆IEMGN) to the maximum integral magnitude across conditions. This method of normalization was used in earlier studies of muscle modes and synergies (Wang et al. 2005, 2014, 2015a, b). Five 50 ms time windows in relation to t 0 were analyzed: T1 {−250, −200 ms}, T2 {−200, −150 ms}, T3 {−150, −100 ms}, T4 {−100, −50 ms}, and T5 {−50, 0 ms}. Different time intervals from −250 ms prior to t 0 up to t 0 allow getting insight into possible time development of the multi-muscle synergies.
Defining M-modes using principal component analysis (PCA)
We extracted groups of muscles (M-modes) from the IEMGN data matrix within the time window in relation to t 0 from −200 ms to t 0 using PCA. For each subject, the IEMGN data formed a matrix of 20 time intervals × 11 muscles × 20 trials = 4400 data points. The correlation matrix among the IEMG was subjected to principal component analysis with Varimax rotation, using procedures from SPSS (SPSS, Inc., Chicago, IL, USA). The factor analysis module with principal component extraction was employed.
For each subject, the obtained eigenvalues and PCs were then considered. To ensure that each muscle was significantly loaded on at least one PC, we chose to accept the first four PCs (described in more detail in “Results”) for further analysis. This was determined by examining of the scree plots and having at least one muscle significantly loaded per PC (Wang et al. 2005; Krishnan et al. 2011). In particular, scree plots displayed an inflection point after the fourth PC; PCs with a number higher than four accounted for similar fractions of the remaining variance per PC. We are going to address these PCs as muscle modes (M-modes) and assume that magnitudes of (coefficients at) the M-modes are manipulated by the controller to produce COPAP shifts. A reciprocal M-mode is defined as a pattern with significant loading coefficients on the ventral muscles (“push-back” mode), or on the dorsal muscles (“push-forward” mode), while a co-contraction M-mode is defined as a pattern with significant loading coefficients on the same M-mode with the same sign for two muscles with opposing action at a particular joint (Asaka et al. 2008).
Defining the Jacobian using multiple regression
Small changes in the magnitudes of M-modes (∆M) were related to the change in the COPAP shifts (∆COPAP) through the Jacobian (J). Multiple linear regression analysis over the trials was used to define the J for each subject separately. The J was estimated as coefficients of multiple linear regression between ∆M and ∆COPAP.
UCM analysis
For each trial of the STNS and STPS tasks, ∆IEMGN were computed and transformed into ∆Ms by multiplying the loadings of the individual M-mode. The mean magnitudes of each ∆M for a selected time interval across a series of ST trials were computed. Since the model relating ∆Ms to ∆COPAP is linear, the mean values were subtracted from each computed value, and the residuals were further analyzed.
The UCM represents different combinations of M-modes that keep the value of ∆COPAP unchanged. The UCM was estimated as the null space of the corresponding J matrix. The null space is spanned by the basis vectors, ɛ i. The vector of individual mean-free ∆Ms was resolved into its projection onto the null space and the orthogonal subspace:
where n is the total number of M-modes (n = 4) and d is the number of degree of freedom describing the task, i.e., one-dimensional COPAP shift (d = 1).
Variance per degree of freedom within the UCM and orthogonal to the UCM across trials was computed as
We computed an index of synergy (ΔV) reflecting the difference between the variance within the UCM and orthogonal to the UCM:
where all variance indices are computed per degree of freedom; V TOT means the total variance. For further analyses, the ΔV values were transformed using a Fisher’s z-transformation (ΔV Z ) adapted to the boundaries of ΔV:
The time of ASA initiation, t ASA, was calculated for the z-transformed values of ∆V (∆V Z ). The rate of change of ∆V Z was computed from t 0 to −250 ms backwards; the time t ASA was defined by two criteria: (1) when the magnitude of this rate was equal to zero (d∆V Z /dt = 0), which was considered as the start of a drop and (2) the drop had to be larger than 20 % of the ∆V Z magnitude at the time of d∆V Z /dt = 0.
Statistics
All descriptive statistics are reported in the text and figures as means and standard deviations. The fractions of variance explained by the first four principal components were transformed into z-scores using standard Fisher’s z-transformation. Paired t tests were performed to analyze differences between the two conditions (STNS and STPS) in the z-scores and the peak COPAP shifts. Two-way repeated measures ANOVA was used with factors condition and interval to analyze possible changes in the ∆V Z values across the conditions. Whenever the Mauchly’s test of sphericity was not met in the ANOVA, Greenhouse-Geisser correction was made. For all statistical analyses, p value less than 0.05 was set as a measure of significance.
Results
EMG and COPAP patterns
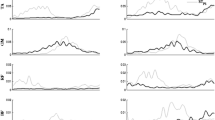
There were common features across the EMG patterns in both conditions. Figure 1 shows the rectified EMGs averaged across trials for a representative subject for selected muscles during the STNS and STPS conditions. Before the EMGs were averaged, the trials were aligned by the time (t 0) of toe off in the foot switch signal. These particular muscles were selected because they showed clear anticipatory postural adjustments across subjects.
Typical EMG patterns averaged across trials by a representative subject for the STNS (dark lines) and STPS (gray lines) conditions. Time zero (t 0) corresponds to the alignment time, the time of toe off. The dorsal muscles showed a decrease in the activity just before the step initiation starting −100 to −50 ms before t 0. In the STPS task, there was a substantial increase in the level of activity of most muscles. The EMGs were recorded in muscles of the right side of the body. The EMG scales are in arbitrary units and time is in ms (TA tibialis anterior, GL lateral head of gastrocnemius, RF rectus femoris, BF biceps femoris)
In the STNS condition, the stepping leg typically showed alternating bursts of activity in the ventral and dorsal muscles. The dorsal muscles showed a decrease in the activity just before the step initiation starting −100 to −50 ms before t 0. We refer to these transient EMG changes as APAs. In the STPS task, there was a substantial increase in the level of activity of most muscles. The regularities in the patterns of activation of the leg and trunk muscles were consistently observed in the STNS and STPS conditions.
Changes in the muscle activity before step initiation occurred earlier in the STNS condition than in the STPS condition. In the STNS condition, the earliest change in the muscle activity in TA, GL, RF, and BF averaged subjects was 91 ± 15 ms before t 0. In the STPS condition, APAs occurred on average 45 ± 11 ms before t 0. The difference was statistically significant (p < 0.05).
In preparation to stepping, subjects shifted the COP in the AP direction backwards. This adjustment allowed to unload the stepping leg and to create a moment of the reactive force rotating the body forward about the ankle joints. In terms of magnitude, anticipatory COPAP displacement in the STNS task (COPAP-NS = −1.91 ± 0.84 cm) was larger as compared to the STPS task (COPAP-PS = −3.95 ± 1.09 cm); the difference was statistically significant (p < 0.05). Negative values correspond to backward displacements.
PCA and multiple regression analysis
On an average, four principal components (PCs) accounted for the 60.8 ± 6.4 % total variance in the muscle activation space in the STNS task and 62.2 ± 6.0 % in the STPS task. The percentage of explained variance in the current study is similar to that observed in previous studies (Klous et al. 2011; Krishnan et al. 2011; Wang et al. 2014). Table 1 shows the loadings for all the muscles on the four factors for a representative subject in the STNS task. The first M-mode showed high loading values (>0.5) for the IEMGN indices of the GL, GM, SOL, ST, BF muscles, which is a “push-back” M-mode. The second M-mode depicted a reciprocal contraction of the thigh muscles (“push-forward” M-mode). Significant loading coefficients for the third M-mode seen in the RA and ES muscles with opposing actions revealed a co-contraction of the trunk muscles (“co-contraction” M-mode). In the fourth M-mode, however, the loading pattern was higher for the ST and BF muscles.
Table 2 shows representative results of the principal component analysis in the STPS task. The first M-mode composition was a “push-back” M-mode. The second M-mode showed a “co-contraction at the hip” pattern. Furthermore, the third M-mode revealed a “push-forward” M-mode. The fourth M-mode again depicted a push-back pattern between the ST and BF muscles. Overall, in the STNS task, 11 M-modes with “co-contraction” M-mode were seen, while in the STPS task, the number of M-modes with co-contraction patterns was 12 from a total of 32 M-modes.
Results of multiple regression analysis were significant in most cases for each of the two tasks. On an average, the analysis accounted for 82.8 ± 12.1 and 81.5 ± 9.8 % of variance in ∆COPAP in the STNS and STPS tasks, respectively. There was no task difference in variations in the magnitudes of the four M-modes accounted for the total variance in ∆COPAP (P > 0.05).
Synergy analysis
We defined multi-M-mode synergies using the framework of the UCM hypothesis analyzing co-variation among the magnitudes of M-modes related to stabilization of the COPAP coordinate. For statistical analysis, ∆V data were log-transformed, resulting in the index ∆V Z (see “Methods”). It has shown that higher ∆V Z values in the STPS condition as compared to those in the STNS condition (Fig. 2). This finding was confirmed by a two-way repeated measures ANOVA condition × interval, which showed a main effect of condition [F (1,70) = 4.27, p < 0.05]. There were no significant main effect of interval [F (4,70) = 0.53, p > 0.05]; no interaction was observed between Condition and Interval [F (4,70) = 0.25, p > 0.05]. This result confirms that COPAP was stabilized by co-variation of M-mode magnitudes.
Mean across subjects ± standard deviation of ΔV Z indices for the control of the COPAP displacement. Adjacent pairs of bars represent the STNS (left, open bars) and STPS (right, gray bars) tasks. ΔV Z indices were averaged over five 50-ms time intervals starting 250 ms prior to t 0 and ending up at t 0. Note the greater ΔV Z values for the STPS condition compared with the STNS condition
Figure 3 shows the ∆V Z time profiles for a representative subject under the STNS and STPS conditions. Visual inspection of the individual ∆V Z profiles showed a consistent pattern across subjects that included a transient drop in ∆V Z which is seen prior to the initiation of changes in the muscle activation level. We refer to this early drop in ∆V Z as ASA. In the STNS condition, the decrease in ∆V Z , on average, occurred earlier (−171 ± 41 ms) than in the STPS condition (−105 ± 48 ms). This difference was statistically significant (Paired t test, p < 0.05).
The time profiles of ∆V Z index for a representative subject in the STNS condition (dark line) and STPS condition (gray line). Time zero (t 0) corresponds to the alignment time, the time of toe off. Note a transient drop in ∆V Z seen prior to the initiation of changes in the muscle activation level. The arrows (1 STNS condition and 2 STPS condition) show two drops of the peaks
Discussion
This study investigated a mechanism of feed-forward control of a multi-muscle postural task, namely anticipatory synergy adjustments (ASAs) prior to a stepping behavior during support surface translation. We analyzed the index of co-variation of muscle modes (M-modes) related to stabilization of COPAP. Our results suggest that muscle synergies stabilize the COPAP coordinate based on co-varied across trials involvement of the M-modes during preparation to taking a step. Indeed, the preparation for a stepping was associated with two types of anticipatory adjustments, ASAs and APAs. ASAs were seen before APAs, and the magnitude of synergy index (∆V Z ) was significantly larger when the support surface was translated.
Bipedal stance in humans is inherently unstable due to the difficulty in maintaining the high center of gravity (2/3 of the body mass is located at or above 54–58 % of the body height from the ground) over the relatively small base of support. Voluntary whole-body actions, interactions with external objects, and in response to balance threats, all contribute to problems of stabilizing the vertical posture (Wang and Asaka 2008; Wang et al. 2006a, b; Krishnan et al. 2011). Standing balance control is complex as a broad range of postural behaviors is available in response to perturbation, such as hip and ankle strategies, or stepping for stabilization (Horak and Nashner 1986). In volitional stepping, the base of support is expanded by taking a step, which is associated with translation of the center of mass even further from the initial position (Winter et al. 1996; Chvatal et al. 2011).
In the current study, the postural perturbations consisted of 5-cm backward translations of the force plate under the subjects’ feet, with peak ramp velocities of 12 cm/s, and peak accelerations of 0.1 g. Perturbations of this speed and magnitude naturally do not elicit a stepping response when subjects respond to the perturbation without any instruction (Mille et al. 2003), and we chose this perturbation based on pilot observations that subjects maintained balance without stepping during support surface translation. Therefore, we chose our perturbation to ensure that EMG changes identified as APAs were not actually responses to platform translation.
Studies of postural adjustments to take a step reported that the synergy index shows a drop starting about 200 ms prior to the stepping foot take-off (Wang et al. 2005, 2006a, b). The assumed purpose of such ASAs is to modify pre-existent synergies stabilizing the COP shift in preparation to an action. Synergies have been defined as neural organizations that ensure stability of the COP coordinate produced by a redundant set of M-modes to maintain and restore balance when perturbed. Though the similarities of ASAs and APAs have been noted, the roles of APAs are different from ASAs. The assumed role of such adjustments has been to generate forces and moments of force that minimizes the effects of expected changes in the environment on posture (Krishnan et al. 2012). APAs reflect early changes in muscle activation levels averaged across repetitive attempts (Klous et al. 2011).
To our knowledge, the current study is the first to quantify multi-muscle synergies that stabilized the COPAP shift during support surface translation. During the time interval started 100–150 ms prior to the stepping foot take-off, we found no significant differences in the magnitudes of the synergy index (∆V Z ) between the two conditions. This result makes sense because the potential for postural instability increases dramatically as the base of support changes from a 2-leg to a 1-leg stance during taking a step. We interpret this as a reflection of a synergy stabilizing COPAP coordinate to avoid collision between the legs at this time interval. We observed that, prior to the take-off of the stepping foot, ∆V Z showed a significant drop before a change in the averaged activation levels across trials in postural muscles. The findings were consistent between both conditions. Earlier studies of multi-muscle tasks with quick changes in a performance variable, such as the COPAP coordinate, have shown that a quick change in that performance variable is commonly associated with weakening of the pre-existent synergy that used to stabilize that variable (Klous et al. 2011; Krishnan et al. 2012). Indeed, anticipatory changes in the ∆V Z index into less positive values were shown as early as 200 ms prior to a self-initiated quick change in the COPAP by a standing person (Krishnan et al. 2012). Since the main purpose of a synergy is to stabilize a value of a performance variable, trying to change this variable in the presence of a synergy may be expected to meet resistance of the synergy opposing the change. Hence, if a controller plans to produce a quick change in the COPAP coordinate, turning the synergy stabilizing the COPAP shift off looks like a sensible strategy. We would like to emphasize that the observations of a drop in the ∆V Z index during the preparation to stepping may be compared to the result of study of multijoint action that reported a drop in the multijoint synergies in response to elbow perturbation (Mattos et al. 2011). Both can be viewed as indices of a relative destabilization of the trajectory.
Previous studies of multi-muscle synergies stabilizing COP shifts failed to show that the preparation for a stepping was associated with two types of anticipatory adjustments, EPAs and APAs (Wang et al. 2005, 2006a, b). There may be two main reasons for this. First, those studies analyzed M-mode synergies based on EMG signals averaged over relatively large time intervals. Second, the timing of changes in the averaged across trials muscle activation levels was not identified. In the current study, we purposefully defined the initiation of burst/inhibition time in the averaged across trials muscle activation to test one of the main hypotheses that ASAs occurred prior to APAs in preparation to stepping.
Comparison of the STNS and STOS conditions showed the composition of M-modes varied depending on the stability of the supporting surface. The observed co-contraction M-mode could be considered as an indication of the increased stiffness of the hip joint for stabilizing the COP displacement when dealing with the instability. Taken together with the literature, the observed adjustments in the composition of M-modes suggest that co-contraction M-mode can be used to augment trunk stiffness thereby increase body stability (Mohapatra et al. 2014; Wang et al. 2015a, b). Due to an inherent limitation of the number of EMG channels we could record simultaneously, we have only assessed anticipatory adjustments on the stepping leg. It is possible that the supporting leg could also be important for increasing the body stability during support surface translation. As such, future studies involving EMG recording from both the sides of the body are needed to overcome this limitation.
Conclusions
This study provides additional quantitative evidence for multi-muscle synergies during support surface translation. Results of the study have revealed that the central nervous system is able to prepare multi-muscle synergies in response to a support surface translation by changing the co-variation of M-modes. Prior to APAs, ASAs reduce stability of COPAP coordinate that is to be adjusted during the APAs. We believe that these findings may help get closer to understanding of physiological mechanism of postural preparation to external perturbation. The parameters of ASAs and APAs could have clinical relevance to identify the risk of falling in daily life.
Abbreviations
- AP:
-
Anterior–posterior
- APAs:
-
Anticipatory postural adjustments
- ASAs:
-
Anticipatory synergy adjustments
- BF:
-
Biceps femoris
- COP:
-
Center of pressure
- ES:
-
Erector spinae
- GL:
-
Lateral head of gastrocnemius
- GM:
-
Medial head of gastrocnemius
- PCA:
-
Principal component analysis
- RA:
-
Rectus abdominis
- RF:
-
Rectus femoris
- SOL:
-
Soleus
- ST:
-
Semitendinosus
- TA:
-
Tibialis anterior
- UCM:
-
Uncontrolled manifold
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Asaka T, Wang Y, Fukushima J, Latash ML (2008) Learning effects on muscle modes and multi-mode postural synergies. Exp Brain Res 184(3):323–338
Belen’kii VE, Gurfinkel VS, Pal’tsev EI (1967) Control elements of voluntary movements. Biofizika 12(1):135–141
Bernstein NA (1967) The coordination and regulation of movements. Pergamon, London
Bertucco M, Cesari P, Latash ML (2013) Fitts’ law in early postural adjustment. Neuroscience 231:61–69
Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH (2011) Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol 106(2):999–1015
Couillandre A, Maton B, Breniere Y (2002) Voluntary toe-walking gait initiation: electromyographical and biomechanical aspects. Exp Brain Res 147:313–321
d’Avella A, Saltiel P, Bizzi E (2003) Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 6:300–308
Elble RJ, Moody C, Leffler K, Sinha R (1994) The initiation of normal walking. Mov Disord 9:139–146
Halliday SE, Winter DA, Frank JS, Patla AE, Prince F (1998) The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture 8(1):8–14
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55(6):1369–1381
Ito T, Azuma T, Yamashita N (2003) Anticipatory control in the initiation of a single step under biomechanical constraints in humans. Neurosci Lett 352(3):207–210
Ivanenko YP, Poppele RE, Lacquaniti F (2004) Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556:267–282
Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F (2005) Coordination of locomotion with voluntary movements in humans. J Neurosci 25:7238–7253
Klous M, Mikulic P, Latash ML (2011) Two aspects of feed-forward postural control: anticipatory postural adjustments and anticipatory synergy adjustments. J Neurophysiol 105(5):2275–2288
Klous M, Mikulic P, Latash ML (2012) Early postural adjustments in preparation to whole-body voluntary sway. J Electromyogr 22(1):110–116
Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM (2004) Muscle modes during shifts of the center of pressure by standing persons: effect of instability and additional support. Exp Brain Res 157(1):18–31
Krishnan V, Aruin AS, Latash ML (2011) Two stages and three components of the postural preparation to action. Exp Brain Res 212(1):47–63
Krishnan V, Latash ML, Aruin AS (2012) Early and late components of feed-forward postural adjustments to predictable perturbations. Clin Neurophysiol 123:1016–1026
Latash ML (2010) Motor synergies and the equilibrium-point hypothesis. Mot Control 14(3):294–322
Lepers R, Breniere Y (1995) The role of anticipatory postural adjustments and gravity in gait initiation. Exp Brain Res 107(1):118–124
Mattos D, Latash ML, Park E, Kuhl J, Scholz JP (2011) Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence. J Neurophysiol 106(3):1424–1436
Mille ML, Rogers MW, Martinez K, Hedman LD, Johnson ME, Lord SR, Fitzpatrick RC (2003) Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol 90(2):666–674
Mohapatra S, Kukkar KK, Aruin AS (2014) Support surface related changes in feedforward and feedback control of standing posture. J Electromyogr Kinesiol 24(1):144–152
Nachshon I, Denno D, Aurand S (1983) Lateral preference of hand, eye and foot: relation to cerebral dominance. Int J Neurosci 18(1–2):1–9
Robert T, Zatsiorsky VM, Latash ML (2008) Multi-muscle synergies in an unusual postural task: quick shear force production. Exp Brain Res 187(2):237–253
Scholz JP, Schöner G (1999) The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126:289–306
Schöner G (1995) Recent developments and problems in human movement science and their conceptual implications. Ecol Psychol 8:291–314
Ting LH (2007) Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res 165:299–321
Torres-Oviedo G, Ting LH (2007) Muscle synergies characterizing human postural responses. J Neurophysiol 98(4):2144–2156
Torres-Oviedo G, Macpherson JM, Ting LH (2006) Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96(3):1530–1546
Wang Y, Asaka T (2008) Muscle synergies involved in shifts of the center of pressure while standing on a narrow support. Brain Res Bull 76(1–2):16–25
Wang Y, Zatsiorsky VM, Latash ML (2005) Muscle synergies involved in shifting the center of pressure while making a first step. Exp Brain Res 167(2):196–210
Wang Y, Asaka T, Zatsiorsky VM, Latash ML (2006a) Muscle synergies during voluntary body sway: combining across-trials and within-a-trial analyses. Exp Brain Res 174(4):679–693
Wang Y, Zatsiorsky VM, Latash ML (2006b) Muscle synergies involved in preparation to a step made under the self-paced and reaction time instructions. Clin Neurophysiol 117:41–56
Wang Y, Kenyon RV, Keshner EA (2010) Identifying the control of physically and perceptually evoked sway responses with coincident visual scene velocities and tilt of the base of support. Exp Brain Res 201(4):663–672
Wang Y, Asaka T, Watanabe K (2013) Multi-muscle synergies in elderly individuals: preparation to a step made under the self-paced and reaction time instructions. Exp Brain Res 226(4):463–472
Wang Y, Watanabe K, Asaka T, Wan FT (2014) Muscle synergies in preparation to a step made with and without obstacle. Eur J Appl Physiol 114(12):2561–2569
Wang Y, Watanabe K, Asaka T (2015a) Muscle synergies in preparation to a step made with obstacle in elderly individuals. J Neuroeng Rehabil 12:10
Wang Y, Watanabe K, Asaka T (2015b) Age effects on multi-muscle modes during voluntary body sway. Res Sports Med 23(1):88–101
Winter DA, Prince F, Frank JS, Powell C, Zabjek KF (1996) Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol 75:2334–2343
Acknowledgments
This study was supported by the National Natural Science Foundation of China [grant #31371207] and the Natural Science Foundation of Tianjin [grant #14JCYBJC43300]. We thank Zhuoyue Zhao and Cuixia Gu for their assistance in the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Fausto Baldissera.
Rights and permissions
About this article
Cite this article
Wang, Y., Watanabe, K. & Asaka, T. Muscle synergies underlying control of taking a step during support surface translation. Eur J Appl Physiol 116, 301–309 (2016). https://doi.org/10.1007/s00421-015-3282-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3282-x