Abstract
Purpose
We explored changes in muscle interactions during healthy aging as a window into neural control strategies of postural preparation to action/perturbation. In particular, we quantified the strength of multi-muscle synergies stabilizing the center of pressure (COP) displacement during the preparation for making a step associated with support surface translations.
Methods
Young and elderly subjects were required to make a step in response to support surface perturbations. Surface muscle activity of 11 leg and trunk muscles was analyzed to identify sets of 4 muscle modes (M-modes). Linear combinations of M-modes and their relationship to changes in the COP displacement in the anterior–posterior direction were then determined. Uncontrolled manifold analysis was performed to determine variance components in the M-mode space and indices of M-mode synergy stabilizing COP displacement.
Results
Prior to the step initiation, the older subjects showed strong synergies that stabilized COP displacement to forward perturbation of the support surface. However, the synergy indices were significantly lower than those of the young subjects during preparation for making a step. The timings of early postural adjustment (EPA) and anticipatory postural adjustment (APA) were consistently earlier in the young subjects as compared to the older subjects. For both groups, the timing of EPA did not change across tasks, while APA showed delayed timing in response to the support surface translations.
Conclusions
We infer that changes in the indices of synergies with age may present challenges for the control of postural preparation to external perturbation in older adults. They may lead to excessive muscle co-contractions and low stability of COP displacement. The results reported here could have clinical relevance when identifying the risk of making a step, which has been linked to an increased risk of falls among the elderly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
When a standing person makes a step, there are feed-forward adjustments in the activation levels of postural muscles starting several 100 ms prior to the takeoff of the leading foot. The purpose of these early adjustments has been assumed to allow unloading of the stepping leg and to create a moment of vertical force rotating the body about the ankle joints (Ito et al. 2003). Following a perturbation to standing balance, the ability to make a step requires controlling the relative motion between the body center of mass and its base of support, with large inertial forces that could potentially threaten stability (Wang et al. 2016). Because even a small error in motor performance during standing may result in catastrophic failure, making a step can become quite a demanding task and may pose great risk in aging adults.
With advanced age comes a decline in the neuromotor system (Iosa et al. 2014; Tsai et al. 2014). This decline can be manifested at different levels and in various functions, including reduction of muscle mass (Shumway-Cook and Woollacott 2000), altered muscle response organization (Tsai et al. 2014), more frequent co-activations of antagonist muscles (Shumway-Cook and Woollacott 2000; Papegaaij et al. 2014) and neuronal loss (Dinse 2006) in a number of structures within the central nervous system (CNS). All these changes can potentially contribute to impaired motor control, gait and balance in older adults, which can be seen across a variety of everyday tasks including less effective postural adjustments to external perturbations (Hicks et al. 2012; Kilby et al. 2014).
Our previous research has shown that the ability to use multi-muscle synergies was reduced in older people during preparations to making a step with obstacles (Wang et al. 2016). Although the ability to recover equilibrium in response to support surface translations is critical to avoiding falls, muscle synergies stabilizing the center of pressure (COP) have previously not been quantified among the elderly. Therefore, we focused on exploring the stability of muscle coordination in healthy older adults exposed to support surface perturbations induced in a sagittal plane. We investigated the organizations of leg and trunk muscles into groups (M-modes), as well as the trial-to-trial variance of M-mode involvement (M-mode synergies), to determine dynamic stability of the elderly throughout the perturbation process.
Recently, the framework of the uncontrolled manifold (UCM) approach has been used to analyze multi-muscle synergies for quantitative assessment (Latash 2010). Within this framework, the trial-to-trial variance of independent elemental variables (e.g., M-modes) is compared in two subspaces: variance within the UCM subspace (V UCM) does not affect the performance variable of interest (e.g., COP trajectory), whereas variance in the subspace orthogonal to the UCM (V ORT) leads to changes in that performance variable. If V UCM > V ORT, most variance reflects the use of a multi-element synergy to stabilize that performance variable (reviewed in Latash et al. 2007).
Previous studies of postural preparation to action/perturbation have shown anticipatory synergy adjustments (ASAs) during two stages of feed-forward postural adjustments: early postural adjustments (EPAs) are seen within a few 100 ms and anticipatory postural adjustments (APAs) occur 80–100 ms prior to the initiation of a voluntary action (Klous et al. 2011; Krishnan et al. 2012). During steady-state standing, postural muscles show high indices of co-variation stabilizing such variables as COP coordinates and the shear force magnitude (Robert et al. 2008; Wang and Asaka 2008; Klous et al. 2011; Krishnan et al. 2011, 2012). If a subject is required to produce a quick action from a steady state, the indices of co-variation drop are delayed until the action initiation (Wang et al. 2006). ASAs have been shown to shift towards the time of action initiation under the simple reaction time instruction (Wang et al. 2006) and are delayed and reduced in magnitude in the elderly (Wang et al. 2013, 2015a, b).
In this study, we investigated the extent to which the coordination of postural muscles contributes to COP stabilization associated with support surface translation and how that coordination changes with aging. The main purpose of the current study was to examine the possible changes of M-modes, their effects on COP shifts, and multi-M-mode synergies in older adults. Based on previous studies of multi-muscle synergies in postural control (Wang et al. 2015a, b, 2016), we hypothesized that, compared with young adults, older adults would show age-related changes in the structure of M-modes.
Previous studies on young adults have shown that the synergy index (∆V, the normalized difference between V UCM and V ORT) is typically high during steady-state standings (Wang and Asaka 2008; Klous et al. 2011; Krishnan et al. 2011, 2012). When a person is preparing for a quick action from a steady state, the synergy index drops prior to the action initiation. These ASAs represent an important reflection of controlled stability that allows combining stability during steady state and agility in transition to a quick action. Hence, our second hypothesis was that elderly adults would show a delayed and lower synergy index, which would decrease their ability to stabilize the COP shifts. This hypothesis has been motivated by reports of age-related decrements in the neuromotor system (Rogers et al. 1992; Woollacott and Manchester 1993; Iosa et al. 2014; Tsai et al. 2014). If the hypotheses are supported, the results from this study could have clinical relevance in identifying step-making risks and increased risk of falls among the elderly.
Methods
Participants
Eight healthy young adults (four males and four females) and eight healthy older adults (four males and four females) without any known neurological or motor disorder volunteered to participate in the study. All participants were right-foot-dominant according to the preferred foot usage when kicking a ball, stepping up on a chair, and leaping off in the long jump (Nachshon et al. 1983). The mean age, height and mass were 24.4 ± 1.8 years, 166.9 ± 6.4 cm and 62.4 ± 9.1 kg for the young subjects and 61.9 ± 2.9 years, 164.9 ± 7.7 cm and 66.4 ± 6.4 kg for the older subjects. The methods used in the study were approved by the Institutional Review Board of Tianjin University of Sport. The experimental procedures were explained and all the subjects signed an informed consent form prior to data collection. Tianjin key lab of exercise physiology and sports medicine was the place of study performed.
Experimental setup and procedures
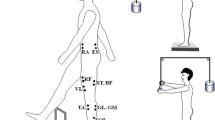
The postural perturbations consisted of 5-cm forward translations of the force plate on which the subject stood, with peak ramp velocities of 5 cm/s, and peak accelerations of 0.05 g. The subjects were instructed to stand with equally distributed weight with their arms hanging loosely by their sides. Sagittal plane balance was perturbed at random intervals by the movable platform in the forward direction.
The experimental condition was taking a step forward with the right leg in response to perturbations from an original posture of side-by-side quiet stance. The task goal was to step forward from a stationary position and subsequently both feet came to rest next to each other. In the initial position, subjects maintained a comfortable posture with their feet placed shoulder width apart while standing on the force plate installed on the movable platform. The initial stance was consistent across trials by having subjects stand within tracings of their foot outlines on the force plate. The subjects were instructed to look straight ahead throughout the trial, and react naturally to forward perturbation of the support surface under the subjects’ feet. In the normal stepping task (STNS), the subjects were free to initiate the step following a ‘get ready’ cue. In the perturbation stepping task (STPS), they were instructed that, at any given time, the platform would suddenly translate forward. The subjects were asked to respond in a natural way to make a step with preferred spatial–temporal dynamics.
Before the start of data collection, 1–3 practice trials in each experimental condition were given to all subjects for familiarization with the task. The subjects were free to choose their preferred pace and distance when taking a step. Data were collected for 5 s per trial, including a 1–2 s quiet standing period prior to platform motion. A total of 20 trials for each condition were performed. Four blocks of normal stepping trials (STNS) were alternated with four blocks of perturbation stepping trials (STPS). Two different conditions were thus presented in blocks of five trials. To prevent fatigue, the subjects were allowed to rest at their request, and rested at least 5 min after 4 blocks (20 trials). For safety precautions, an assistant stood in reaching distance behind the subject to prevent a fall in each perturbation trial.
Data collection
Electromyographical (EMG) activity was recorded from eleven lower limb and trunk muscles of the subject’s right side. After the skin was shaved and cleaned with alcohol, bipolar, pre-gelled Ag/AgCl surface electrodes were placed on the muscle bellies and were oriented in the direction of the muscle fibers. The surface EMGs (Noraxon Telemyo 2400T V2, Scottsdale, AZ, USA) were recorded from the tibialis anterior (TA), lateral head of gastrocnemius (GL), medial head of gastrocnemius (GM), soleus (SOL), rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), biceps femoris (BF), semitendinosus (ST), rectus abdominis (RA), and erector spinae (ES). The electrodes were placed in pairs with the center-to-center distance of 25 mm; the ground electrode was placed over the epicondyle of the tibia. EMG signals were band-pass filtered at 10–500 Hz and sampled at 1500 Hz with a 16-bit resolution. Kinetic data were collected at 1500 Hz from a force platform (model 9281B, Kistler Instrumente AG, Winterthur, Switzerland) under the feet. A foot switch was attached under the heads of the metatarsal bones of right foot to measure the timing of toe off and thin socks were used to secure the sensor in place.
Data analysis
All signals were processed offline using MATLAB (The MathWorks, Natick, MA) version R2015b. Raw EMG signals were first rectified and low-pass filtered at 50-Hz (fourth-order zero-lag Butterworth filter). Signals from the force plate were low-pass filtered at 20 Hz. The ‘time zero’ (t 0) was defined by the toe off time using the signal from the foot switch. Then, all trials were aligned to t 0 and integrals of EMGs were calculated. To do this, the data for further analysis were selected in the range from −1000 ms (before t 0) to t 0, out of which -1000 ms to −900 ms of the data in the STNS condition were taken for the baseline activity.
To identify the muscle onset for EMG data, we defined t EMG as the instant in time when the average muscle activation across trials for each condition differed by more than ±2 standard deviations from the baseline activity for at least 25 ms continuously. Each muscle was assigned one t EMG value that corresponded to either a burst or an inhibition.
Rectified EMG signals for each muscle for each subject were integrated over 10 ms intervals in a time window from −600 ms to t 0. These EMG integrals for each of 10 ms were corrected by subtracting the EMG integrals of the averaged 10 ms baseline activity (IEMG). ∆IEMG indices were further normalized (∆IEMGN) to the maximum integral magnitude across conditions. This method of normalization was used in earlier studies of muscle modes and synergies (Wang et al. 2005, 2014, 2015a, b).
Defining M-modes
For the STNS condition, we extracted groups of muscles (M-modes) from the IEMGN data matrix within the time window in relation to t 0 from −200 ms to t 0 using the principal component analysis (PCA). For each subject, the IEMGN data formed a matrix of 20 time intervals × 11 muscles × 20 trials = 4400 data points. The correlation matrix among the IEMG was subjected to principal component analysis with Varimax rotation, using procedures from SPSS (SPSS, Inc., Chicago, IL, USA). The factor analysis module with principal component extraction was employed.
For each subject, the obtained eigenvalues and principal components (PCs) were then considered. The first four PCs were selected for further analysis. This was determined by examining the scree plots and having at least two muscles significantly loaded per PC. We address these PCs as muscle modes (M-modes) and assume that the magnitudes of coefficients at the M-modes are manipulated by the controller to produce the COP shifts in the AP direction (COPAP).
Defining the Jacobian
Since the support surface perturbations were induced in the sagittal plane, only the COPAP shifts were analyzed to explore covariation among the magnitudes of M-modes (∆M) related to stabilization of the COPAP coordinate. Small changes in the magnitudes of ∆Ms were related to the change in the COPAP shifts (∆COPAP) through the Jacobian matrix (J). Multiple linear regression analysis over the trials was used to define the J for each subject separately. The J was estimated as coefficients of multiple linear regression between ∆M and ∆COPAP.
Synergy analysis
For each trial of the STNS tasks, ∆IEMGN was computed and transformed into ∆Ms by multiplying the loadings of the individual M-mode. The mean magnitudes of each ∆M for a selected time interval across a series of ST trials were computed. Since the model relating ∆Ms to ∆COPAP is linear, the mean values were subtracted from each computed value and the residuals were further analyzed.
The UCM represents different combinations of M-modes that keep the value of ∆COPAP unchanged. The UCM was estimated as the null space of the corresponding J matrix. The null space is spanned by the basis vectors, ɛ i . The vector of individual mean-free ∆Ms was resolved into its projection onto the null space and the orthogonal subspace:
where n = 4 and d = 1 are the total number of M modes and the number of degree of freedom describing the task; i.e., 1-dimensional COPAP shift.
Variance per degree of freedom within the UCM and orthogonal to the UCM across trials was computed as:
We computed an index of synergy (ΔV) reflecting the difference between the variance within the UCM and orthogonal to the UCM:
where all variance indices are computed per degree of freedom; and V TOT means the total variance. For further analyses, the ΔV values were transformed using a Fisher’s z-transformation (ΔV Z ) adapted to the boundaries of ΔV:
Anticipatory synergy adjustments (ASAs) were identified as a drop in the ΔV Z time profile. The time of ASA initiation, t ASA, was calculated for the z-transformed values of ∆V (∆V Z ). The rate of change of ∆V Z was computed from t 0 to −600 ms backwards; the time t ASA was defined by two criteria: (1) when the magnitude of this rate was equal to zero (d∆V Z /dt = 0), which was considered as the start of a drop; and (2) the drop had to be larger than 20% of the ∆V Z magnitude at the time of d∆V Z /dt = 0. All subjects showed 2 such anticipatory drops in the ∆V Z time profiles before t 0. The outcomes of t ASA were grouped into two time intervals with respect to t 0, {−600; −200} ms (EPAs, early postural adjustments) and {−200; 0} ms (APAs, anticipatory postural adjustments).
Statistical analysis
Descriptive statistics was used to compute the means and standard deviations for the outcome variables. The fractions of variance explained by the first four principal components were transformed into z-scores using standard Fisher’s z-transformation. A paired t test was used for comparing the z-scores and the normalized peak COPAP shifts between STNS and STPS conditions. To compare the indices of synergies (∆V Z ) stabilizing the COP shift, a three-way analysis of variance was performed on ∆V Z with group, condition, and time as factors. In the EPA and APA time intervals, to compare the initiation times of the synergy index (∆V Z ), two-way analysis of variances were applied with group and condition as factors. For all statistical tests, a p value less than 0.05 was set as a measure of significance.
Results
General EMG patterns
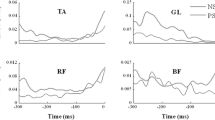
There were common features across the EMG patterns in both conditions and subject groups. Figure 1 shows the rectified EMGs averaged across trials for representative young and older subjects for selected muscles for the STNS and STPS conditions, respectively. Before the EMGs were averaged, the trials were aligned by the time (t 0) of toe off in the foot switch signal. These particular muscles were selected because they showed clear anticipatory postural adjustments across subjects. In the STNS condition, the stepping leg typically showed alternating bursts of activity in the ventral and dorsal muscles. In the STPS condition, there was a substantial increase in the level of activity in most muscles. The regularities in the patterns of activation of the leg and trunk muscles were consistently observed in the STNS and STPS conditions. Note that the muscle activity of the elderly subjects was typically higher than that of the young subjects.
Typical EMG patterns averaged across trials for representative young and older subjects for the STNS (dark lines) and STPS (gray lines) conditions, respectively. Time zero (t 0) corresponds to the alignment time, which is the time of toe off. The EMGs were recorded in muscles on the right side of the body. The EMG scales are in arbitrary units and time is in ms (TA tibialis anterior, GM medial head of gastrocnemius, RF rectus femoris, BF biceps femoris)
Figure 2 illustrates onsets of EMG activity averaged across subjects for the STNS and STPS conditions in the young and elderly groups. In the STPS condition, onsets of EMG activity were observed in both groups. However, anticipatory activity of the trunk and leg muscles in the young subjects occurred earlier as compared to the elderly subjects. Particularly, early EMG onsets were seen in the TA and RF muscles. The TA onset was −156.5 ± 45.1 ms in the older group and −118.6 ± 45.0 ms in the young group [F (1,14) = 11.447, p = 0.004]. The RF onset was −186.9 ± 65.0 ms in the older group and −134.7 ± 51.2 ms in the young group [F (1,14) = 5.643, p = 0.032]. Moreover, TA and ER muscles showed delayed onsets in the STPS condition as compared to the STNS condition (p = 0.003, p < 0.001, respectively).
Onsets of EMG activity averaged across subjects, plotted for the STNS and STPS conditions in the young and elderly groups. Onset of muscle activation is plotted in ms and the mean ± SD is shown (TA tibialis anterior, SOL soleus, GL lateral head of gastrocnemius, GM medial head of gastrocnemius, RF rectus femoris, VL vastus lateralis, VM vastus medialis, BF biceps femoris, ST semitendinosus, RA rectus abdominis, ES erector spinae)
In preparation for stepping, subjects shifted the COP in the AP direction backwards. This adjustment allowed unloading the stepping leg and creating a moment of the reactive force rotating the body forward about the ankle joints. In the STNS task, the amplitude of the peak COPAP displacement normalized by foot length (COPAP-NS = −0.083 ± 0.025) was smaller for the young group than for the elderly group (COPAP-PS = −0.105 ± 0.019); the difference was statistically significant (p < 0.05). Note that the negative values correspond to backward displacements. In the STPS task, the amplitude of the peak COPAP displacements normalized by foot length was COPAP-NS = −0.228 ± 0.027 and COPAP-PS = −0.202 ± 0.028 for the young and elderly groups, respectively. The difference was statistically significant (p < 0.05).
PCA and multiple regression analysis
On average, the first four principal components (PCs) accounted for about 62.3 ± 2.8% of the total variance in the muscle activation space in the young group and 62.2 ± 4.2% in the older group. Figure 3 illustrates the average amount of variance explained by each M-mode (M1 through M4) across subjects. For the data of the young subjects, the average amount of variance explained by the first four PCs was 21.4 ± 2.2, 16.7 ± 2.1, 13.0 ± 1.3 and 11.3 ± 1.4%, respectively. For the data of the older subjects, the average amount of variance explained by the first four PCs was 20.9 ± 2.4, 16.2 ± 2.6, 13.5 ± 1.9 and 11.6 ± 1.3%, respectively. z-scores for the fractions of variance explained were computed to assess differences between groups. A one-way ANOVA with the factor Group (young and older) run for each PC data separately showed no significant differences between groups.
There was considerable variability across the subjects in the M-mode composition. Table 1 shows the loadings for all the muscles on the four factors for a representative young subject. Such typical M-modes did not show co-contraction M-modes. A co-contraction M-mode is defined as a pattern with significant loading coefficients on the same M-mode with the same sign for two muscles with opposing action at a particular joint (Asaka et al. 2008). Table 2 presents a representative result of the PCA of the older subject. The second M-mode depicted a co-contraction pattern between the RA (ventral) and the ES (dorsal) muscles.
For each subject, the number of times that reciprocal M-modes, co-contraction M-modes, and mixed M-modes were seen under the STNS condition was summed (Table 3). Wilcoxon’s test showed that the number of times the co-contraction and mixed M-modes were seen in the older subjects was significantly larger than that of the young subjects (p < 0.05), while the number of times the reciprocal M-modes occurred in the older subjects was significantly smaller than the young subjects (p < 0.05).
Multiple regression analysis was performed to define the Jacobian mapping of small changes in the M-mode magnitudes onto COPAP shifts. Results of multiple regression analysis were significant in most cases for each of the two groups. On average, the analysis accounted for 86.3 ± 3.6 and 84.3 ± 4.4% of variance in ∆COPAP in the young and older groups, respectively. There was no group difference in variations in the magnitudes of the four M-modes accounting for the total variance in ∆COPAP (p > 0.05).
Synergy analysis
The synergy index (∆V) was computed as the normalized difference between V UCM and V ORT. Positive ∆V values indicated the existence of a multi-M-mode synergy in the M-mode space that stabilized the average COPAP shift, while zero or negative ∆V meant no such synergy. For further analysis, ∆V data were log-transformed, resulting in the index ∆V Z . To perform an across-subjects analysis of M-mode synergies to stabilize the COPAP shift prior to stepping, ΔV Z indices were averaged over six 100-ms time intervals starting 600 ms prior to t 0 and ending up at t 0. The selection of these time windows was based on earlier studies which showed that M-mode synergies take time to emerge and develop prior to action initiation (Krishnan et al. 2011, 2012). The ∆V Z values computed for the COPAP shift as the performance variable became smaller for the older subjects than for the young subjects, and the trend of ∆V Z across time was similar for both groups. This finding was supported by a three-way Group × Condition × Time ANOVA. There was a significant effect of Group [F (1,14) = 6.858, p = 0.020] with the young subjects (∆V Z = 1.11 ± 0.48) showing higher values than the older subjects (∆V Z = 0.91 ± 0.50). The main effect of Condition [F (1,70) = 6.015, p = 0.028] confirmed that, for the older and young groups, ∆V Z in the STNS condition had significant higher values than in the STPS condition. None of the time effects or interactions was significant. Overall, the results showed that strong M-mode synergies stabilized the average COPAP shift in both subject groups.
The ∆V Z time profiles computed for the STNS and STPS conditions in representative young and older subjects are shown in Fig. 4. In both groups and conditions, the ∆V Z values remained positive during preparation for making a step associated with support surface translations. The two ∆V Z profiles started with positive values and included a transient drop in ∆V Z during the EPA time interval followed by another drop during the APA time interval. The timing of the drop varied across subjects such that averaged across subjects data did not show consistent behavior. For statistical analysis, we focused on the two drops in ∆V Z computed using the criterion mentioned in the “Methods”. The time of the ∆V Z drop averaged across subjects for the EPA and APA time intervals are presented in Fig. 5. A two-way ANOVA on the timing indices for the EPA period with the factors Group and Condition showed a main effect of Group [F (1,14) = 5.028, p = 0.042] without any other effects. There were significantly earlier timing indices in the younger group than in the older group. In contrast, for the APA time intervals, the time of ∆V Z drop occurred earlier in the STNS condition than in the STPS condition. This finding was supported by a two-way Group × Condition ANOVA, which showed a significant effect of Condition [F (1,14) = 24.200, p < 0.001] with the STNS condition showing significantly earlier timing indices than the STPS condition. The main effect of Group [F (1,14) = 5.715, p = 0.031] confirmed that for both conditions, the time of ∆V Z drop occurred significantly earlier in the younger group than in the older group.
Time profiles of the ∆V Z index for representative young and elderly subjects in the STNS (dark line) and STPS (gray line) conditions. The two ∆V Z profiles started with positive values and included a transient drop in ∆V Z during the EPA time interval followed by another drop during the APA time interval (gray-shaded area). The arrows represent drops of anticipatory synergy adjustment (ASA). Time zero (t 0) corresponds to the alignment time, which is the time of toe off
Discussion
This study investigated the organization of multi-muscle postural control associated with support surface perturbations in both young and old subjects. We analyzed whether M-mode synergies stabilizing the COPAP displacement during the preparation of making a step were influenced by age and support surface translation. The particular focus was an examination of the role of anticipatory synergy adjustments (ASAs) in the regulation of feed-forward postural control. Initiating a step with the constraints imposed by support surface perturbation involves multi-muscle postural control for balance stabilization, which must effectively manipulate the COP displacement to accelerate the entire body’s center of mass forward. The mechanisms of balance maintenance while making a step with preferred spatial–temporal dynamics provide insight into the neural control of older adults in anticipation of a perturbation or an action.
We used PCA as the means of defining independent (orthogonal) elemental variables; i.e., M-modes. In other studies, other matrix factorization methods have been used to identify muscle groupings that do not require orthogonality of the components (Sabatini 2002; Tresch et al. 2006; Ting 2007). In those studies, synergies were associated with such muscle groupings. Our approach is different, viewing such groupings only as a set of elemental variables, which is the first step in identifying synergies. PCA defines orthogonal M-modes, which is preferred for computational reasons, because such M-mode sets correspond to equal amounts of variance in all directions in the M-mode space when in the absence of an organizing (synergetic) action. Using non-orthogonal sets of variables may be able to account for more variance in the original data at the first step of analysis (M-mode identification). However, it complicates interpretation of the second step (the UCM analysis) since differences in the amounts of variance in different subspaces may be due to the non-orthogonal axes rather than to a control strategy.
Within the UCM framework, M-modes represent combinations of muscle activations that reduce the number of degrees of freedom manipulated by the CNS. It has been suggested that the CNS has the ability to combine M-modes in different ways as the situation warrants to achieve performance stability (Latash et al. 2007). In most studies of whole-body actions, a few (3–5) M-modes have been identified: two of them united leg and trunk muscles crossing several joints on the ventral (“push- forward” M-mode) and dorsal (“push-back” M-mode) surfaces of the body. In more challenging conditions and in older people, agonist–antagonist muscle pairs entered the same M-mode with the same signs of the loading coefficients, which is a “co-contraction” M-mode (Krishnamoorthy et al. 2003; Asaka et al. 2008; Robert et al. 2008; Klous et al. 2010, 2011; Wang et al. 2013, 2015a, b). The reciprocal M-modes may effectively move the body forward or backwards, while the co-contractions M-modes may improve performance by stiffening the joints to resist perturbations.
The findings showed that the older subjects had more co-contraction M-modes than the younger subjects. There were only a few cases of co-contraction M-modes in the younger subjects despite the fact that the older subjects showed that similar variances were accounted for by four M-modes, which were similar to the younger subjects. It has been shown that there is a predominance of muscle co-contraction patterns in postural tasks in persons with an impaired postural control due to aging (Hatzitaki et al. 2005). Further, previous research on voluntary body sway has shown a more common occurrence of co-contraction M-modes in older people (Wang et al. 2015a, b). Therefore, more co-contraction M-modes among the elderly subjects found here reflect the generally observed co-contraction patterns with aging. This finding may be related to the preference for co-contraction motor patterns in the elderly, which replace the more typical reciprocal patterns that require accurately timed production of muscle forces (Benjuya et al. 2004). However, more co-contraction M-modes could be also a reflection of increased caution among the elderly to securely perform the task.
In previous studies, M-mode compositions and the corresponding Jacobian matrices were defined over different tasks (body sway and shoulder flexion) and over different time intervals within the shoulder flexion task (Klous et al. 2011; Krishnan et al. 2011). Despite major differences in the M-mode compositions, when different sets of M-modes were used to analyze the whole data set, striking consistency in both magnitudes and time profiles of the synergy indices was observed. Therefore, in this study, the M-mode composition and the Jacobian matrix were defined with respect to the APA time interval within the STNS condition, but were later applied to the whole time of data analysis in both conditions.
Based on the UCM hypothesis, muscle synergies were defined as neural organizations that ensure stability of the COP coordinate produced by a redundant set of M-modes to maintain and restore balance when perturbed. There were similarities and differences in the M-mode synergies in the young and older subjects. Both groups were able to demonstrate M-mode co-variation stabilizing the magnitude of the COPAP shift. Both groups showed higher synergy indices for the STNS condition than for the STPS condition. The main difference between groups was in the significantly lower values of the synergy indices for the COPAP shift. Earlier studies documented lower synergy indices in older subjects during step initiation that involved the obstacle-negotiation and reaction time task settings (Wang et al. 2006, 2015a, b). These observations implied that aging is associated with weakening of postural muscle synergies in response to support surface translations. This may be a reflection of a general trend of a shift from more complex, synergic control to more element-based control, possibly related to the progressive death of neurons at different levels of the neural axis (Eisen et al. 1996).
EPAs and APAs have been known for a long time (Belen’kii et al. 1967; Elble et al. 1994) and are viewed as reflections of feed-forward processes at the stage of planning a movement (Bertucco et al. 2013). However, APAs have frequently been used to describe adjustment seen prior to an expected perturbation that might or might not be time-linked to a voluntary action (Massion 1992). Recent studies have documented that EPAs and APAs are two separate phenomena of postural preparation with different purposes and variable timing (Klous et al. 2011; Krishnan et al. 2011, 2012). In particular, EPAs do not show consistent changes in their timing across different conditions of a task, while the timing of APAs changes. Our current study provides further support for these two postural adjustments. Our study is unique in documenting two stages of anticipatory changes in indices of multi-muscle synergies in older persons during preparation to making a step associated with support surface translation. Until now, such synergic adjustments (ASAs) have only been reported in healthy young subjects in standing postures (Klous et al. 2011; Krishnan et al. 2011, 2012) and they have been assumed to reflect purposeful destabilization of the COP coordinate in preparation to its quick change. We believe that our study is the first observation of age effects on EPAs and APAs in preparation for a step during support surface translation.
We observed two clearly different ASAs time intervals in preparation for making a step: EPAs started 400–500 ms prior to the stepping foot liftoff followed by APAs, 100 ms or so prior to the liftoff. Indeed, EPAs and APAs were seen in each of the subjects and the sequence was preserved with changes in stepping tasks (STNS, STPS). The timing of EPAs did not change across tasks, while APAs showed delayed timing in response to support surface translations. The findings were observed in both young and older groups. The timings of EPAs and APAs were consistently earlier in young subjects as compared to older subjects. These findings may have received contributions from major changes in the neuro-muscular system associated with aging. In particular, older adults show progressive loss of muscle coordination between posture and intended actions. Since the main purpose of a synergy is to stabilize a value of a performance variable, trying to change this variable in the presence of a synergy may be expected to meet resistance of the synergy opposing the change. Hence, if a controller plans to produce a change in the COP coordinate, turning the synergy that is stabilizing the COP displacement off seems like a sensible strategy.
Conclusions
Prior to the step initiation, older subjects showed strong synergies that stabilized COP displacement to forward perturbation of the support surface. However, the synergy indices were significantly lower and delayed as compared to the young subjects during preparation for making a step. We infer that changes in the indices of synergies with age may present challenges for the control of postural preparation to external perturbation in older adults. They may lead to excessive muscle co-contractions and low stability of the COP displacement. The results reported here could have clinical relevance to identify the risk of making a step, which has been linked to an increased risk of falls among the elderly.
Abbreviations
- AP:
-
Anterior-posterior
- APAs:
-
Anticipatory postural adjustments
- ASAs:
-
Anticipatory synergy adjustments
- BF:
-
Biceps femoris
- COP:
-
Center of pressure
- EPAs:
-
Early postural adjustments
- ES:
-
Erector spinae
- GL:
-
Lateral head of gastrocnemius
- GM:
-
Medial head of gastrocnemius
- PCA:
-
Principal component analysis
- RA:
-
Rectus abdominis
- RF:
-
Rectus femoris
- SOL:
-
Soleus
- ST:
-
Semitendinosus
- TA:
-
Tibialis anterior
- UCM:
-
Uncontrolled manifold
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Asaka T, Wang Y, Fukushima J, Latash ML (2008) Learning effects on muscle modes and multi-mode postural synergies. Exp Brain Res 184(3):323–338
Belen’kii VE, Gurfinkel VS, Pal’tsev EI (1967) Control elements of voluntary movements. Biofizika 12(1):135–141
Benjuya N, Melzer I, Kaplanski J (2004) Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J Gerontol A Biol Sci Med Sci 59:166–171
Bertucco M, Cesari P, Latash ML (2013) Fitts’ law in early postural adjustments. Neuroscience 231:61–69
Dinse HR (2006) Cortical reorganization in the aging brain. Prog Brain Res 157:57–80
Eisen A, Entezari-Taher M, Stewart H (1996) Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurol 46:1396–1404
Elble RJ, Moody C, Leffler K, Sinha R (1994) The initiation of normal walking. Mov Disord 9:139–146
Hatzitaki V, Amiridis IG, Arabatzi F (2005) Aging effects on postural responses to self-imposed balance perturbations. Gait Posture 22:250–257
Hicks GE, Shardell M, Alley DE (2012) Absolute strength and loss of strength as predictors of mobility decline in older adults: The InCHIANTI study. J Gerontol A Biol Sci Med Sci 67A(1):66–73
Iosa M, Fusco A, Morone G, Paolucci S (2014) Development and decline of upright gait stability. Front Aging Neurosci 6:14
Ito T, Azuma T, Yamashita N (2003) Anticipatory control in the initiation of a single step under biomechanical constraints in humans. Neurosci Lett 352(3):207–210
Kilby MC, Slobounov SM, Newell KM (2014) Aging and the recovery of postural stability from taking a step. Gait Posture 40(4):701–706
Klous M, Danna-dos-Santos A, Latash ML (2010) Multi-muscle synergies in a dual postural task: evidence for the principle of superposition. Exp Brain Res 202:457–471
Klous M, Mikulic P, Latash ML (2011) Two aspects of feed-forward postural control: anticipatory postural adjustments and anticipatory synergy adjustments. J Neurophysiol 105(5):2275–2288
Krishnamoorthy V, Goodman S, Zatsiorsky V, Latash ML (2003) Muscle synergies during shifts of the center of pressure by standing persons: identification of muscle modes. Biol Cybern 89:152–161
Krishnan V, Aruin AS, Latash ML (2011) Two stages and three components of the postural preparation to action. Exp Brain Res 212(1):47–63
Krishnan V, Latash ML, Aruin AS (2012) Early and late components of feed-forward postural adjustments to predictable perturbations. Clin Neurophysiol 123:1016–1026
Latash ML (2010) Motor synergies and the equilibrium-point hypothesis. Mot Control 14(3):294–322
Latash ML, Scholz JP, Schöner G (2007) Toward a new theory of motor synergies. Mot Control 11:276–308
Massion J (1992) Movement, posture and equilibrium—interaction and coordination. Prog Neurobiol 38:35–56
Nachshon I, Denno D, Aurand S (1983) Lateral preference of hand, eye and foot: relation to cerebral dominance. Int J Neurosci 18(1–2):1–9
Papegaaij S, Taube W, Baudry S, Otten E, Hortobágyi T (2014) Aging causes a reorganization of cortical and spinal control of posture. Front Aging Neurosci 6:28
Robert T, Zatsiorsky VM, Latash ML (2008) Multi-muscle synergies in an unusual postural task: quick shear force production. Exp Brain Res 187(2):237–253
Rogers MW, Kukulka CG, Soderberg GL (1992) Age-related changes in postural responses preceding rapid self-paced and reaction time arm movements. J Gerontol 47:M159–M165
Sabatini AM (2002) Identification of neuromuscular synergies in natural upper-arm movements. Biol Cybern 86:253–262
Shumway-Cook A, Woollacott M (2000) Motor control: theory and practical applications, 2nd edn. Lippincott Williams & Wilkins, Philadelphia
Ting LH (2007) Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res 165:299–321
Tresch MC, Cheung VC, D’Avella A (2006) Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol 95(4):2199–2212
Tsai YC, Hsieh LF, Yang S (2014) Age-related changes in posture response under a continuous and unexpected perturbation. J Biomech 47(2):482–490
Wang Y, Asaka T (2008) Muscle synergies involved in shifts of the center of pressure while standing on a narrow support. Brain Res Bull 76(1–2):16–25
Wang Y, Zatsiorsky VM, Latash ML (2005) Muscle synergies involved in shifting the center of pressure while making a first step. Exp Brain Res 167(2):196–210
Wang Y, Zatsiorsky VM, Latash ML (2006) Muscle synergies involved in preparation to a step made under the self-paced and reaction time instructions. Clin Neurophysiol 117:41–56
Wang Y, Asaka T, Watanabe K (2013) Multi-muscle synergies in elderly individuals: preparation to a step made under the self-paced and reaction time instructions. Exp Brain Res 226(4):463–472
Wang Y, Watanabe K, Asaka T, Wan FT (2014) Muscle synergies in preparation to a step made with and without obstacle. Eur J Appl Physiol 114(12):2561–2569
Wang Y, Watanabe K, Asaka T (2015a) Age effects on multi-muscle modes during voluntary body sway. Res Sports Med 23(1):88–101
Wang Y, Watanabe K, Asaka T (2015b) Muscle synergies in preparation to a step made with obstacle in elderly individuals. J Neuroeng Rehabil 12:10
Wang Y, Watanabe K, Asaka T (2016) Muscle synergies underlying control of taking a step during support surface translation. Eur J Appl Physiol 116(2):301–309
Woollacott MH, Manchester DL (1993) Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? J Gerontol 48:M64–M70
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant #31371207) and the Natural Science Foundation of Tianjin (Grant #14JCYBJC43300). We thank Shanshan Jiao, Huihua Lan, Yunteng Liu, and Teng Ma for their assistance in the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Benedicte Schepens.
Rights and permissions
About this article
Cite this article
Wang, Y., Watanabe, K. & Asaka, T. Aging effect on muscle synergies in stepping forth during a forward perturbation. Eur J Appl Physiol 117, 201–211 (2017). https://doi.org/10.1007/s00421-016-3514-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3514-8