Abstract
Purpose
We sought to determine if expiratory flow limitation influences intensive aerobic exercise performance in mild hypoxia.
Methods
Fourteen trained male cyclists were separated into flow-limited (FL, n = 7) and non-FL (n = 7) groups based on the extent of expiratory flow limitation exhibited during maximal exercise in normoxia. Participants performed two self-paced 5-km cycling time trials, one in normoxic (F IO2 = 0.21) and one in mild hypoxic (F IO2 = 0.17) conditions in a randomized, balanced order with the subjects blinded to composition of the inspirate. Percent change from normoxia to hypoxia in average power output (%ΔP TT) and time to completion (%ΔT TT) were used to assess performance.
Results
Hypoxia resulted in a significant decline in estimated arterial O2 saturation and decrements in performance in both groups, although FL had a significantly smaller %ΔP TT (−4.0 ± 0.5 vs. −9.0 ± 1.8 %) and %ΔT TT (1.3 ± 0.3 vs. 3.7 ± 0.9 %) compared to non-FL. At the 5th km of the time trial, minute ventilation did not change from normoxia to hypoxia in FL (3.4 ± 3.1 %) or non-FL (2.3 ± 3.7 %), but only the non-FL reported a significantly increased dyspnea rating in hypoxia compared to normoxia (~9 %). Non-FL athletes did not utilize their ventilatory reserve to defend arterial oxygen saturation in hypoxia, which may have been due to an increased measure of dyspnea in the hypoxic trial.
Conclusion
FL athletes experience less hypoxia-related aerobic exercise performance impairment as compared to non-FL athletes, despite having less ventilatory reserve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The degree to which performance and maximal oxygen consumption, VO2max, are impaired during endurance exercise at altitude is not uniform between athletes, and the inter-individual variability can be substantial (Romer et al. 2007; Chapman et al. 1999; Dill and Adams 1971; Tucker et al. 1984; Chapman 2013). A number of potential factors accounting for this variability have been suggested, and the maintenance of arterial oxyhemoglobin saturation (SaO2), due to in part to the ventilatory response to exercise and hypoxia, appears to play a primary role (Lawler et al. 1988; Gore et al. 1996; Terrados et al. 1985; Chapman et al. 1999; Chapman 2013). A greater minute ventilation (V E) increases alveolar partial pressure of oxygen (PAO2), which is the driving pressure for O2 diffusion across the alveolar–capillary membrane. Due to the relationship between alveolar ventilation, PAO2, and SaO2, it has been suggested that increasing V E during exercise at altitude is a positive response (Lawler et al. 1988; Sutton et al. 1988).
The magnitude of the ventilatory response to hypoxia and/or exercise is dependent upon a number of factors, ranging from mechanical to chemical to perceptual. For example, achievement of the mechanical limit imposed by the chest wall and conducting airways, classically termed expiratory flow limitation, results in some athletes being unable to increase ventilation during maximal or near maximal exercise in hypoxia (Johnson et al. 1992; Chapman et al. 1998). In contrast to aged and diseased individuals, these athletes “suffer” not from a reduced ventilatory capacity per se, but from an increased ventilatory demand that approaches the capacity of the respiratory system. In many cases, these athletes display a substantially large minute ventilation during maximal exercise, often exceeding 160 l/min (Johnson et al. 1992; Chapman et al. 1998; McClaran et al. 1999). As a consequence of expiratory flow limitation, these athletes are unable to substantially increase V E in an effort to defend SaO2 over already large minute volumes during an all-out endurance exercise task in hypoxia. If so, expiratory flow-limited athletes (FL) may be at greater risk of performance declines during hypoxic exercise than athletes without limitations to expiratory flow.
It follows that non-expiratory flow-limited athletes (non-FL) may be less impaired during heavy exercise in hypoxia, as increases in V E lead to increases in PAO2, helping to defend SaO2 in hypoxia. However, added respiratory muscle work (Harms et al. 2000) and increased dyspnea (Iandelli et al. 2002) during heavy exercise have been shown to negatively impact exercise performance. Data from our laboratory (Chapman et al. 1998) indicate that, despite having the mechanical reserve to increase V E, non-FL athletes allow SaO2 to fall during heavy and maximal exercise in mild hypoxia (F IO2 = 18.7 %). These data suggest that non-FL athletes may sub-consciously regulate V E during hypoxic exercise in an attempt to optimize the work of breathing and/or dyspnea around some critical threshold that the athlete is willing to tolerate.
When an exercise task allows for pacing, such as a performance time trial (TT), athletes regulate work output during the task based on a number of sensory feedback cues for review see (St Gibson et al. 2006; Ulmer 1996). Many of the primary regulated feedback cues monitored during exercise are associated with ventilation (Noble et al. 1973; Robertson 1982). Specifically, sensations of ventilatory effort and/or breathlessness, i.e., dyspnea, during exercise have been shown to be a key determinant of exercise performance in healthy (Iandelli et al. 2002; Volianitis et al. 2001) and diseased populations (O’Donnell 2006). Ultimately, it is not known whether performance at altitude in a self-paced TT for a set distance is impaired more or less in FL (without the mechanical reserve to increase V E over already high levels in hypoxia) or non-FL (who may reduce power output to limit or clamp a tolerable level of dyspnea and/or work of breathing). Therefore, the purpose of this study was to examine exercise performance during a self-paced maximal endurance exercise task in acute hypoxia as a function of expiratory flow limitation. We hypothesized that non-FL athletes would increase V E, whereas FL would not, when exposed to hypoxia during a 5-km cycling trial. Additionally, due to a balance between the potential benefits and costs of increasing V E, FL and non-FL athletes would incur a similar decrement to cycling performance when exposed to a hypoxic inspirate.
Methods
Subjects
Prior to initiation, the study protocols and procedures were approved by Indiana University’s Institutional Review Board for the Protection of Human Subjects. Fourteen apparently healthy men engaged in a current cycling regime gave written, informed consent before any participation in the study. All subjects were advised orally, and in writing, as to the nature of the experiments and gave written informed consent to participate in the study. A potential subject was eligible to be included as part of the study if VO2max while cycling was ≥60 mL kg−1 min−1 and pulmonary function was classified as normal, as defined by a ≥80 % of predicted (Hankinson et al. 1999) forced vital capacity (FVC), forced expired volume in one second (FEV1), and FEV1/FVC according to the American Thoracic Society (ATS) standards (American Thoracic Society 1995).
Study design
Each subject participated in four experimental testing sessions, separated by a minimum of 48 h and a maximum of 7 days. During all visits, subjects reported to the lab 2 h post-prandial and were instructed not to exercise intensely within 24 h of each session. Session 1 included pulmonary function tests, familiarization with inspiratory capacity (IC) maneuvers (Babb 1997b), and a progressive maximal exercise test on an electronically braked cycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA, USA).
During session 2, subjects were thoroughly familiarized and acclimated to the experimental procedures and equipment, with specific focus given to the reproduction of IC maneuvers at rest and during exercise. After performing 3–5 pre-exercise FVC maneuvers to obtain maximum flow-volume loops (MFVLs) (American Thoracic Society 1995), subjects were encouraged to complete a 5 km TT in the fastest time possible. A 5-km TT was chosen for the performance measure as it has been shown to be highly reproducible (Jeukendrup et al. 1996; Hickey et al. 1992; Amann et al. 2006) and elicits high ventilatory and metabolic loads (Amann et al. 2006). MFVLs were collected again within 4 min following completion of the TT.
Subjects completed identical testing procedures in sessions 3 and 4 as performed in the session 2 familiarization trial. All subjects were blinded to the testing condition, and completed a 5 km TT randomized to either a normoxic (F IO2 = 0.21) or hypoxic (F IO2 = 0.17) condition. To enhance the blinding, subjects breathed gas at rest and during exercise from a meteorological balloon for both the normoxic and hypoxic gas trials.
Instrumentation
Pulmonary function testing
Pulmonary function tests were conducted while the subject was seated on a cycle ergometer and consisted of a FVC maneuver, from which FEV1, peak expiratory flow rate (PEFR), mid-expiratory flow between 25 and 75 % expiration (MEF25–75) were obtained. Additionally, construction of a MFVL pre- and post-exercise was acquired with FVC maneuvers. Pulmonary testing was performed in triplicate per ATS standards (American Thoracic Society 1995), with a secured silicone oro-nasal mask (Hans Rudolph #7450V2, Shawnee, KS, USA), with the largest FVC, FEV1, PEFR, and MEF25–75 being selected. Flow and volume values were corrected to body temperature, pressure, saturated (BTPS).
Progressive maximal exercise test
Subjects performed a maximal exercise test to determine fitness (VO2max) eligibility, classification of expiratory flow limitation, and to provide descriptive data. VO2max was determined as the highest 1-min average VO2 achieved during exercise while meeting two of the following three criteria: (1) a heart rate (HR) ≥90 % age-predicted maximum, and (2) a respiratory exchange ratio (RER) ≥1.10, and 3) a plateau in VO2 ≤ 150 mL with an increased workload. Following a 5-min warm-up (1.5 W kg body weight−1), the subject began an adapted incremental exercise test (100 W + 25 W min−1) (Amann et al. 2006) on an electronically braked cycle ergometer. Subjects continued until the power output could no longer be maintained (i.e., RPM’s drop >10 % and could not be recovered or volitional exhaustion) or the subject voluntarily ended the test. Ventilatory threshold was established, to demonstrate matched fitness levels in each group, using the ventilatory equivalent for oxygen (V E/VO2) method described previously (Amann et al. 2004).
Metabolic and ventilatory measurements
The metabolic and ventilatory data were collected continuously on a computer equipped with a data acquisition software (DASYLab 10.0, National Instruments, Norton, MA) sampling at 50 Hz during all exercise tests. An original acquisition script was used to report 15 s averages of all metabolic and ventilatory measures during TT performance. At each km, the previous minute of data was combined. Minute ventilation was measured using a calibrated pneumotachograph (Hans Rudolph Inc. #3813, Shawnee, KS, USA) on the inspired side. Ventilatory capacity (V Emax, CAL) was calculated using a previously described method (Babb and Rodarte 1993) and is an estimate of the maximum theoretical ventilation an individual can achieve at a given lung volume. Ventilation as a percent of V Emax, CAL (V E/V Emax, CAL) was calculated at each km to describe the proportion of ventilatory capacity being utilized and to estimate the ventilatory reserve present. Subjects respired through a facemask attached to a two-way non-rebreathing valve (Hans Rudolph #2700, Shawnee, KS, USA), and connected by a large bore hose, one meter in length, on the expired side to a five-liter mixing chamber. Fractional inspired and expired O2 (F IO2) and carbon dioxide (F ECO2) were measured by two sets of Applied Electrochemistry S-3A/I and CD-3A analyzers (AEI Technologies, Naperville, IL), respectively. Gas samples were drawn continuously at a rate of 300 mL min−1 through low-resistance tubing and a desiccant (Drierite, Xenia, OH, USA) to remove moisture before reaching the gas analyzers. A calibration gas within the physiological range was used to calibrate the analyzers before each testing session. Heart rate (HR) was measured using a Polar heart rate monitor and the minute average prior to the km was reported. SpO2 was continuously measured on the pinna of the left ear using non-invasive pulse oximetry (Masimo Radical SET v 4, Masimo Corporation, Irvine, CA, USA). The oximeter was calibrated prior to each exercise test per manufacturer’s instructions.
Flow-volume relationships
Flow-volume loops were constructed during the last minute of the progressive maximal exercise test and at every km of each TT to determine the extent of expiratory flow limitation. Airflow and volume were measured on both the inspired and expired side using linear pneumotachographs (Hans Rudolph Inc. #3813, Shawnee, KS, USA). Temperature of expired gas proximal to the pneumotachograph on the expired side was measured to allow for the conversion of ventilatory values to BTPS.
MFVL’s were constructed in triplicate from FVC maneuvers pre- and post-exercise as previously described with ATS standards (Bartlett and Phillips 1962; Chapman et al. 1998; American Thoracic Society 1995; Johnson et al. 1992; Babb 1997a). The maneuver with the largest FVC and FEV1 was chosen for the MFVL, as we have done previously (Duke et al. 2014). Specifically, post-exercise FVCs produced the largest MFVL in each subject, likely due to exercise-induced bronchodilation. Subjects were extensively instructed on how to perform IC prior to exercise sessions. An IC maneuver was performed every 30 s during the maximal aerobic capacity test and approximately 30 s prior to and at each km during the TT. The purpose of the IC maneuver was to determine and correct for electronic and physiologic drift in the volume signal when placing the exercise tidal FVL within the MFVL. Approximately 10–15 representative tidal breaths were averaged for each stage and placed into the MFVL as previously described (Chapman et al. 1998; Derchak et al. 2000). Loops were considered ‘representative’ if they had similar volume and flow characteristics as breaths prior to the first IC maneuver (Babb and Rodarte 1992). The percent of flow limitation (%TVFL) was determined as the percentage of exercise tidal volume that either met or exceeded the boundary of the MFVL (Johnson et al. 1991; Johnson et al. 1992). Subjects were placed a priori into distinct groups according to the extent of expiratory flow limitation achieved during the last minute of the maximal aerobic capacity test: non-FL (0 % expiratory flow limitation) or FL (≥40 % expiratory flow limitation) (Chapman et al. 1998). Any subjects with expiratory flow limitation during the maximal aerobic capacity test between ≥5 and ≤40 % were excluded from the study. These parameters were chosen as criteria to group subjects into FL and non-FL based on previous research (Chapman et al. 1998) to prevent any ambiguity in subject expiratory flow limitation. Additionally, any overestimation of expiratory flow limitation would be considered minor as post-exercise MFVLs result in a similar estimation of expiratory flow limitation as has been shown to occur with graded submaximal FVC efforts (used to prevent an influence of thoracic gas compression) (Guenette et al. 2010). By these standards, it is unlikely that someone classified as FL was actually non-FL.
5-km TT
For the experimental trials, following 5 min of rest while breathing room air, an additional 8 min of resting data was collected, while subjects breathed either a normoxic (F IO2 = 21 %) or hypoxic (F IO2 = 17 %) humidified gas mixture from a large balloon reservoir. The TT began from a stationary start and subjects remained seated throughout the exercise. Distance traveled and verbal encouragement were continuously provided. Subjects provided a rating of dyspnea (0–10 modified Borg scale) (Borg 1982) at the completion of each km. This scale has been demonstrated to be a highly reproducible measure of breathlessness and strongly correlates with the level of V E (Wilson and Jones 1991). Subjects were told that a rating of 0 implied that there was no “noticeable breathing effort above what occurs at rest” and a rating of 10 implied that there was “maximal ventilatory effort” and/or “severe breathlessness”. Time for completion and average power output at each km were analyzed using WKO+software (Peaksware LLC, Lafayette, CO, USA). Each subject was allowed to recover for 2–3 min before performing a second set of MFVL maneuvers.
Data analysis
A two-way mixed model ANOVA with a priori planned comparisons (t tests, two tailed) was computed for the 5th km to determine if a significant effect occurred on various physiological variables between groups and inspired gases. This time point was chosen for analysis as previous literature has demonstrated that the largest metabolic and ventilatory perturbations occur at the 5th km (Amann et al. 2006). A priori planned comparisons were conducted on (1) the percentage change in metabolic, ventilatory, and performance data from normoxia to hypoxia within FL athletes and (2) within non-FL athletes, and (3) the percentage change from normoxia to hypoxia between FL and non-FL athletes. To maintain a family-wise error rate (α) of 0.05, we used the Holm-Bonferroni adjustment. A follow-up 2 × 5 ANOVA (inspirate × distance) was performed to examine the development of primary variables (i.e. V E, dyspnea, and power) during the TT. All data are presented as mean ± SEM unless otherwise noted. Statistical analyses were performed using PASW version 19.0 for Windows (IBM, Chicago, IL, USA).
Results
Subjects
Of the initial thirty-one subjects screened, fourteen completed the entire study. Reasons for exclusion included: not meeting the aerobic fitness criteria (n = 9), falling between the defined expiratory flow limitation categories (n = 5), inability to consistently perform the IC maneuver (n = 2), and voluntary withdrawal (n = 1). Descriptive data for all fourteen subjects, by group, are presented in Table 1. Subject groups were well matched for age, weight, and VO2max. Non-FL athletes were taller than FL (183.0 ± 2.4 vs. 174.5 ± 2.3 cm, respectively), but no differences in baseline pulmonary function were detected.
Maximal aerobic capacity test
Metabolic and ventilatory data from the maximal aerobic capacity test are summarized in Table 2. As designed, the FL athletes presented with a significantly higher (P < 0.05) mean tidal expiratory flow limitation compared to the non-FL athletes at VO2max (52 ± 5 vs. 0 ± 0 %, respectively). There was no significant difference (P > 0.05) in any other ventilatory or metabolic measurement taken at VO2max between the FL and non-FL groups.
5-km time-trial performance
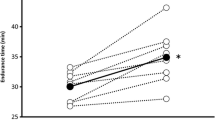
TT order was randomized and there was no condition by order effect (P = 0.55). Subjects in both the FL and non-FL had a significant (P < 0.05) ΔT TT and ΔP TT from normoxia to hypoxia (Table 3). Compared to non-FL, the FL group had a significantly smaller increase (P < 0.05) in ΔT TT (1.3 ± 0.2 vs. 3.7 ± 0.9 %, Fig. 1) and smaller reduction in ΔP TT (−4.0 ± 0.5 vs. −9.0 ± 1.8 %) indicating a larger worsening in performance in the non-FL group.
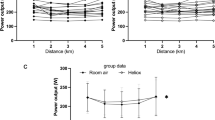
Further statistical analysis was conducted to elucidate the development of our primary variables (V E, dyspnea, and power) during the 5 km TT. While there was a main effect for V E across distance in both groups (P < 0.001), there was no effect of inspirate within either group (P > 0.79; Fig. 2). Despite the unchanging V E from normoxia to hypoxia, there was a main effect for distance (P < 0.01) and inspirate (P < 0.01) with regards to dyspnea in the non-FL. In contrast, only an effect of distance (P < 0.01) was observed in the FL for dyspnea. Additionally, non-FL subjects had a significantly reduced (P < 0.05) mean power output in hypoxia compared to normoxia during the 1st, 2nd, 3rd, 4th, and 5th km while the FL athletes had a significantly diminished (P < 0.05) power output in hypoxia only at the 5th km (Fig. 2).
Ventilatory and metabolic data during experimental TT
Ventilatory and metabolic data for the 5th km are presented in Table 3. Hypoxia resulted in a significant decrease (P < 0.05) in VO2 (−12.2 ± 3.6 and −13.3 ± 2.4 %), VCO2 (−6.4 ± 1.6 and −7.7 ± 1.6 %), and SpO2 (−7.0 ± 0.8 and −9.6 ± 1.2 %) compared to normoxia in FL and non-FL athletes, respectively. The magnitude of decline from normoxia to hypoxia in the aforementioned variables was not statistically different (P > 0.05) between groups.
V E for the 5th km did not significantly change between trials in either FL (3.4 ± 3.0 %) or non-FL (2.3 ± 3.7 %) and were not different between groups. Subsequently, V E/V Emax, CAL, expiratory reserve volume (ERV), inspiratory reserve volume (IRV), tidal volume (V T), and breathing frequency (F B) were not significantly different (P > 0.05) between normoxia and hypoxia in either group (Table 3). However, V E/VO2 (19.1 ± 6.6 and 17.0 ± 5.3 %) and V E/VCO2 (10.3 ± 2.3 and 9.2 ± 2.5 %) were significantly augmented from normoxia to hypoxia (P < 0.05) in the FL and non-FL groups, respectively. Neither the change in V E/VO2 nor V E/VCO2 was significantly different (P > 0.05) between groups. Additionally, in hypoxia, the non-FL athletes reported a significantly higher (P < 0.05) rating of dyspnea at the 5th km than normoxia.
During the normoxic TT, the majority of FL athletes began exhibiting expiratory flow limitation at the 2nd km (6 out of 7 athletes, 23 ± 7 % expiratory flow limitation). In hypoxia, FL athletes had a similar pattern of expiratory flow limitation (5 out of 7, 22 ± 6 % expiratory flow limitation). In contrast, expiratory flow limitation was absent in the non-FL athletes in both conditions at every km (Fig. 3).
Discussion
The purpose of this study was to examine whether the performance of athletes who exhibit expiratory flow limitation during intensive aerobic exercise at sea level is compromised to a greater or lesser extent in a mild hypoxic environment as compared to those who do not exhibit expiratory flow limitation at sea level. Our data demonstrate that FL athletes experience less hypoxia-related aerobic exercise performance impairment when compared with non-FL athletes, as evidenced by a smaller increase in time to completion of a 5-km time trial and a smaller reduction in power when compared to normoxia. Our data suggest that the greater performance decrement of non-FL athletes may have been due to higher ventilatory sensations at the 5th km incurred with hypoxia.
This study was the first to make comparisons between FL and non-FL athletes using a “realistic” exercise challenge that allowed them to self-select and regulate their own pacing strategies while breathing a mild hypoxic gas. Previous studies have utilized progressive maximal or constant load exercise tests, rather than a self-paced exercise protocol. The latter of which allows for the integration of sensory feedback to influence exercise performance (Marino 2012; Ulmer 1996). Specifically, incremental maximal protocols progressively increase the external workload and thereby demand ventilatory and metabolic adaptations until task failure. In contrast, the goal of a self-selected maximal exercise (i.e., TT) is to reach the finish line in as little time as possible, without resulting in premature exhaustion. As a consequence, TTs rely on a complex integration of both feed-forward and feedback processes to produce optimal performance. In the present study, FL and non-FL athletes increased time to completion (1.3 and 3.7 %) and reduced average power output (−4 and −9 %) in hypoxia, with the FL athletes having a significantly smaller decrement in performance. The overall decrease in average power output in our study (6.5 % collectively) is comparable to others using a similar protocol at mild altitude (580 m; (Gore et al. 1997)) and breathing an F IO2 = 0.168 (Koskolou and McKenzie 1994).
In addition to the reduction in exercise performance, the hypoxic inspirate in the present study diminished VO2 ~ 13 % at the 5th km in both groups. This is similar to the reduction previously reported in trained subjects cycling at sea level and acute hypobaric exposure corresponding to 1500 m (Terrados et al. 1985). As the hypoxic inspirate did not significantly alter V E in either group (see below), V E/VO2 was elevated ~18 %, indicating both groups experienced a hypoxia-induced hyperventilation.
Surprisingly, the non-FL athletes in the present study did not increase absolute V E in hypoxia above normoxic values as expected (see Fig. 2) despite having a substantial ventilatory reserve (V E/V Emax, CAL = 69 %). This is in stark contrast to previous studies examining the ventilatory response to maximal exercise in non-FL and FL individuals (Chapman et al. 1998; Babb 1997b). However, those investigations utilized an incremental exercise protocol to exhaustion, in which the stimuli influencing V E may be starkly different from a TT effort where workload can be continually and consciously adjusted by the athlete. Increasing V E during a TT in hypoxia has theoretical advantages and disadvantages. It could result in an increased arterial O2 saturation and subsequent O2 delivery to the working musculature, improving performance. On the other hand, increasing V E could also increase the work of breathing and/or the perception of breathlessness, ultimately hindering performance. The lack of an increase in V E in hypoxia in the non-FL athletes, despite the presence of a ventilatory reserve, suggests that this cohort regulated the absolute V E during the TT in hypoxia by altering exercise intensity (i.e. reducing power output).
Previous reports have suggested that a heightened intensity of breathlessness hastens exercise termination in healthy individuals (Harms et al. 2000; Iandelli et al. 2002) and in patients with obstructive lung disease (Guenette et al. 2012). Non-FL athletes in the current study reported a higher end-exercise dyspnea rating in the hypoxia trial than the normoxia trial, even though V E was not different and power output was reduced. Additionally, previous literature has demonstrated an exaggerated development of respiratory muscle fatigue during exercise in hypoxia, which induced a SpO2 ~80 %, possibly leading to the reduction in performance and increased dyspnea (Verges et al. 2010). The increased dyspnea may be due to the increased ventilatory drive for the metabolic work rate in response to the 10 % reduction in SpO2 during exercise in hypoxia, even though V E was unaltered. In support of this, previous literature from our laboratory has demonstrated a positive relationship between the hypoxic ventilatory response and SpO2 in non-FL athletes, while no relationship existed for the FL (Derchak et al. 2000). However, instead of increasing ventilation, which could theoretically defend this fall in SpO2, the non-FL athletes in the current study reduced their average power output during every km compared to normoxia, effectively clamping V E (Fig. 2).
In contrast to the non-FL athletes, the FL athletes demonstrated no change in V E or dyspnea between trials and had a significant reduction in power at only the 5th km, suggesting there were no changes in pacing strategy in this group between the normoxic and hypoxic trials. In agreement with previous literature, there was no change in V E from normoxia to hypoxia in the FL athletes, who utilized 90 and 93 % V E/V Emax, CAL in normoxia and hypoxia respectively. Interestingly, the majority of the FL athletes in the present study experienced expiratory flow limitation beginning at the 2nd km in both conditions (Fig. 3), demonstrating that FL athletes perform the bulk of a high-intensity self-selected exercise trial exhibiting expiratory flow limitation. Nevertheless, the FL athletes demonstrated a smaller hypoxia-induced performance decrement while reporting no changes in the perception of respiratory efforts, in contrast to their non-FL group counterparts, further illustrating the perceptual response to exercise is different between the groups. It is important to note that while expiratory flow limitation has been classically viewed as a negative ventilatory constraint, healthy athletes with expiratory flow limitation can still operate at mechanically optimal lung volumes and muscle lengths while maintaining adequate alveolar ventilation (Johnson et al. 1992). Additionally, while lung dynamic hyperinflation is associated with exercise intolerance, the presence of expiratory flow limitation alone does not always result in an increase in end expiratory lung volume and hyperinflation (Taylor et al. 2013). Ultimately, we would agree with the conclusions of others (Johnson et al. 1992; Chapman et al. 1998) that the increased ventilatory requirement of highly endurance-trained, flow-limited athletes during heavy exercise is causing the athlete to reach a mechanical limit to expiratory flow, rather than mechanical limitation constraining maximal ventilation in this specific population. As we have suggested previously (Chapman et al. 1998), it may be better to describe these high ventilating endurance athletes as ‘flow maximized’ rather than FL. As such, these athletes are regularly exposed to high levels of ventilation, and consequently work of breathing, which could be argued to act as a training stimuli thereby making this group less susceptible to fatigue. While an intriguing proposal, further investigation needs to be performed to determine its validity.
During dynamic exercise, a number of physiological factors, both central and peripheral, are integrated to regulate exercise performance (i.e., pacing strategy) in a process termed teleoanticipation (Ulmer 1996; Hampson et al. 2001). Current physiological thinking holds that these factors are integrated at the level of the brain to prevent: (a) fatigue prior to task completion, and/or (b) detrimental disturbances in systemic homeostasis (St Gibson et al. 2006; Noakes 2000). Specifically, but not exclusively, the maintenance of SaO2 (Romer et al. 2007), regulation of ventilatory work (Harms et al. 2000), and sensations of breathlessness (Iandelli et al. 2002) have been demonstrated to have a profound influence on exercise performance. Although hypoxia resulted in a reduction in SaO2 compared to normoxia, our FL and non-FL groups desaturated to the same extent. Cycling 5-km power output has been shown to fall, and recover, rapidly and congruently with changing SaO2 (Johnson et al. 2009), indicating that if the non-FL had defended their declining SaO2 via accessing their available ventilatory reserve, they theoretically would have been able to subsequently maintain power output. In our study, only the presence of expiratory flow limitation and changes in the sensation of dyspnea and exercise performance between normoxia and hypoxia were different between groups. Although the trial conditions were blinded and there were no differences in baseline resting measures between groups, it appears that the non-FL group “sensed” the effects of the hypoxic inspirate as early as the first km of the time trial, and as others have suggested (de Koning et al. 2011), integrated that signal with the amount of distance remaining. Despite having the mechanical room to increase ventilation in an effort to defend SpO2 in the hypoxic trial, we believe the non-FL group ultimately down-regulated power output early and throughout the exercise bout in an effort to keep ventilation fixed at the same tolerable level as in the normoxic trial.
Ultimately, FL athletes did not change V E or dyspnea at any time point and experienced a smaller decrement in exercise performance in hypoxia, while the non-FL athletes reported a 9 % higher rating of dyspnea at the 5th km and greater performance decrement overall, even though V E was unchanged. The latter suggests that the non-FL are regulating their ventilatory response to prevent excessive discomfort. Subsequently, we propose that the perception of ventilatory effort is a key modifier of exercise work output for non-FL athletes during a self-paced 5-km TT.
Abbreviations
- ERV:
-
Expiratory reserve volume
- F B :
-
Frequency of breathing
- F IO2 :
-
Fraction of inspired O2
- F ECO2 :
-
Fraction of expired CO2
- FEV1 :
-
Forced expired volume in 1 s
- FEV1/FVC:
-
Forced expired volume in 1 s to forced vital capacity ratio
- FL:
-
Flow limited
- FVC:
-
Forced vital capacity
- HR:
-
Heart rate
- IC:
-
Inspiratory capacity
- IRV:
-
Inspiratory reserve volume
- MEF25–75 :
-
Maximal expiratory flow between 25 and 75 % of forced vital capacity
- MFVL:
-
Maximal flow volume loop
- PAO2 :
-
Partial pressure of alveolar oxygen (O2)
- PEFR:
-
Peak expired flow rate
- P TT :
-
Power output during the 5-km time trial
- RPM:
-
Revolutions per minute
- RER:
-
Respiratory exchange ratio
- SaO2/SpO2 :
-
Arterial oxygen saturation/estimated arterial oxygen saturation
- TT:
-
Time trial
- T TT :
-
Time to completion for the 5-km time trial
- V E :
-
Minute ventilation
- V E/VO2 :
-
Ventilatory equivalents for oxygen (O2)
- V E/VCO2 :
-
Ventilatory equivalents for carbon dioxide (CO2)
- VO2max :
-
Maximal oxygen consumption
- V T :
-
Tidal volume
- W:
-
Watts
References
Amann M, Subudhi AW, Walker J, Eisenman P, Shultz B, Foster C (2004) An evaluation of the predictive validity and reliability of ventilatory threshold. Med Sci Sports Exerc 36(10):1716–1722
Amann M, Eldridge M, Lovering A et al (2006) Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575(3):937–952
American Thoracic Society (1995) Standardization of Spirometry, 1994 update. Am J Respir Crit Care Med 152(3):1107–1136
Babb TG (1997a) Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir Physiol 109(1):15–28
Babb TG (1997b) Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol 82(3):746–754
Babb TG, Rodarte JR (1992) Exercise capacity and breathing mechanics in patients with airflow limitation. Med Sci Sports Exerc 24(9):967–974
Babb TG, Rodarte JR (1993) Estimation of ventilatory capacity during submaximal exercise. J Appl Physiol (1985) 74(4):2016–2022
Bartlett RG Jr, Phillips NE (1962) The velocity volume loop: a composite pulmonary function test. Dis Chest 42:482–488
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Chapman RF (2013) The individual response to training and competition at altitude. Br J Sports Med 47(Suppl 1):i40–i44
Chapman RF, Emery M, Stager JM (1998) Extent of expiratory flow limitation influences the increase in maximal exercise ventilation in hypoxia. Respir Physiol 113(1):65–74
Chapman RF, Emery M, Stager JM (1999) Degree of arterial desaturation in normoxia influences VO2max decline in mild hypoxia. Med Sci Sports Exerc 31(5):658–663
de Koning JJ, Foster C, Bakkum A, Kloppenburg S, Thiel C, Joseph T, Cohen J, Porcari JP (2011) Regulation of pacing strategy during athletic competition. PLoS One 6(1):e15863
Derchak PA, Stager JM, Tanner DA, Chapman RF (2000) Expiratory flow limitation confounds ventilatory response during exercise in athletes. Med Sci Sports Exerc 32(11):1873–1879
Dill DB, Adams WC (1971) Maximal oxygen uptake at sea level and at 3,090-m altitude in high school champion runners. J Appl Physiol 30(6):854–859
Duke JW, Stickford JL, Weavil JC, Chapman RF, Stager JM, Mickleborough TD (2014) Operating lung volumes are affected by exercise mode but not trunk and hip angle during maximal exercise. Eur J Appl Physiol 114(11):2387–2397
Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, Campbell DP, Emonson DL (1996) Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol (1985) 80(6):2204–2210
Gore CJ, Little SC, Hahn AG, Scroop GC, Norton KI, Bourdon PC, Woolford SM, Buckley JD, Stanef T, Campbell DP, Watson DB, Emonson DL (1997) Reduced performance of male and female athletes at 580 m altitude. Eur J Appl Physiol Occup Physiol 75(2):136–143
Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND, Sheel AW (2010) Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol 170(3):279–286
Guenette JA, Webb KA, O’Donnell DE (2012) Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J 40(2):322–329
Hampson DB, St Clair Gibson A, Lambert MI, Noakes TD (2001) The influence of sensory cues on the perception of exertion during exercise and central regulation of exercise performance. Sports Med 31(13):935–952
Hankinson JL, Odencrantz JR, Fedan KB (1999) Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159(1):179–187
Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA (2000) Effects of respiratory muscle work on exercise performance. J Appl Physiol (1985) 89(1):131–138
Hickey MS, Costill DL, McConell GK, Widrick JJ, Tanaka H (1992) Day to day variation in time trial cycling performance. Int J Sports Med 13(6):467–470
Iandelli I, Aliverti A, Kayser B, Dellaca R, Cala SJ, Duranti R, Kelly S, Scano G, Sliwinski P, Yan S, Macklem PT, Pedotti A (2002) Determinants of exercise performance in normal men with externally imposed expiratory flow limitation. J Appl Physiol (1985) 92(5):1943–1952
Jeukendrup A, Saris WH, Brouns F, Kester AD (1996) A new validated endurance performance test. Med Sci Sports Exerc 28(2):266–270
Johnson BD, Reddan WG, Pegelow DF, Seow KC, Dempsey JA (1991) Flow limitation and regulation of functional residual capacity during exercise in a physically active aging population. Am Rev Respir Dis 143(5 Pt 1):960–967
Johnson BD, Saupe KW, Dempsey JA (1992) Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol (1985) 73(3):874–886
Johnson BD, Joseph T, Wright G, Battista RA, Dodge C, Balweg A, de Koning JJ, Foster C (2009) Rapidity of responding to a hypoxic challenge during exercise. Eur J Appl Physiol 106(4):493–499
Koskolou MD, McKenzie DC (1994) Arterial hypoxemia and performance during intense exercise. Eur J Appl Physiol Occup Physiol 68(1):80–86
Lawler J, Powers SK, Thompson D (1988) Linear relationship between VO2max and VO2max decrement during exposure to acute hypoxia. J Appl Physiol (1985) 64(4):1486–1492
Marino FE (2012) The limitations of the constant load and self-paced exercise models of exercise physiology. Comp Exerc Physiol 7(4):173–178
McClaran SR, Wetter TJ, Pegelow DF, Dempsey JA (1999) Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol (1985) 86(4):1357–1366
Noakes TD (2000) Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand J Med Sci Sports 10(3):123–145
Noble BJ, Metz KF, Pandolf KB, Cafarelli E (1973) Perceptual responses to exercise: a multiple regression study. Med Sci Sports 5(2):104–109
O’Donnell DE (2006) Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3(2):180–184
Robertson RJ (1982) Central signals of perceived exertion during dynamic exercise. Med Sci Sports Exerc 14(5):390–396
Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF, Dempsey JA (2007) Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol 292(1):R598–R606
St Gibson AC, Lambert EV, Rauch LH, Tucker R, Baden DA, Foster C, Noakes TD (2006) The role of information processing between the brain and peripheral physiological systems in pacing and perception of effort. Sports Med 36(8):705–722
Sutton JR, Reeves JT, Wagner PD, Groves BM, Cymerman A, Malconian MK, Rock PB, Young PM, Walter SD, Houston CS (1988) Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 64(4):1309–1321
Taylor BJ, How SC, Romer LM (2013) Expiratory muscle fatigue does not regulate operating lung volumes during high-intensity exercise in healthy humans. J Appl Physiol 114:1569–1576
Terrados N, Mizuno M, Andersen H (1985) Reduction in maximal oxygen uptake at low altitudes; role of training status and lung function. Clin Physiol 5(Suppl 3):75–79
Tucker A, Stager JM, Cordain L (1984) Arterial O2 saturation and maximum O2 consumption in moderate-altitude runners exposed to sea level and 3,050 m. JAMA 252(20):2867–2871
Ulmer HV (1996) Concept of an extracellular regulation of muscular metabolic rate during heavy exercise in humans by psychophysiological feedback. Experientia 52(5):416–420
Verges S, Bachasson D, Wuyam B (2010) Effect of acute hypoxia on respiratory muscle fatigue in healthy humans. Respir Res 11:109
Volianitis S, McConnell AK, Koutedakis Y, Jones DA (2001) Specific respiratory warm-up improves rowing performance and exertional dyspnea. Med Sci Sports Exerc 33(7):1189–1193
Wilson RC, Jones PW (1991) Long-term reproducibility of Borg scale estimates of breathlessness during exercise. Clin Sci (Lond) 80(4):309–312
Acknowledgments
The authors would like to express their gratitude to the individuals who volunteered to participate in this study and the colleagues who helped with data collection and manuscript preparation.
Conflict of interest
No author claims any conflict of interest from this study or has any relationships with companies or manufacturers. No company will benefit directly from the results of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carsten Lundby.
Rights and permissions
About this article
Cite this article
Weavil, J.C., Duke, J.W., Stickford, J.L. et al. Endurance exercise performance in acute hypoxia is influenced by expiratory flow limitation. Eur J Appl Physiol 115, 1653–1663 (2015). https://doi.org/10.1007/s00421-015-3145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3145-5