Abstract
Purpose
This study examined the effect of graded hypoxia during exhaustive intermittent cycling on subsequent exercise performance and neuromuscular fatigue characteristics in normoxia.
Methods
Fifteen well-trained cyclists performed an exhaustive intermittent cycling exercise (EICE 1; 15 s at 30% of anaerobic power reserve interspersed with 45 s of passive recovery) at sea level (SL; FiO2 ~ 0.21), moderate (MH; FiO2 ~ 0.16) and severe hypoxia (SH; FiO2 ~ 0.12). This was followed, after 30 min of passive recovery in normoxia, by an identical exercise bout in normoxia (EICE 2). Neuromuscular function of the knee extensors was assessed at baseline, after EICE 1 (post-EICE 1), and EICE 2 (post-EICE 2).
Results
The number of efforts completed decreased with increasing hypoxic severity during EICE 1 (SL: 39 ± 30, MH: 22 ± 13, SH: 13 ± 6; p ≤ 0.02), whereas there was no difference between conditions during EICE 2 (SL: 16 ± 9, MH: 20 ± 14, SH: 24 ± 17; p ≥ 0.09). Maximal torque (p = 0.007), peripheral (p = 0.02) and cortical voluntary activation (p < 0.001), and twitch torque (p < 0.001) decreased from baseline to post-EICE 1. Overall, there were no significant difference in any neuromuscular parameters from post-EICE 1 to post-EICE 2 (p ≥ 0.08).
Conclusion
Increasing hypoxia severity during exhaustive intermittent cycling hampered exercise capacity, but did not influence performance and associated neuromuscular responses during a subsequent bout of exercise in normoxia performed after 30 min of rest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle fatigue can be defined as a decline in force generating capacity of the exercising musculature that is reversible with sufficient rest (Gandevia 2001). The force decrement can be attributed to the interaction between biochemical changes within exercising muscles (i.e. peripheral fatigue) and an incomplete neural drive to the active musculature (i.e. central fatigue) (Amann 2011).
Reductions in oxygen availability negatively influence fatigability during the completion of exhaustive whole-body exercise (e.g. cycling time to exhaustion), with severe hypoxia triggering premature exercise cessation (Amann et al. 2007; Goodall et al. 2012). For instance, Amann et al. (2007) showed that cycling in severe hypoxia (fraction of inspired oxygen [FiO2] ~ 0.10) resulted in one-third less peripheral fatigue at task failure compared to normoxia and moderate hypoxia with reoxygenation significantly alleviating cerebral oxygenation (assessed with near-infrared spectroscopy; NIRS), and allowing individuals to continue cycling. This suggests that concomitant increases in hypoxic severity and cerebral deoxygenation may induce a shift from a predominantly peripheral origin of fatigue to a hypoxia-sensitive source of inhibition within the central nervous system (Amann et al. 2007; Millet et al. 2012). Accordingly, it is likely that the severity of hypoxia alters the neuromuscular fatigue characteristics during exhaustive exercise, which in turn, influence requirement for recovery, and ultimately subsequent exercise performance.
An experimental approach to understand the interplay between central and peripheral factors during exercise in graded hypoxia is to manipulate hypoxic severity during an initial exercise bout (Girard et al. 2016; Soo et al. 2020a) and identify factors limiting performance during a subsequent exercise bout in normoxia. To date, most studies using such protocols have selected tasks with a “closed-loop” design (Girard et al. 2016; Soo et al. 2020a) where the number of efforts (Townsend et al. 2020) or distance (Girard et al. 2016) to be completed is known. In this instance, participants may consciously/subconsciously modulate their performance through pacing strategies (Billaut et al. 2011). Accordingly, using an “open-looped” design, in which exercise is performed at a fixed work rate until exhaustion (Amann et al. 2007) may resolve the issue of pacing.
This study examined the effects of hypoxia severity during an initial exhaustive intermittent cycling exercise trial on subsequent performance and associated neuromuscular fatigue characteristics in normoxia. We hypothesised that the most severe hypoxic condition limits exercise capacity during the initial exercise bout primarily due to more pronounced central fatigue when compared to normoxia or moderate hypoxia. We further anticipated that, due to premature exercise cessation in severe hypoxia (i.e. less work done), increased exercise performance will be evident following completion of a subsequent exercise bout (in normoxia). To examine the potential role of cerebral deoxygenation in development of central fatigue in severe hypoxia, prefrontal cortex and muscle oxygenation was concomitantly measured throughout the trials. A better understanding of how fatigue-related residual or “carry over” effects of an initial exercise bout performed at various hypoxic levels influence performance during a subsequent exercise in normoxia is important to optimise training quality when athletes are breathing different oxygen concentrations within the same session.
Methods
Ethical approval
The experimental protocol was conducted according to the Declaration of Helsinki, and approved by Shafallah Medical Genetics Center Ethics Committee, Doha, Qatar (Institutional review board project number no. 2011–011). All participants gave their informed, written consent prior to the commencement of the experiment.
Participants
Fifteen well-trained male cyclists (38.4 ± 7.1 years; 181.7 ± 7.7 cm; 81.9 ± 13.8 kg; 8.1 ± 2.5 h cycling per week) participated in the study. All participants were born and raised at < 1500 m and had not travelled to elevations > 1000 m in the 3 months prior to investigation.
Experimental design
Each participant completed one familiarisation session and three experimental trials in a randomised, double-blind design. All tests were completed in a normobaric hypoxic chamber (Colorado Mountain Room System; Colorado Altitude Training, Boulder, CO). The experimental trials were separated by at least 5 days, performed at the same time of the day (± 2 h) in temperate ambient conditions (air temperature: ~ 24 °C; relative humidity: 40%). Participants avoided vigorous exercise for 24 h, caffeine for 12 h, and food for 2 h, before each trial. They were permitted to drink ad libitum during testing.
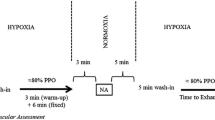
The experimental session was conducted as follows: (1) participants were seated for 15 min to rest and allow for instrumentation; (2) standardised warm-up (i.e. 5 min of continuous cycling at 50% of power associated with V̇O2max, immediately followed by 2 min at 100% of power associated with maximal oxygen uptake [V̇O2max; 357 ± 46 W] and, after 2 min of rest, 15 s of cycling at 30% of the anaerobic power reserve [618 ± 123 W] with a pedalling frequency of 110 rpm) in normoxia; (3) climatic chamber entrance and 2 min seated rest on the cycle ergometer (wash-in period) before the start of exercise; (4) an exhaustive intermittent cycling exercise (EICE 1) conducted either near sea level (SL; simulated altitude/ 0 m/ FiO2 ~ 0.21), at moderate (MH; ~ 2200 m/ ~ 0.16) or severe (SH; ~ 4200 m/ ~ 0.12) hypoxia in random order (see below); (5) 30 min of passive rest (normoxia) including neuromuscular function assessment (post-EICE 1) initiated 7 min after completion of EICE 1; (5) completion of an identical exercise test (EICE 2), always in normoxia; (6) neuromuscular function assessment (post-EICE 2) initiated 7 min after completion of EICE 2.
Baseline testing session
Participants were accustomed to all neuromuscular testing procedures during the first visit. Optimal levels of stimulation intensities to the motor cortex and femoral nerve were determined (see below) and kept constant during the subsequent experimental sessions. Baseline neuromuscular function assessment was performed after ~ 15 min of rest, and this served as the pre-test (Baseline) comparison for all conditions. This was done to ensure all participants were performing the first exhaustive intermittent cycling exercise (EICE 1) without the need for completing the extensive neuromuscular testing battery prior to cycling. Thereafter, participants performed an incremental cycling test on an electromagnetically braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands), while breathing room air to determine maximal aerobic power output (last completed stage in full) and maximal oxygen uptake (V̇O2max). Workload increased at a ramped rate of 25 W.min−1 until exhaustion, as indicated by volitional cessation of exercise, or failure to maintain a pedal cadence of 70 rpm despite strong verbal encouragement. After 20 min of passive rest, participants performed three single 10-s cycling sprints (peak power output = 1202 ± 262 W), with 2 min of rest between efforts. Anaerobic power reserve was calculated as the difference between peak power output (i.e. highest peak power output of the three single 10-s sprints) and maximal aerobic power output (Buchheit and Laursen 2013a).
Exhaustive intermittent cycling exercise
The exercise protocol for EICE 1 and EICE 2 consisted of performing intermittent cycling at supramaximal intensity until exhaustion; 15 s at 30% of the anaerobic power reserve (618 ± 123 W) with a pedalling frequency of 110 rpm (visual and verbal feedback, achieved after ~ 3–4 s) (Abbiss et al. 2009), interspersed with 45 s of passive rest (Fig. 1). EICE 1 and EICE 2 were separated by a 30-min recovery period, to allow significant perceptual recovery from EICE 1, but only a partial recovery of neuromuscular function, both likely to influence subsequent exercise performance (Minett and Duffield 2014). Exercise was terminated when pedal cadence dropped below 70 rpm for > 5 s. Unfinished cycling efforts were excluded from analysis.
Protocol overview. Participants performed an exhaustive intermittent cycling protocol EICE 1 (15 s of cycling at 30% of anaerobic power reserve, interspersed with 45 s of passive recovery) in either normoxia (SL; FiO2 ~ 0.21), moderate (MH; FiO2 ~ 0.16) or severe hypoxia (SH; FiO2 ~ 0.12). This was followed, after 30 min of passive recovery, by an identical exercise bout in normoxia (EICE 2). Neuromuscular function of the knee extensor was assessed 7 min after EICE 1 and 2
Responses to exercise
Heart rate (HR), monitored via a wireless monitoring system (Polar Electro Oy, Kempele, Finland), pulse oxygen saturation (SpO2), estimated non-invasively via pulse oximetry with a finger probe (Palmsat 2500, NONIN Medical Inc., Plymouth, MI, USA) and rating of perceived exertion (RPE), obtained using the 6–20 Borg scale, were recorded 10 s following each exercise bout. As the total exercise duration differed between participants and conditions, exercise data were time-normalised on a scale using 20% intervals over the individual total exercise duration (1–20, 21–40, 41–60, 61–80 and 81–100% of the time to exhaustion).
Prefrontal cortex and muscle oxygenation responses
Uninterrupted measurements of cerebral and muscle tissue oxygenation trends were obtained via NIRS (Oxymon MkIII, Artinis, The Netherlands). One NIRS emitter–detector pair was placed over the left prefrontal lobe, between Fp1 and F3 (international EEG 10–20 system). A second emitter–detector pair was placed on the distal part of the right vastus lateralis (VL), approximately 15 cm above the proximal border of the patella. Spacing between optodes were fixed at 45 mm using a black, plastic spacer held in place via double-sided tape. A modified form of the Beer–Lambert law was used to determine the tissue saturation index (TSI; oxyhaemoglobin/[oxyhaemoglobin + deoxyhaemoglobin] × 100). For each individual, NIRS signals were time-normalised on a scale using 20% intervals over the total exercise duration. TSI values at the beginning of exercise and near task failure (i.e. 1–20% and 81–100% of the time to exhaustion, respectively) for EICE 1 and EICE 2 are reported. Differential path length factors were fixed at 5.93 for cerebral and 3.83 for muscle tissues. NIRS data were acquired at 10 Hz and down sampled to 1 Hz for analysis.
Neuromuscular function
Neuromuscular test battery
Isometric knee extensor torque of the right leg was measured during voluntary and evoked contractions on an isokinetic dynamometer (Biodex; Isokinetic Dynamometer, Shirley, NY). Participants were seated with their hip joint angles set at 90° (0° is full extension) and their chest and working leg tightly fixed against the chair. The axis of the dynamometer was aligned with the knee flexion–extension axis, and the lever arm was attached to the shank around the ankle with a strap. Participant position information was recorded and replicated for all subsequent tests.
Neuromuscular assessment included six sets (recovery = 1 min) of three brief contractions (~ 5 s; MVC, 50% MVC and 75% MVC; recovery = 6 s) of the knee extensors (Girard et al. 2013). The intensities for the submaximal contractions were calculated from the preceding MVC, and the feedback of the target torque was provided via a computer monitor. During contractions, transcranial magnetic stimulations (TMS) or peripheral motor nerve (PMN) stimulations were alternatively delivered ~ 1.5 s after the plateau (3 sets each for TMS and PMN). Additionally, a potentiated twitch was evoked 5 s after each MVC with PMN.
Motor nerve stimulation
Single supramaximal electrical stimuli (max voltage 400 V, rectangular pulse of 200 ms) were delivered to the right femoral nerve using a high-voltage, constant-current, stimulator (Digitimer DS7AH, Welwyn Garden City, Hertfordshire, UK). The cathode ball electrode was manually pressed into the femoral triangle (3–5 cm below the inguinal ligament) by the experimenter and the anode (5 × 9 cm) was positioned in the gluteal fold opposite the cathode. The intensity of stimulation was determined during the familiarisation session by delivering single stimuli with increments of 10 mA until plateaus occurred in twitch amplitude and M-wave. Supramaximal stimulation was ensured by increasing the final intensity by 50% (mean current: 116 ± 54 mA; range: 40–220 mA).
Transcranial magnetic stimulation
A magnetic stimulator (Magstim 200, The Magstim Company, Dyfed, UK) was used to stimulate the motor cortex. A single TMS pulse (1-ms duration) was delivered via a concave double-cone coil (13 cm diameter) maintained manually over the vertex of the scalp. The coil was adjusted to activate the left motor cortex (contralateral to the right leg) until the largest motor evoked potential in the VL during 50% MVC contractions were observed with a stimulation intensity of 60% of the maximal stimulator power output (Girard et al. 2013). Motor threshold occurred at 41 ± 10% of maximum stimulator output. During each experimental session, TMS was delivered at 140% of the motor threshold (61 ± 10% of maximum stimulator output; range: 49–77%).
Analysis of neuromuscular parameters
Voluntary torque was recorded during 1 s of plateau. Peripheral motor nerve voluntary activation (VAPMN) was assessed using the twitch interpolation method and quantified using the equation: VAPMN (%) = (1 – [superimposed twitch/potentiated twitch]) × 100. Voluntary activation using transcranial magnetic stimulation (VATMS) was assessed by measuring the force responses to motor cortex stimulations during submaximal and maximal contractions (Goodall et al. 2009). VATMS (%) was quantified using the equation: (1 – [superimposed twitch/estimated resting twitch]) × 100. Muscle contractility was assessed via the electrically evoked resting twitch as peak twitch amplitude (Qtw-pot; highest value of twitch tension produced). The resting twitch evoked by TMS was estimated. Briefly, a linear regression analysis was performed between the amplitude of the superimposed twitch evoked by TMS against voluntary torque recorded during 50%, 75% and 100% of MVC (Todd et al. 2003). The estimated resting twitch evoked by TMS was determined as the y-intercept from the linear regression analysis (Todd et al. 2003).
Statistical analysis
Data are expressed as means ± SD. Two-way repeated-measures analysis of variance (ANOVA) was used to compare (1) differences in the number of cycling efforts completed (time: EICE 1, EICE 2; condition: SL, MH and SH); (2) differences in cerebral and muscle oxygenation (time: beginning of exercise–EICE 1, task failure–EICE 1, beginning of exercise–EICE 2, and task failure–EICE 2; condition: SL, MH and SH); and (3) differences in exercise responses (i.e. HR, RPE and SpO2) for both EICE 1 and EICE 2 (time: 1–20, 21–40, 41–60, 61–80 and 81–100%; condition: SL, MH and SH). To assess differences in neuromuscular variables, a two-way repeated-measures analysis of covariance (ANCOVA) was used (time: post-EICE 1, post-EICE 2; condition: SL, MH and SH), with the baseline neuromuscular values included as the covariate. To assess the effect of hypoxia severity on neuromuscular variables post-EICE 1, differences between conditions (SL, MH and SH) at the post-EICE 1 time point were explored within the two-way ANCOVA model, and the mean difference from baseline presented with 95% confidence intervals (95% CI; [lower, upper]). Mauchly’s test of sphericity was used to assess for assumptions of variance within each model, and the Greenhouse–Geisser correction was used to adjust the degree of freedom if an assumption was violated. Post hoc comparisons with Bonferroni-adjusted p values were performed if a significant interaction or main effect was observed. Partial eta-squared (η2) was calculated as measures of effect size (presented in parentheses in figures). Effect size values of 0.01, 0.06 and > 0.14 were considered as small, medium and large, respectively (Cohen 2013). All statistical calculations were performed using SPSS statistical software V.24.0 (IBM Corp., Armonk, NY, USA). The significance level was set at p ≤ 0.05.
Results
Exercise capacity
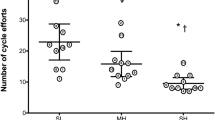
There were fewer cycling efforts completed during EICE 1 in MH (22 ± 13; p = 0.02) and SH (13 ± 6; p = 0.003), compared with SL (39 ± 30) (Fig. 2). However, the total number of efforts completed in EICE 2 did not differ between conditions (SL: 16 ± 9, MH: 20 ± 14, SH: 24 ± 17; p ≥ 0.09). The total number of efforts (i.e. sum of EICE 1 and EICE 2) was significantly lower in SH than SL (36 ± 21 vs. 55 ± 36; p = 0.02), whereas it was not different in MH (42 ± 27; p ≥ 0.08) compared to other conditions.
Number of efforts during exhaustive intermittent cycling exercise (EICE) during the initial (EICE1 at sea level [SL], moderate [MH] or severe hypoxia [SH]) and the subsequent exercise bout (EICE 2 always at SL). Data are mean ± SD for 15 participants. C, T, and C × T, respectively, refer to ANOVA main effects of condition, time, and interaction between these two factors with p value and partial eta-squared presented in the brackets. * Significantly different from SL (p < 0.05). γ significantly different from MH (p < 0.05) for EICE 1. ‡Significantly different from EICE 1 performance in SL (p < 0.05). # Significantly different from EICE 1 performance in SH (p < 0.05)
Neuromuscular responses
There was a significant interaction effect in VAPMN (p = 0.005; Fig. 3 C), with an increased VAPMN post-EICE 2 in the SH condition (1.5 ± 2.3%; p = 0.003) compared with post-EICE 1, but not in the SL ( − 0.4 ± 3.7%; p = 0.38) or MH (1.3 ± 4.2%; p = 0.19) conditions. There were no significant differences in maximal torque, Qtw-pot or VATMS between post-EICE 1 and post-EICE 2 (all p > 0.08; Fig. 3 A, B and D).
Quadriceps potentiated twitch torque (Qtw-pot; A), voluntary torque (B), peripheral motor nerve (VAPMN; C) and transcranial magnetic stimulation (VATMS; D) at baseline, after the first (post-EICE 1 at sea level [SL], moderate [MH] or severe hypoxia [SH]) and the second (post-EICE 2 [always at SL]) exhaustive intermittent cycling exercise. Data are mean ± SD for 15 participants. Neuromuscular function tests were performed 7 min after EICE 1 and EICE 2, always in normoxia near SL. C, T, and C × T, respectively refer to ANOVA main effects of condition, time, and interaction between these two factors with p value and partial eta-squared presented in the brackets. # Significantly different from post-EICE 1 in SH (p < 0.05)
There were no significant differences in any neuromuscular factors between conditions at the post-EICE 1 time point (all p ≥ 0.26). Relative to baseline, there were consistent reductions in maximal torque (mean difference [95% CI]; 27.3 [7.3, 47.3] Nm), Qtw-pot (21.5 [17.7, 25.4] Nm), VAPMN (1.9 [0.3, 3.6] %) and VATMS (3.7 [2.1, 5.4] %) within each condition.
Cerebral and muscle oxygenation
At the beginning of EICE 1, cerebral TSI was significantly lower with increasing hypoxia severity (67.9 ± 9.2%, 61.9 ± 6.7% and 52.9 ± 12.3% in SL, MH and SH, respectively; p ≤ 0.04) (Fig. 4 A). Cerebral TSI was significantly lower in MH and SH compared with SL at near task failure in EICE 1 (45.0 ± 10.0% and 36.7 ± 13.7% vs. 54.4 ± 12.5%; p ≤ 0.04), with no difference between MH and SH (p = 0.20). Cerebral TSI declined from the beginning of EICE 2 to near task failure (62.3 ± 11.1% vs. 49.5 ± 15.3%; p ≤ 0.01), with no differences between conditions (p > 0.05).
Cerebral (A) and muscle (B) tissue saturation index (TSI) during EICE 1 (at sea level [SL], moderate [MH] and severe hypoxia [SH]) and EICE 2 (always at SL). Data are mean ± SD for 12 participants. Data were obtained at the beginning of exercise and near task failure (1–20% and 81–100% of time to exhaustion, respectively). C, T, and C × T, respectively, refer to ANOVA main effects of condition, time, and interaction between these two factors with p value and partial eta-squared presented in the brackets. * Significantly different from sea level (p < 0.05). γ Significantly different from MH (p < 0.05). + and α Significantly different from the beginning of exercise (EICE 1) in SL and SH, respectively.
Muscle TSI at the beginning of EICE 1 did not differ between conditions (pooled values: 79.7 ± 4.9%, p > 0.05) (Fig. 4 B). However, muscle TSI near task failure became significantly lower in SH compared with SL (54.6 ± 14.8% vs. 62.0 ± 11.6%; p = 0.02). During EICE 2, muscle TSI decreased from the beginning of EICE 2 to near task failure (pooled values: 80.6 ± 5.6% vs. 64.3 ± 10.5%, p < 0.001), with no differences between conditions (p > 0.05).
Physiological and perceptual responses
SpO2 decreased with increasing hypoxia severity (SL: 96.1 ± 2.0%, MH: 90.7 ± 1.2%, SH: 82.5 ± 3.3%; p < 0.001) at the beginning of EICE 1 (Fig. 5 A). During EICE 2, SpO2 remained unchanged across all time points in all conditions (p > 0.05). During EICE 1, HR increased similarly at each time point, except for the 21–40% interval with significantly lower values in SH (121 ± 15 bpm) than SL (138 ± 18 bpm; p = 0.003) and MH (136 ± 17 bpm; p = 0.03) (Fig. 5 B). During EICE 2, HR significantly increased similarly at each time point from the beginning to near task failure (pooled values: 113 ± 20 vs. 152 ± 15 bpm). RPE increased at each time point from the beginning to near task failure during both EICE 1 (pooled values: 13.0 ± 1.9 vs. 19.5 ± 0.5) and EICE 2 (14.1 ± 2.2 vs. 19.6 ± 0.4), with no significant differences between conditions (p = 0.88) (Fig. 5 C).
Arterial oxygen saturation (SpO2; A), heart rate (HR; B) and ratings of perceived exertion (RPE; C) during EICE 1 (at sea level [SL], moderate [MH] and severe hypoxia [SH]) and EICE 2 (always at SL). Data are mean ± SD for 11 participants. Data were time normalised on a scale 0–100% using 20% intervals (1–20, 21–40, 41–60, 61–80 and 81–100% of total exercise duration). C, T, and C × T, respectively, refer to ANOVA main effects of condition, time, and interaction between these two factors with p value and partial eta-squared presented in the brackets. * Significantly different from sea level (p < 0.05). γ Significantly different from MH (p < 0.05)
Discussion
This study examined the effects of graded hypoxia during exhaustive intermittent cycling on subsequent performance and associated neuromuscular consequences during an identical normoxic exercise. Our main findings were: (1) despite shorter exercise duration with graded hypoxia during EICE 1, the magnitude of central and peripheral adjustments post-EICE 1 did not differ across conditions; (2) the number of efforts completed during EICE 2 did not differ between conditions; (3) muscle fatigue characteristics were largely similar between EICE 1 and EICE 2. Despite differences in the total number of cycling efforts completed across conditions, the associated neuromuscular fatigue characteristics between conditions at post-EICE 1 and post-EICE 2 were similar.
Graded hypoxia limits exercise performance during exhaustive intermittent cycling exercise
As expected, increasing hypoxic severity limited exercise capacity during EICE 1. Interestingly, the reduction in Qtw-pot ( − 53 ± 10%) post-EICE 1 was similar between conditions, which seemed to align with the concept of an individual critical threshold of peripheral fatigue (Amann 2011). That said, it should also be noted that the challenge to homeostasis (decrease in SpO2 to ~ 80%) during exhaustive intermittent exercise in O2-deprived conditions likely exert disruptions to other physiological regulatory systems. Accordingly, the increased physiological solicitation to restore homeostasis during exercise when oxygen availability is challenged would likely combine to increase the perception of effort (Thomas et al. 2018). This is evidenced in our study by near maximal RPE values (i.e. ~ 19) reached near task failure in all conditions. Since hypoxia exerts an additional systemic stress during exhaustive intermittent cycling, it is likely that both physiological (e.g. HR) and perceptual factors (higher-than-normal exercise sensations), rather than peripheral fatigue per se, influenced an individual’s tolerance limit and exercise capacity during EICE 1 (Thomas et al. 2018). In our study, exercise was performed at the same absolute intensity (or sustained mechanical output) across conditions. Therefore, it cannot be excluded that decreased performance in hypoxia during EICE 1 may have also been due to a higher relative intensity, independent from the effects of acute hypoxia. In this instance, exhaustive cycling bouts in hypoxia performed at the same relative intensity as in normoxia may provide further clarity on the effects of hypoxia on performance (Mira et al. 2020).
Despite a significant decline in SpO2 and cerebral TSI during EICE 1 in SH, the decrease in VAPMN and VATMS during MVCs from baseline to post-EICE 1 were not different between conditions. It was previously reported that decreased cerebral oxygenation, during constant load cycling (at ~ 80% of maximal work rate) at comparable hypoxia severity (FiO2 0.13) is associated with an increased component of supraspinal fatigue (Goodall et al. 2012). Contrastingly, no differences in muscle activation capacity (i.e. VAPMN and VATMS) was observed during an exhaustive continuous cycling task performed at different hypoxic severity (SpO2 clamped at ~ 98%, ~ 85% and ~ 70%) (Mira et al. 2020). This uncertainty regarding the influence of hypoxia on central fatigue may be due to the severity of hypoxemia (Amann et al. 2007), the type and/or intensity of exercise. Accordingly, while the decreases in VATMS and VAPMN during MVCs suggest that exhaustive intermittent cycling exercise (~ 3 min of exercise, excluding passive rest) induced central fatigue with a supraspinal component that was independent of hypoxia severity, it should be noted that these decrements were generally modest.
Exhaustive intermittent cycling at graded hypoxia does not influence exercise performance in normoxia
We observed that the number of efforts completed during EICE 2 in SH was not different between conditions. This is in contrast to our hypothesis that, compared to either SL or MH, earlier exercise termination (less work being completed) in SH due to exacerbated central fatigue during EICE 1 may in turn increase the number of cycling efforts completed during EICE 2. Our initial assumption was based on previous works showing that increasing hypoxic severity significantly elevates cerebral deoxygenation during exercise (Goodall et al. 2010), causing a shift of the primary locus of neuromuscular fatigue from the muscle to the central nervous system (Goodall et al. 2010; Amann et al. 2007). Consequently, the exacerbated central fatigue (and substantially less peripheral fatigue) induces earlier task failure, whilst hyperoxia (FiO2 1.0) at task failure prolongs exercise performance (Goodall et al. 2010; Amann et al. 2007). For instance, Amann et al. (2007) demonstrated that constant load cycling to exhaustion was prematurely terminated (due to lower SpO2 values, likely < 70–75%, on central motor output) in severe hypoxia (FiO2 0.10) compared with normoxia. As described above, the decreased SpO2 (~ 80% throughout exercise in SH) and cerebral TSI (proxy for cerebral hypoxia) was not accompanied by additional central and/or supraspinal fatigue development such that participants terminated EICE 1 at or close to their limit of tolerance. Our data tend to indicate that decreased work done during EICE 1 (due to increasing hypoxic severity) resulted in improved exercise performance during EICE 2. However, this finding did not reach significance, which may be due to a large variability in performance during exercise in hypoxia. That said, unlike the aforementioned study (Amann et al. 2007), our exercise protocol was intermittent in nature. Exercise interspersed with rest periods tends to be performed for longer duration compared with continuous exercise of the same intensity (Grossl et al. 2012). Thus, compared with continuous exercise, the rest intervals between efforts might have allowed participants to persist longer, narrowing differences in exercise performance between conditions during EICE 2, and resulting in larger than anticipated physiological strain and neuromuscular fatigue.
In the SL condition, the number of efforts completed during EICE 2 (always in normoxia) was lower than during EICE 1 suggesting that the 30-min recovery period was insufficient to fully restore exercise capacity. Recently, we examined the effect of ten, 4-s repeated-sprint cycling at graded hypoxia (FiO2 of 0.21, 0.16 and 0.13) on a subsequent set of five sprints (Soo et al. 2020a) and showed that maximal power output was restored during the first sprint of the second set, independent of hypoxic severity. However, repeated-sprint ability was impaired compared with sea level and moderate hypoxia following exercise in severe hypoxia. In this instance, the recovery duration would likely influence exercise performance during EICE 2. Additionally, the present findings also suggest that the relative effect of residual fatigue may only become more prominent when the subsequent exercise task is performed to the limit of exhaustion.
Neuromuscular fatigue patterns following EICE 2
An unexpected finding from our study was a small increase in VAPMN following EICE 2 in SH when compared with EICE 1. The increase in VAPMN, however, was of small magnitude (+ 1.5%) and probably not of physiological significance, and could have been influenced by experimental conditions (e.g. time delay in the evaluation of neuromuscular function). In this instance, alterations in voluntary activation following high intensity, short duration exercise are generally modest (Brownstein et al. 2020). Comparatively, significant reduction in Qtw-pot (~ 54% lower, relative to baseline) was evidenced post-EICE 2 in all conditions. This was despite of lower total mechanical work (i.e. sum of cycling efforts completed during EICE 1 and 2) performed in MH and SH compared to SL. While this finding supports previous studies (Amann et al. 2007, 2013) suggesting that peripheral fatigue is regulated to a task specific individual critical threshold, the influence of peripheral fatigue on exercise performance remains contentious (Thomas et al. 2018). Nonetheless, the substantial decrease in Qtw-pot at post-EICE 2 do suggest that neuromuscular alterations were largely of peripheral origins.
Additional considerations and limitations
A limitation of conducting muscle assessments during whole-body exercise is the difficulty in measuring neuromuscular function integrity during and/or immediately (within seconds) after exercise. As the intermittent cycling test was performed to the limit of exhaustion, additional time was required to allow participants to move from the cycle ergometer to the neuromuscular test ergometer located outside the climatic chamber in normoxia. While measurements of neuromuscular function conducted immediately after exercise would more accurately reflect the true nature of alterations within the central nervous system, a 7 min recovery period post-exercise was implemented before neuromuscular testing to minimise the risk of vagal syncope. As such, given that corticospinal excitability may recover substantially after less than 1 min of rest (Goodall et al. 2012), the magnitude of central fatigue observed here was likely underestimated. Nonetheless, we ensured that the time taken (exactly 7 min after task failure) to assess neuromuscular fatigue was consistent throughout all trials/conditions.
There is growing interest in the use of high intensity intermittent training in combination with hypoxia to maximise physical performance and/or health benefits (Li et al. 2020). Consequently, better understanding of the neuromuscular consequences associated with different exercise designs (e.g. exercise-to-rest ratio) and methods of administering the hypoxic stimulus (i.e. SpO2 vs. FiO2) will be useful to optimise training periodisation (Buchheit and Laursen 2013b). In particular, large interindividual variability in SpO2 to a given FiO2 (Hamlin et al. 2010) may have influenced exercise performance (Chapman et al. 2011) and possibly neuromuscular responses. Accordingly, an index (e.g. SpO2 to FiO2 ratio) that considers both the internal and external stimuli could be used to prescribe hypoxia based on an individualised approach (Soo et al. 2020b).
Conclusion
This study examined the effect of graded hypoxia during exhaustive intermittent cycling on subsequent performance and associated neuromuscular responses during exercise in normoxia. Neuromuscular fatigue pattern (large peripheral fatigue development) was not different across conditions at post-EICE 1, despite performance being hypoxia severity-dependent during EICE 1. Exercise performance during EICE 2 was not statistically different between conditions. Additionally, neuromuscular fatigue characteristics following EICE 2 were largely similar between conditions. We conclude that exhaustive intermittent cycling performed at increasing hypoxia severity did not influence performance and associated neuromuscular responses, during completion of a subsequent exercise of similar nature in normoxia.
Abbreviations
- ANCOVA:
-
Analysis of covariance
- ANOVA:
-
Analysis of variance
- EICE:
-
Exhaustive intermittent cycling exercise
- FiO2 :
-
Fraction of inspired oxygen
- HR:
-
Heart rate
- MH:
-
Moderate hypoxia
- MVC:
-
Maximal voluntary contraction
- NIRS:
-
Near-infrared spectroscopy
- PMN:
-
Peripheral motor nerve
- Qtw-pot:
-
Peak twitch amplitude
- SH:
-
Severe hypoxia
- SL:
-
Sea level
- SpO2 :
-
Arterial oxygen saturation
- TMS:
-
Transcranial magnetic stimulation
- TSI:
-
Tissue saturation index
- VA:
-
Voluntary activation
- VL:
-
Vastus lateralis
References
Abbiss CR, Peiffer JJ, Laursen PB (2009) Optimal cadence selection during cycling. Int SportMed J 10(1):1–15
Amann M (2011) Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc 43(11):2039–2045. https://doi.org/10.1249/MSS.0b013e31821f59ab
Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA (2007) Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581(1):389–403. https://doi.org/10.1113/jphysiol.2007.129700
Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, Richardson RS (2013) Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol 115(3):355–364. https://doi.org/10.1152/japplphysiol.00049.2013
Billaut F, Bishop DJ, Schaerz S, Noakes TD (2011) Influence of knowledge of sprint number on pacing during repeated-sprint exercise. Med Sci Sports Exerc 43(4):665–672. https://doi.org/10.1249/MSS.0b013e3181f6ee3b
Brownstein CG, Millet GY, Thomas K (2020) Neuromuscular responses to fatiguing locomotor exercise. Acta Physiologica. e13533. https://doi.org/10.1111/apha.13533
Buchheit M, Laursen PB (2013a) High-intensity interval training, solutions to the programming puzzle. Sports Med 43(5):313–338. https://doi.org/10.1007/s40279-013-0029-x
Buchheit M, Laursen PB (2013b) High-intensity interval training, solutions to the programming puzzle: part II: anaerobic energy, neuromuscular load and practical applications. Sports Med 43(10):927. https://doi.org/10.1007/s40279-013-0066-5
Chapman RF, Stager JM, Tanner DA, Stray-Gundersen J, Levine BD (2011) Impairment of 3000-m run time at altitude is influenced by arterial oxyhemoglobin saturation. Med Sci Sports Exerc 43(9):1649–1656. https://doi.org/10.1249/MSS.0b013e318211bf45
Cohen J (2013) Statistical power analysis for the behavioral sciences. Academic press, Cambridge
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789. https://doi.org/10.1152/physrev.2001.81.4.1725
Girard O, Bishop DJ, Racinais S (2013) Neuromuscular adjustments of the quadriceps muscle after repeated cycling sprints. PLoS ONE 8(5):e61793. https://doi.org/10.1371/journal.pone.0061793
Girard O, Bula S, Faiss R, Brocherie F, Millet GY, Millet GP (2016) Does altitude level of a prior time-trial modify subsequent exercise performance in hypoxia and associated neuromuscular responses? Physiol Rep 4(14):e12804
Goodall S, Romer L, Ross E (2009) Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp Physiol 94(9):995–1004. https://doi.org/10.1113/expphysiol.2009.047902
Goodall S, Ross EZ, Romer LM (2010) Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee-extensor contractions. Am J Physiol-Heart Circ Physiol 109(6):1842–1851
Goodall S, González-Alonso J, Ali L, Ross EZ, Romer LM (2012) Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590(11):2767–2782. https://doi.org/10.1113/jphysiol.2012.228890
Grossl T, de Lucas RD, de Souza KM, Guglielmo LGA (2012) Time to exhaustion at intermittent maximal lactate steady state is longer than continuous cycling exercise. Appl Physiol Nutr Metab 37(6):1047–1053. https://doi.org/10.1139/H2012-088
Hamlin MJ, Marshall HC, Hellemans J, Ainslie PN (2010) Effect of intermittent hypoxia on muscle and cerebral oxygenation during a 20-km time trial in elite athletes: a preliminary report. Appl Physiol Nutr Metab 35(4):548–559. https://doi.org/10.1139/H10-044
Li SN, Hobbins L, Morin J-B, Ryu JH, Gaoua N, Hunter S, Girard O (2020) Running mechanics adjustments to perceptually-regulated interval runs in hypoxia and normoxia. J Sci Med Sport 23(11):1111–1116. https://doi.org/10.1016/j.jsams.2020.04.001
Millet GY, Muthalib M, Jubeau M, Laursen PB, Nosaka K (2012) Severe hypoxia affects exercise performance independently of afferent feedback and peripheral fatigue. J Appl Physiol 112(8):1335–1344. https://doi.org/10.1152/japplphysiol.00804.2011
Minett GM, Duffield R (2014) Is recovery driven by central or peripheral factors? A role for the brain in recovery following intermittent-sprint exercise Front Physiol 5 24 ARTN2410.3389/fphys.2014.00024
Mira J, Floreani M, Savoldelli A, Amery K, Koral J, Oranchuk DJ, Messonnier LA, Rupp T, Millet GY (2020) Neuromuscular fatigue of cycling exercise in hypoxia. Med Sci Sports Exerc 52(9):1888–1899. https://doi.org/10.1249/mss.0000000000002331
Soo J, Billaut F, Bishop DJ, Christian RJ, Girard O (2020a) Neuromuscular and perceptual responses during repeated cycling sprints—usefulness of a “hypoxic to normoxic” recovery approach. Eur J Appl Physiol 120(4):1–14. https://doi.org/10.1007/s00421-020-04327-3
Soo J, Girard O, Ihsan M, Fairchild T (2020b) The Use of the SpO2 to FiO2 Ratio to Individualize the Hypoxic Dose in Sport Science, Exercise, and Health Settings. Frontiers in Physiology. 11 (1415). https://doi.org/10.3389/fphys.2020.570472
Thomas K, Goodall S, Howatson G (2018) Performance fatigability is not regulated to a peripheral critical threshold. Exerc Sport Sci Rev 46(4):240–246
Todd G, Taylor JL, Gandevia S (2003) Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551(2):661–671
Townsend N, Brocherie F, Millet GP, Girard O (2020) Central and peripheral muscle fatigue following repeated-sprint running in moderate and severe hypoxia. Exp Physiol. https://doi.org/10.1113/EP088485
Author information
Authors and Affiliations
Contributions
This project was completed within the Athlete Health and Performance Research Center, Aspetar Orthopaedic and Sports Medicine Hospital, Doha, Qatar. OG, SR and MB: were involved in conception and design of the work as well as data collection. All authors were involved in data analysis and interpretation of results. JS, TF, MI and OG: drafted the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All person designated as authors qualify for authorship, and all those who qualify are listed.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest, source of funding, or financial ties to disclose and no current or past relationship with companies or manufacturers who could benefit from the results of the present study. The authors report no conflict of interest. At the time of the experiment, Olivier Girard was employed by Aspetar Hospital (Qatar), where the experiment was performed.
Ethical approval
The experimental protocol was approved by the Shafallah Medical Genetics Center Ethics Committee, Doha, Qatar.
Consent to participate
All participants gave their informed, written consent prior to the commencement of the experiment.
Additional information
Communicated by Guido ferrati.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soo, J., Racinais, S., Fairchild, T.J. et al. Effects of graded hypoxia during exhaustive intermittent cycling on subsequent exercise performance and neuromuscular responses. Eur J Appl Physiol 121, 3539–3549 (2021). https://doi.org/10.1007/s00421-021-04809-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04809-y