Abstract
Purpose
The alpha method enables the dissociation of the passive (i.e., tendinous tissues) and active (i.e., fascicles) part of muscle–tendon stiffness. It is based on two main assumptions (i.e., a constant tendon stiffness and a muscle stiffness proportional to the torque produced), which have not been approved in vivo. The purpose of this study was to validate these two assumptions using ultrafast ultrasound, and to compare fascicle and tendon stiffness as determined by both methods.
Methods
Ten healthy males performed a fast-stretch experiment on the ankle plantar flexors. The mathematical model of the alpha method allowed to estimate the stiffness of muscle and tendinous tissues on the basis of the assumptions associated to the behaviors of muscle fascicles and tendinous tissues. Muscle and tendon stiffness of the gastrocnemius medialis were also calculated from ultrafast ultrasound measurements.
Results
Muscle stiffness measured by the ultrasound method increased from 217 ± 83 to 720 ± 265 N/mm with an increasing level of force (from 30 to 90 % MVC). Tendinous stiffness measured by the ultrasound method remained constant across the force level (P < 0.001). However, the stiffness values obtained with both methods were significantly different (P < 0.001).
Conclusions
In conclusion, these findings validate the two main assumptions underlying the alpha method using ultrasound. Differences in stiffness values obtained with alpha and ultrasound methods could be associated with physiological and geometrical differences between the whole plantar flexors muscles characterized by the alpha method and the gastrocnemius medialis assessed with the ultrasound method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanical properties of muscle–tendon unit highly influence storage–restitution of elastic energy and force transmission, and play an important functional role in several frameworks (Pearson and McMahon 2012; Wilson et al. 1991). Thus, several methods developed to characterize stiffness for isolated muscles were adapted in vivo. These include sinusoidal perturbation (Cornu et al. 1997), quick release (Cornu et al. 1998; Lambertz et al. 2001; Goubel and Pertuzon 1973) and short-range stiffness experiments (Cook and McDonagh 1996; Rack and Westbury 1973). Considering the latter method, muscle–tendon compliance (i.e., inverse of stiffness) is assumed to be the sum of active (i.e., contractile elements) and passive (i.e., tendon-aponeurosis structures) parts of the series elastic component (SEC) compliances (Ettema and Huijing 1994) associated mainly with the behaviors of the muscle cross-bridges and tendinous tissues, respectively (Fouré et al. 2010).
In the past 15 years, tendon stiffness (i.e., the main structure included in the passive part of the SEC) was assessed non-invasively from the force–elongation relationship determined with ultrasound measurements during an isometric contraction (Kubo et al. 1999; Maganaris and Paul 1999; Magnusson et al. 2001; 2008). In this way, the effects of training (Fouré et al. 2012a, b; Kubo et al. 2010; Lenskjold et al. 2013), aging (Carroll et al. 2008; Stenroth et al. 2012) or tendinopathy (Arya and Kulig 2010; Helland et al. 2013) on both Achilles and patellar tendon stiffness were characterized. In contrast, muscle stiffness remains more difficult to characterize non-invasively in vivo.
Currently, only one method, the “alpha method” (Morgan 1977) enables the simultaneous assessment of the specific behaviors of both the active and passive parts of the SEC based on short-range stiffness experiments. Measurements using the alpha method were shown to be reproducible in vivo (Fouré et al. 2010) and allowed for the study of the effects of training (Fouré et al. 2011, 2013), gender (Fouré et al. 2012a) and neuromuscular disorders (Svantesson et al. 2000). The alpha method is based on two main assumptions: (i) beyond 30 % of the maximal force tendon stiffness is constant (Proske and Morgan 1987) and (ii) muscle compliance is inversely proportional to the force produced. Although the experimental conditions for tendon stiffness measurements (i.e., incremental isometric contraction) were quite different compared to the short-range experiments (i.e., fast stretch during constant isometric contractions), the first hypothesis has been discussed previously based on ultrasound measurements (Fouré et al. 2010, 2013). In order to experimentally check the second assumption, a more direct validation based on muscle and tendon behaviors could be very useful in assessing the validity of the alpha method applied in vivo.
Recently, ultrafast ultrasound (Deffieux et al. 2006, 2008; Tanter and Fink 2014) has been used to analyze the behavior of muscle fascicles (Nordez et al. 2009; Hauraix et al. 2013; Farcy et al. 2014) during different tasks and modalities of contractions. Thus, the contributions of fascicles and tendinous tissues to the global compliance of the muscle–tendon unit could be assessed using ultrafast ultrasound. Additionally, a direct validation of assumptions associated with the alpha method with direct measurements using ultrafast ultrasound could facilitate the assessment of specific muscle and tendon stiffness in clinical and athletic contexts with a commercial isokinetic device as a unique requirement.
Therefore, the aims of the current study were to: (i) check the two main assumptions associated with the alpha method (i.e., a constant tendon stiffness and a muscle stiffness proportional to the force produced) by measuring fascicle behavior with ultrafast ultrasound during fast-stretch experiments in vivo, and, (ii) compare muscle and tendon stiffness determined by the alpha method and the ultrafast ultrasound.
Materials and methods
Subjects
Ten healthy males (27 ± 4 years, 180 ± 6 cm, 74 ± 7 kg) volunteered to participate in the present study. Subjects were fully informed about the nature and aim of the study, before signing a written informed consent form to be included in the study. This study was in conformity with the Declaration of Helsinki and has been approved by the local ethics committee.
Mechanical and ultrafast ultrasound measurements
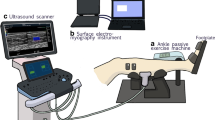
A Biodex System 3 research dynamometer (Biodex Medical, Shirley, New York, USA) and research toolkit were used to measure ankle angle, angular velocity and torque. Subjects lay in a prone position with legs fully extended. The thighs and hips were secured by adjustable lap belts, and held in position. The right ankle was securely strapped to a footplate connected to the dynamometer’s lever arm (with 90° corresponding to the foot being perpendicular to the leg and 0° to full plantar flexion). The input axis of the dynamometer was carefully adjusted to the ankle’s axis of rotation. Mechanical signals of torque, joint angle and angular velocity were sampled at a frequency of 1,000 Hz (Delsys Inc, Boston, MA, EU).
The probe (5–12 MHz; 55 mm) of an ultrafast ultrasound scanner (Aixplorer, Supersonic Imagine, Aix en Provence, France) was placed on the medial gastrocnemius at 30 % of the distance between the popliteal crease and the center of the lateral malleolus (Kawakami et al. 1998). The probe was aligned vertically to the midline of the muscle in the same plane of the muscle fascicles, to obtain the longest possible fascicles’ length and to minimize measurement error (Bénard et al. 2009). A trigger output signal was used to synchronize the mechanical data with the raw ultrasound signal, both sampled at 1,000 Hz.
Experimental protocol
The protocol started with a 3-min warm-up consisting of submaximal isometric plantar flexions. After 2 min of rest, two trials of maximal voluntary contraction (MVC) in plantar flexion were performed at 75° of ankle angle. Two trials at 30 and 60 % MVC were conducted to familiarize subjects with the fast-stretch experiment. Subjects had to maintain a submaximal isometric plantar flexion prior to a fast dorsiflexion (i.e., 250° s−1) applied by the dynamometer on a range of motion of 20° (i.e., from 75° to 95°). The fast-stretch experiments (14 trials) were performed at 7 levels of submaximal torque in random order (two trials for each torque level every 10 % MVC from 30 to 90 % MVC) with 2-min of rest between trials.
Data analysis
All data were processed with standardized Matlab Scripts (The Mathworks, Natick, MA). Mechanical data (i.e., torque, ankle angle and angular velocity) were filtered using a low-pass (98 Hz), zero-phase second-order Butterworth filter. The torque measured by the dynamometer was corrected for inertia and the weight of the dynamometer attachment to obtain the external torque. The moment arm and length of the muscle–tendon unit were estimated from the ankle and knee joint angles using the anthropometric model proposed by Grieve et al. (1978). The force was calculated from the external torque divided by the moment arm. As described previously in a fast-stretch experiment (Fouré et al. 2010), the first 60 ms of stretch was considered for analysis of mechanical and ultrasound data.
B-mode images were created from ultrasonic raw data by a conventional beam formation, i.e., using a time-delay operation to compensate for the travel time differences. The changes in the length of the muscle fascicles were obtained via an automatic fascicle tracking method (Cronin et al. 2011; Gillett et al. 2013). When the muscle fascicles length was not fully visible, an extrapolation was performed based on the interception with superficial and deep aponeurosis. The pennation angle corresponded to the angle between fascicle length and deep aponeurosis. The horizontal fascicle length (L fh) was calculated as the fascicle length multiplied by the cosinus of the pennation angle. The change in length of tendinous tissues (ΔL tt) was calculated from the difference between the change in length of muscle–tendon unit (ΔL) and L fh.
Muscle and tendon stiffness was calculated using both the alpha method and ultrasound measurements, thereafter referred as to the “ultrasound method”.
Muscle and tendon stiffness estimated using the alpha method
The muscle–tendon stiffness (S) corresponded to the ratio between changes in force (ΔF) and ΔL (Eq. 1).
Then, the alpha method is a mathematical method using the fast-stretch experiment (Fouré et al. 2010; Morgan 1977). Muscle and tendon are assumed to be placed in series, and thus the muscle–tendon stiffness could be expressed as (Eq. 2):
Assuming that tendon and muscle stiffness (S tendon and S muscle, respectively) are constant and proportional to the force, respectively, it could be written as (Eq. 3):
where α 0 represents a muscle stiffness index (Eq. 4):
In addition, α was defined as (Eq. 5):
A linear regression was performed on the α-F relationship. Then, the tendon stiffness (S tendon) was determined as the inverse of the slope of the α-F relationship (Fig. 1). The α 0 parameter was obtained as the inverse of the y-intercept of the α-F relationship.
Muscle and tendon stiffness using the ultrasound method
The ultrasound method was based on data from the automatic fascicle tracking (Fig. 2). The muscle stiffness was calculated from the ratio between the global ΔF and the horizontal fascicles length change (ΔL fh). The tendinous tissues’ stiffness was calculated from the ratio between ΔF and the tendinous tissues’ length change (ΔL tt). Therefore, this calculation implicitly considers that the change in length of fascicles and tendinous tissues was representative of the whole plantar flexors.
Typical example of muscle horizontal fascicle length during fast-stretch experiment. The ultrafast ultrasound was used to measure the fascicle length (white dotted line) and the horizontal fascicle length (white arrows) during a maintained isometric contraction at 40 % of maximal torque level—i.e., prior to quick stretch (A) and 60 ms after its beginning (B)
Statistical analysis
After checking the normal distribution of data using a Shapiro–Wilk test, parametric statistical tests were performed using Statistica® software (Statsoft Inc., Tulsa, OK, USA). Two-way multivariate analysis of variance (ANOVA) (force level × method) was performed to assess the statistical changes in the muscle and tendon stiffness measured by both the alpha method and the ultrafast ultrasound method. When appropriate, a Tukey HSD post hoc analysis was performed. The level of significance was set at P < 0.05.
Results
Stiffness of tendinous tissues
The significant main effect for the “method” factor (P < 0.001) showed that the tendinous tissues stiffness measured by the ultrasound method was significantly lower than that calculated with the alpha method (258 ± 40 and 401 ± 97 N/mm, respectively). Although a main effect was determined for “force” factor (P = 0.026), post hoc analysis revealed no significant difference between force levels. Thus, the tendinous tissues stiffness was considered as constant, regardless the torque level (Fig. 3). No significant interaction between “force level” and “method” factors was found.
Stiffness of muscle fascicles
The “force” effect (P < 0.001) indicated that the stiffness of the muscle fascicles increased with force level (Fig. 4) (mean R 2: 0.82 ± 0.11 and 0.97 ± 0.02 for the ultrasound and alpha methods, respectively). The “method” effect (P < 0.001) indicated that fascicle stiffness measured by the ultrasound method was significantly higher than muscle stiffness determined by the alpha method. “Force” and “method” factors significantly interacted (P < 0.001) and showed that the increase in stiffness was more important for the ultrafast ultrasound method than that estimated by the alpha method (218 ± 83 to 721 ± 266 N/mm and 139 ± 34 to 337 ± 97 N/mm, respectively). Higher muscle fascicle stiffness was determined with the ultrasound method compared to muscle stiffness estimated by the alpha method for elevated force level (≥50 % MVC). In contrast, muscle fascicle stiffness was not significantly different between both methods (P < 0.05) for low force levels (i.e., 30 and 40 % MVC).
Discussion
The ultrasound method showed that tendon stiffness was not significantly different in the range of force from 30 to 90 % MVC, and that muscle fascicle stiffness increased linearly with the force level. Therefore, the main novelty of the paper was the in vivo validation of the assumptions associated to the alpha method during fast stretches by direct ultrasound measurement of fascicle behavior. Tendon stiffness measured by the ultrasound method was significantly lower than the stiffness obtained by the alpha method. Inversely, muscle fascicle stiffness determined by the ultrasound method was significantly higher than muscle stiffness obtained by the alpha method, and this latter difference increased with force level.
The sampling frequency of ultrafast ultrasound scanner used in the present study was 1,000 Hz, which is largely higher than conventional ultrasound scanner (i.e., 30–170 Hz) (Cronin and Lichtwark 2012). First, a low temporal resolution of 10 ms (i.e., 100 images per seconds) obtained with conventional ultrasound could induce variability in the fascicle length measurements 60 ms after the beginning of the stretch leading to biased stiffness assessment. Second, considering the high velocity reached during the stretches (about 250° s−1), it would not be possible to perform automatic tracking using conventional ultrasound. The manual processing is time consuming and requires to average some trials to obtain a sufficient reliability of fascicle length measurements (Cronin and Lichtwark 2012) while the automatic tracking is a “robust, repeatable and time-efficient method” to measure the fascicle behavior during contraction (Cronin et al. 2011). Therefore, ultrafast ultrasound measurements are useful to use automatic tracking to minimize the errors of measurement in fascicles length changes.
The experimental protocol used in the present study was similar to those described by Fouré et al. (2010), who demonstrated the reproducibility of stiffness measurements during fast-stretch experiments and using the alpha method. A global joint stiffness was measured during the fast stretch, and this stiffness was considered to represent the muscle–tendon stiffness of plantar flexors. It implicitly means that the role of non-musculotendinous structures (e.g., joint capsule, ligaments, skin) was neglected. While this assumption is quite classical for stiffness measurements performed in vivo during contractions (e.g., Blanpied and Smidt 1992; Zee and Voigt 2001), further studies could be required to better understand the contributions of the latter structures to the joint stiffness.
Considering the stretch applied, the velocity used in the present study was higher than in previous in vivo studies (Blanpied and Smidt 1992; Blanpied and Smidt 1993), and similar to the velocity applied in ex vivo studies (Morgan 1977; Morgan et al. 1978). In addition, the relative range of the stretch was also similar to the range used in the study of Morgan (1977) (about 1.3 % of the total muscle–tendon length vs. 1.8 % in the present study). Finally, it was previously reported that stiffness assessment was not influenced by changes in muscle activation considering that reflex activity starts at least 45 ms or more after the beginning of the stretch (Fouré et al. 2013) and electromechanical delay is higher than 15 ms in experimental conditions used in the present study (Muraoka et al. 2004; Wu et al. 2009). Therefore, the influence of reflex activity on external torque and stiffness assessed in the present study is likely to be minimal.
Values of stiffness determined in the present study using the alpha method were in the same range as those obtained by Fouré et al. (2010) (ie., 0.13 ± 0.03°−1 vs. 0.17 ± 0.04°−1, for the active part of the SEC and 14.3 ± 3.2 N/m vs. 12.2 ± 3.1 N/m for the passive part of the SEC). Values of tendinous tissue stiffness found in the present study using the ultrasound method (ranged from 226 to 300 N/mm) were in accordance with studies that assessed Achilles tendon stiffness using ultrasonography (ranged from 140 to 375 N/mm) (Arya and Kulig 2010; Fletcher et al. 2013; Fouré et al. 2013; Kubo et al. 2007; Peltonen et al. 2013; Rosager et al. 2002; Zhao et al. 2009).
The alpha model is based on two main assumptions from ex vivo experiments (Morgan 1977): (i) tendon stiffness remains constant beyond 30 % of the torque level, and (ii) muscle stiffness is proportional to the torque level. These assumptions were established from studies that used isolated muscle, considering that “above 20–30 % of maximal isometric tension, tendon stiffness is more nearly constant than proportional to the tension” (Proske and Morgan 1987). Then, the alpha method has been adapted in vivo to dissociate muscle and tendon stiffness in humans (Fouré et al. 2010). So far, no study has directly checked these assumptions in vivo during fast-stretch experiments using ultrafast ultrasound. Our results obtained in vivo with the ultrasound method show that tendon stiffness of the gastrocnemius medialis remains almost constant with increasing torque level (i.e., from 30 to 90 % MVC). Moreover, muscle stiffness was found to be proportional to the force level confirming the second assumption associated to the alpha method.
We found that stiffness values for muscle and tendon were different using the ultrasound and the alpha methods (Fig. 3). We think that it could be explained by the differences between both methods. On the one hand, the alpha method characterizes the whole plantar flexor muscles (i.e., mainly the gastrocnemius medialis, the gastrocnemius lateralis and the soleus) using global torque and angle measurements. On the other hand, the ultrasound method was focused on one fascicle located in a restricted area of the gastrocnemius medialis muscle belly. Since the global stiffness is the sum of the stiffness of muscles (i.e., muscles could be considered as in parallel), it actually does not make sense to compare the stiffness of several muscles (i.e., alpha method) with the stiffness of one muscle (i.e., ultrasound). Thus, to perform the comparison, we used the total force of plantar flexors to calculate a global stiffness using the ultrasound method, by implicitly assuming that the fascicle length measured using ultrasound was representative of the whole plantar flexors. This assumption might not be valid for at least three reasons. First, the architectural characteristics between plantar flexors muscles (i.e., muscle volume, tendon length, muscle fascicle length, and pennation angle) are very different (Albracht et al. 2008; Fukunaga et al. 1996). Second, the alpha method evaluated tendinous tissue stiffness of the whole plantar flexors, whereas the ultrasound method used in the present study was only focused on the tendinous tissues of the gastrocnemius medialis (Arya and Kulig 2010; Fouré et al. 2010, 2012a, b; Kubo et al. 2007, 2010; Lenskjold et al. 2013; Magnusson et al. 2001; Stenroth et al. 2012). Moreover, the soleus represents 62 % of the global volume of the triceps surae (Albracht et al. 2008), and its tendon length is significantly shorter than the gastrocnemius medialis tendon length (55 ± 9 vs. 192 ± 24 mm, respectively; Kongsgaard et al. 2011; Stenroth et al. 2012). Third, since the strain (ΔL/L) was not calculated in the present study, the stiffness calculated in our study corresponds to an apparent stiffness (ΔF/ΔL). Thus, tendon length largely influenced calculated stiffness. Kongsgaard et al. (2011) showed that free Achilles tendon stiffness (i.e., soleus tendon) was significantly higher than the values of gastrocnemius medialis tendon stiffness (2622 ± 534 N/mm vs. 140–375 N/mm, respectively). Therefore, the assumption used for the comparison was probably not valid and it could largely explain the differences between methods found in the present study. However, it should be noted that the estimation of the force (i.e., force of all plantar flexors or force of one muscle) is also problematic in several frameworks such as for the characterization of tendon mechanical properties (Seynnes et al. 2015). While it is very relevant and important, no consensus currently exists on that topic in the literature.
Conclusion
In summary, the present study validated the two main assumptions underlying the alpha method using the ultrafast ultrasound method. Thus, the alpha method is a simple and reliable experiment for assessing the specific mechanical properties of muscles and tendons after training, neuromuscular disorders and to compare populations (e.g., gender and aging). However, the values of stiffness obtained between both methods are significantly different considering the various characterized structures (one muscle–tendon vs. the whole plantar flexor muscles) by the two methods (local vs. global). Further studies are therefore required to better understand these relative differences.
Abbreviations
- ANOVA:
-
Analysis of variance
- L fh :
-
Horizontal fascicle length
- MVC:
-
Maximal voluntary contraction
- S :
-
Muscle-tendon stiffness
- SEC:
-
Series elastic component
- S muscle :
-
Muscle stiffness
- S tendon :
-
Tendinous stiffness
- ΔF :
-
Change in force
- ΔL :
-
Muscle-tendon unit length change
- ΔL fh :
-
Horizontal fascicle length change
- ΔL tt :
-
Tendinous tissues length change
References
Albracht K, Arampatzis A, Baltzopoulos V (2008) Assessment of muscle volume and physiological cross-sectional area of the human triceps surae muscle in vivo. J Biomech 41(10):2211–2218. doi:10.1016/j.jbiomech.2008.04.020
Arya S, Kulig K (2010) Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol 108:670–675. doi:10.1152/japplphysiol.00259.2009
Bénard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT (2009) Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve 39:652–665. doi:10.1002/mus.21287
Blanpied P, Smidt GL (1992) Human plantarflexor stiffness to multiple single-stretch trials. J Biomech 25(1):29–39
Blanpied P, Smidt GL (1993) The difference in stiffness of the active plantarflexors between young and elderly human females. J Gerontol 48(2):M58–63
Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA (2008) Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105(6):1907–1915. doi:10.1152/japplphysiol.00059.2008
Cook CS, McDonagh MJ (1996) Measurement of muscle and tendon stiffness in man. Eur J Appl Physiol Occup Physiol 72(4):380–382
Cornu C, Almeida Silveira MI, Goubel F (1997) Influence of plyometric training on the mechanical impedance of the human ankle joint. Eur J Appl Physiol Occup Physiol 76(3):282–288
Cornu C, Goubel F, Fardeau M (1998) Stiffness of knee extensors in Duchenne muscular dystrophy. Muscle Nerve 21(12):1772–1774
Cronin NJ, Lichtwark G (2012) The use of ultrasound to study muscle-tendon function in human posture and locomotion. Gait Posture 37(3):305–312
Cronin NJ, Carty CP, Barrett RS, Lichtwark G (2011) Automatic tracking of medial gastrocnemius fascicle length during human locomotion. J Appl Physiol 111:1491–1496. doi:10.1152/japplphysiol.00530.2011
Deffieux T, Gennisson JL, Tanter M, Fink M, Nordez A (2006) Ultrafast imaging of in vivo muscle contraction using ultrasound. Appl Phys Lett 89:184107–184111. doi:10.1063/1.2378616
Deffieux T, Gennisson JL, Tanter M, Fink M (2008) Assessment of the mechanical properties of the musculoskeletal system using 2-D and 3-D very high frame rate ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 55:2177–2190. doi:10.1109/TUFFC.917
Ettema GJ, Huijing PA (1994) Skeletal muscle stiffness in static and dynamic contractions. J Biomech 27(11):1361–1368
Farcy S, Nordez A, Dorel S, Hauraix H, Portero P, Rabita G (2014) Interaction between gastrocnemius medialis fascicle and Achilles tendon compliance: a new insight on the quick-release method. J App Physiol 116(3):259–266. doi:10.1152/japplphysiol.00309.2013
Fletcher JR, Pfister TR, Macintosh BR (2013) Energy cost of running and Achilles tendon stiffness in man and woman trained runners. Physiol Rep 16(6):678–687. doi:10.1002/phy2.178
Fouré A, Nordez A, Cornu C (2010) In vivo assessment of both active and passive parts of the plantarflexors series elastic component stiffness using the alpha method: a reliability study. Int J Sports Med 31(1):51–57. doi:10.1055/s-0029-1241210
Fouré A, Nordez A, McNair P, Cornu C (2011) Effects of plyometric training on both active and passive parts of the plantarflexors series elastic component stiffness of muscle-tendon complex. Eur J Appl Physiol 111(3):539–548. doi:10.1007/s00421-010-1667-4
Fouré A, Cornu C, McNair P, Nordez A (2012a) Gender differences in both active and passive parts of the plantar flexors series elastic component stiffness and geometrical parameters of the muscle-tendon complex. J Orthop Res 30(5):707–712. doi:10.1002/jor.21584
Fouré A, Nordez A, Cornu C (2012b) Effects of plyometric training on passive stiffness of gastrocnemii muscles and Achilles tendon. Eur J Appl Physiol 112(2):2849–2857. doi:10.1007/s00421-011-2256-x
Fouré A, Nordez A, Cornu C (2013) Effects of eccentric training on mechanical properties of the plantar flexor muscle-tendon complex. J Appl Physiol 114(5):523–537. doi:10.1152/japplphysiol.01313.2011
Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Edgerton VR (1996) Specific tension of human plantar flexors and dorsiflexors. J Appl Physiol 80(1):158–165
Gillett JG, Barrett RS, Lichtwark GA (2013) Reliability and accuracy of an automated tracking algorithm to measure controlled passive and active muscle fascicle length changes from ultrasound. Comput Methods Biomech Biomed Eng 16(6):678–687. doi:10.1080/10255842.2011.633516
Goubel F, Pertuzon E (1973) Evaluation of the elasticity of muscle in situ by the quick-release method. Arch Int Physiol Biochim 81(4):697–707
Grieve D, Pheasant S, Cavanagh PR (1978) Prediction of gastrocnemius length from knee and ankle joint posture. In: Asmussen E, Jorgensen K (eds) Biomechanics VI-A. University Park Press, Baltimore, pp 405–412
Hauraix H, Nordez A, Dorel S (2013) Shortening behavior of the different components of muscle-tendon unit during isokinetic plantar flexions. J Appl Physiol 115(7):1015–1024. doi:10.1152/japplphysiol.00247.2013
Helland C, Bojsen-Møller J, Raastad T, Seynnes OR, Moltubakk MM, Jakobsen V, Visnes H, Bahr R (2013) Mechanical properties of the patellar tendon in elite volleyball players with and without patellar tendinopathy. Br J Sports Med 47(13):862–868. doi:10.1136/bjsports-2013-092275
Kawakami Y, Ichinose Y, Fukunaga T (1998) Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol 85(2):398–404
Kongsgaard M, Nielsen CH, Hegnsvad S, Aagaard P, Magnusson SP (2011) Mechanical properties of the human Achilles tendon, in vivo. Clin Biomech 26(7):772–777. doi:10.1016/j.clinbiomech.2011.02.011
Kubo K, Kawakami Y, Fukunaga T (1999) Influence of elastic properties of tendon structures on jump performance in humans. J Appl Physiol 87(6):2090–2096
Kubo K, Morimoto M, Komuro T, Tsunoda N, Kanehisa H, Fukunaga T (2007) Influences of tendon stiffness, joint stiffness, and electromyographic activity on jump performances using single joint. Eur J Appl Physiol 99(3):235–243
Kubo K, Tabata T, Ikebukuro T, Igarashi K, Yata H, Tsunoda N (2010) Effects of mechanical properties of muscle and tendon on performance in long distance runners. Eur J Appl Physiol 110(3):507–514. doi:10.1007/s00421-010-1528-1
Lambertz D, Perot C, Kaspranski R, Goubel F (2001) Effects of long-term spaceflight on mechanical properties of muscles in humans. J Appl Physiol 90(1):179–188
Lenskjold A, Kongsgaard M, Larsen JO, Nielsen RH, Kovanen V, Aagaard P, Kjaer M, Magnusson SP (2013) The influence of physical activity during youth on structural and functional properties of the Achilles tendon. Scand J Med Sci Sports. doi:10.1111/sms.12143
Maganaris CN, Paul JP (1999) In vivo human tendon mechanical properties. J Physiol 521:307–313
Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M (2001) Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 531:277–288
Magnusson SP, Narici MV, Maganaris CN, Kjaer M (2008) Human tendon behaviour and adaptation, in vivo. J Physiol 586(1):71–81
Morgan DL (1977) Separation of active and passive components of short-range stiffness of muscle. Am J Physiol 232(1):45–49
Morgan DL, Proske U, Warren D (1978) Measurements of muscle stiffness and the mechanism of elastic storage of energy in hopping kangaroos. J Physiol 282:253–261
Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H (2004) Influence of tendon slack on electromechanical delay in the human medial gastrocnemius in vivo. J Appl Physiol 96(2):540–544
Nordez A, Gallot T, Catheline S, Guével A, Cornu C, Hug F (2009) Electromechanical delay revisited using very high frame rate ultrasound. J Appl Physiol 106(6):1970–1975. doi:10.1152/japplphysiol.00221.2009
Pearson SJ, McMahon J (2012) Lower limb mechanical properties: determining factors and implications for performance. Sports Med 42(11):929–940. doi:10.2165/11635110-000000000-00000
Peltonen J, Cronin NJ, Stenroth L, Finni T, Avela J (2013) Viscoelastic properties of the Achilles tendon in vivo. Springerplus 2(1):212
Proske U, Morgan DL (1987) Tendon stiffness: methods of measurement and significance for the control of movement. A review. J Biomech 20(1):75–82
Rack PM, Westbury DR (1973) The short range stiffness of active mammalian muscle. J Physiol 229(1):16–17
Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP (2002) Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12(2):90–98
Seynnes OR, Bojsen-Møller J, Albracht K, Arndt A, Cronin NJ, Finni T, Magnusson SP (2015) Ultrasound-based testing of tendon mechanical properties: a critical evaluation. J Appl Physiol
Stenroth L, Peltonen J, Cronin NJ, Sipilä S, Finni T (2012) Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113:1537–1544. doi:10.1152/japplphysiol.00782.2012
Svantesson U, Takahashi H, Carlsson U, Danielsson A, Sunnerhagen KS (2000) Muscle and tendon stiffness in patients with upper motor neuron lesion following a stroke. Eur J Appl Physiol 82(4):275–279
Tanter M, Fink M (2014) Ultrafast Imaging in biomedical ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 61(1):102–119. doi:10.1109/TUFFC.2014.6689779
Wilson GJ, Wood GA, Elliott BC (1991) Optimal stiffness of series elastic component in a stretch-shorten cycle activity. J Appl Physiol 70(2):825–833
Wu YK, Lien YH, Lin KH, Shih TT, Wang TG, Wang HK (2009) Relationships between three potentiation effects of plyometric training and performance. Scand J Med Sci Sports 20(1):80–86
Zee M, Voigt M (2001) Moment dependency of the series elastic stiffness in the human plantar flexors measured in vivo. J Biomech 34(11):1399–1406
Zhao H, Ren Y, Wu Y, Liu SQ, Zhang L (2009) Ultrasonic evaluations of Achilles tendon mechanical properties poststroke. J Appl Physiol 106:843–849. doi:10.1152/japplphysiol.91212.2008
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Olivier Seynnes.
Rights and permissions
About this article
Cite this article
Hauraix, H., Fouré, A., Dorel, S. et al. Muscle and tendon stiffness assessment using the alpha method and ultrafast ultrasound. Eur J Appl Physiol 115, 1393–1400 (2015). https://doi.org/10.1007/s00421-015-3112-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3112-1