Abstract
Objectives
To investigate age-related changes on passive muscle stiffness in healthy individuals and measure the shear modulus in different age groups.
Methods
Shear wave elastography (SWE) movies of gastrocnemius medialis (GM) were collected during passive stretching induced by ankle rotation from plantarflexion (PF) to dorsiflexion (DF). A series of SWE images at ankle angles of PF 40°, PF 30°, PF 20°, PF 10°, 0°, DF 10°, DF 20°, and DF 30° were collected and shear moduli measured accordingly for analyses.
Results
Eighty-six healthy volunteers (27 children, 31 middle-aged adults, and 28 older people) were recruited. No significant difference was observed in the shear modulus between the three groups at ankle angles of PF 40°, PF 30°, PF 20°, PF 10°, and 0° (p > 0.05). The difference in the shear modulus among the three groups became significant as DF increased. At ankle angles of DF 10°, DF 20°, and DF 30°, the shear modulus was the greatest in the older group, followed by the middle-aged group and then the children group (p = 0.007, 0.000, and 0.000, respectively).
Conclusions
Passive muscle stiffness increases with age, and the difference between age groups was pronounced only after reaching a certain degree of stretching.
Key Points
• The influence of age on passive muscle stiffness becomes pronounced only after reaching a certain degree of stretching.
• Age should be considered when evaluating passive muscle stiffness in muscular disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Passive muscle stiffness is a promising indicator for evaluating the mechanical properties of skeletal muscle tissue due to the strong linear relationship with passive muscle force [1,2,3]. Quantification of passive muscle stiffness is particularly relevant in clinical settings because increased stiffness contributes to muscle adaptation and decreased passive range of motion (RoM) in joints, which disrupts motor function over time, and affects the quality of life adversely [4, 5]. Increased muscle stiffness is correlated with disease stage [6]. Noninvasive measurements of passive muscle stiffness could provide a longitudinal assessment of muscle function in monitoring disease progression and treatment response [7, 8].
Various elastography methods have been developed to estimate muscle stiffness: magnetic resonance elastography, vibration elastography, compression elastography, and shear wave elastography (SWE) [1]. Compared with other methods, SWE has gained attention: (i) for its low dependence on operator skills; because external compression or vibration is not needed [1, 9]; (ii) because it is superior to magnetic resonance elastography with regard to cost and accessibility [1, 2]. Furthermore, SWE tracking is sufficiently fast that it can be implemented on the scanner to provide real-time quantitative metrics of tissue stiffness [10]. SWE has been applied for muscle diseases, including Duchenne muscular dystrophy, GNE-related myopathy, amyotrophic lateral sclerosis, stroke, muscle atrophy, muscle injury, and cerebral palsy [4,5,6, 11,12,13].
Muscle disorders can affect people of all ages. Changes in passive muscle stiffness are not only pathologic, but also affected by the maturation and aging of muscle [14, 15]. Age-related changes in muscle stiffness may contribute to understanding of the development of musculoskeletal disorders with age. However, there are minimal data on passive muscle stiffness and how it alters with age [14, 15].
When measuring passive muscle stiffness, muscle stretching is conducted by adopting specific static postures or dynamic movements [3, 8, 9, 16]. The calf muscle crosses both the knee and ankle joints, so the extent of stretching of gastrocnemius medialis (GM) is determined by the position of both joints. Various stretching methods have been applied to study the passive muscle stiffness of GM. Chino and colleagues [16] quantified the stiffness of GM with that of the ankle in 30° plantarflexion (PF), a neutral anatomic position, and 20° dorsiflexion (DF) with a fully extended knee in 52 healthy young adults. Nordaz and coworkers [9] carried out dynamic passive stretching at a very slow velocity (0.5° s−1) from 40° in PF until 95% of the maximal RoM with a fully extended knee in nine healthy young male adults. Maïsetti and collaborators [3] measured the passive stiffness of GM by undertaking slow (2°/s) passive loading/unloading cycles between 40° of PF and 80% of the maximal RoM in DF at different knee angles (0°, 15°, 30°, 45°, 60°, and 80°, where 0° = knee is fully extended) in seven healthy young men. Studies have evaluated few cases and not covered the age range of children, adults, and older patients. Moreover, comparing the results from different studies is difficult because of the inconsistency in the stretching protocol.
We investigated age-related changes on passive muscle stiffness in healthy people and the shear modulus of different age groups.
Methods
Ethical approval of the study protocol
All procedures were approved by the Ethics Review Board of Shenzhen Hospital of Guangzhou University of Chinese Medicine (KYLS20190201) in Shenzhen, China. Written informed consent was obtained from all participants. In the children group, written informed consent was obtained from both children and their parent or guardian.
Study design
This study had a cross-sectional design. We recruited healthy participants from three age groups: children (< 16 years), middle-aged (30–40 years), and older (> 55 years). We tested the passive stiffness of GM at a series of stretching levels in a single visit. Assessments were conducted from July 2019 to January 2020.
Participants
In this prospective study, participants’ eligibility was based on the following: (i) age (as detailed above); (ii) normal motor function of both legs. Individuals were excluded if they (i) were professional athletes and (ii) reported a history of neuromuscular/musculoskeletal disease, or injury/surgery to the lower extremities. We recorded the circumference of the left calf at the area of the greatest bulk. Height and weight were measured, and the body mass index (BMI) was calculated accordingly.
Instrumentation
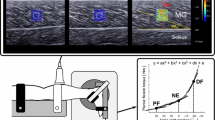
A passive exercise machine (YTK-E; Zhengda Medical Co., Ltd.) was used to control the ankle angle and angular velocity of the joint during stretching by a microchip program (Fig. 1a). Surface electromyography (Trigno™ Wireless EMG) ensured muscle relaxation during stretching (Fig. 1b). An ultrasound scanner (Resona v7.0; Mindray Bio-Medical Electronics Co., Ltd.) with a multifrequency linear transducer (L11-3U; frequency range = 3–11 MHz) was used to measure the shear modulus of GM (Fig. 1c).
Experimental setup. a A passive exercise machine was used to control the ankle angle and joint angular velocity during stretching. b Surface electromyography was used to ensure muscle relaxation during stretching. c An ultrasound scanner (Resona v7.0) with a linear ultrasound transducer (L11-3U) was applied to collect SWE images
Pretest
To ensure safety and confirm the preset parameters covered the maximum available RoM, a passive stretching pretest was imposed on four volunteers (two middle-aged authors of this manuscript and their children aged 8 years) using the passive exercise machine. The cycle of ankle movement was carried out from PF 40° to DF 30° and back at a constant velocity of 2°/s (Fig. 2) [11]. The knee was bent at 30° flexion to avoid GM overstretching at DF positions [11, 17]. An ankle angle of 0° was defined when the sole of the foot was perpendicular to the longitudinal axis of the calf.
Experimental setup for SWE
Participants lay supine on a flat bed with their left leg relaxed on the passive exercise machine and their left foot secured firmly to the footplate by two straps. The axes of the footplate and ankle were aligned visually as close as possible. Participants were instructed to stay as relaxed as possible through passive stretching.
The stretching cycle was repeated thrice with the first cycle regarded as “preconditioning” [9]. Each cycle started with a B-mode acquisition over the area of the greatest GM bulk under the musculoskeletal preset. Copious amounts of gel were applied to minimize the pressure of the transducer on the skin. The transducer was held manually and oriented perpendicular to the skin and parallel to GM fascicles [18] consistently during stretching. Once the B-mode image had been optimized, a rectangular elastography window (13 × 7 mm2) was superimposed in the center of the GM belly. SWE movies were captured synchronously with each stretching cycle. Two stretching cycles produced two sets of SWE movies for further analyses. The procedure was carried out by two examiners with > 10 years’ experience of analyzing ultrasound findings of musculoskeletal areas and 4 years’ experience of evaluating SWE images.
During collection of SWE movies, an investigator monitored real-time surface electromyography continuously to ensure that there was no subtle movement of the leg or unconscious contraction of GM.
Measurement of the shear modulus
SWE readings were represented as shear modulus (kPa). The shear modulus was measured at the exact moment correspondent with a series of ankle PF–DF angles (PF 40°, PF 30°, PF 20°, PF 10°, 0°, DF 10°, DF 20°, DF 30°) (Table 1). A 6-mm circular region of interest [8] was positioned in the elastography box, carefully avoiding tendons, aponeuroses, and fascial tissue. The scanner provided the corresponding shear modulus automatically. The shear modulus was measured in the two sets of SWE movies and the average value of the two measurements adopted for analyses. GM thickness was also measured.
Evaluation of intra-observer and inter-observer reproducibility
Intra-observer and inter-observer agreement of measurements were assessed in 30 participants selected randomly. The same observer (L.X.) measured the elastic modulus twice to assess intra-observer reproducibility. Assessment of intra-observer agreement was undertaken during different sessions. The second measurement was undertaken 1 week after the first measurement, and the information of the participant was blinded to the observer. To assess inter-observer reproducibility, shear modulus measurements were made by a second observer (P.M.) who was blinded to the results of the first observer (L.X.).
Statistical analyses
Statistical analyses were undertaken using SPSS v21.0 (IBM) and MedCalc v18.2.1 (MedCalc). The difference in shear modulus measurements between males and females was compared with the independent t test. One-way ANOVA was used to determine if there was a significant difference in passive muscle stiffness among the three age groups, with post hoc Tukey-corrected pairwise multiple comparisons to highlight differences between one group and another group. The normal distribution was tested by the Kolmogorov–Smirnov test. Homogeneity was examined by Levene’s test. Also, 95% confidence intervals (95% CIs) and the intra-class correlation coefficient were used to assess the reproducibility of results of the shear modulus test. P < 0.05 was considered significant.
Results
A total of 86 healthy people (44 males and 42 females) were enrolled consecutively. There were 27 children (6–12 years), 31 middle-aged individuals (30–40 years), and 28 older people (55–66 years). Demographic characteristics (age, sex) and physical measurements (height, weight, body mass index (BMI), calf circumstance, GM thickness) are presented in Table 2.
The shear modulus and 95% CIs at each ankle angle of the three groups are listed in Table 3. In the three groups, passive muscle stiffness increased as the ankle DF increased (Table 3 and Fig. 3), except for a low ebb of shear modulus that appeared at PF 20° in the middle-aged group (Table 3). There was no significant difference between sexes (Table 4).
The one-way ANOVA results of the muscle assessments in Table 3 showed no significant difference in passive muscle stiffness among the three age groups at ankle angles of PF 40°, PF 30°, PF 20°, PF 10°, and 0° (p > 0.05). As the ankle underwent DF and the stretching level of GM increased, a significant difference was observed among the three age groups at ankle angles of DF 10°, DF 20°, and DF 30° (p < 0.05). Further analyses via post hoc multiple comparisons revealed that the difference was not significant between the children group and middle-aged group at an ankle angle of DF 10°, and that the shear modulus increased significantly in the older group compared with that in the children group and middle-aged group. At ankle angles of DF 20° and DF 30°, the shear modulus was the greatest in the older group, followed by the middle-aged group and children group (p < 0.05). The shear modulus (in kPa) was 24.28 ± 7.72, 28.39 ± 6.85, and 34.89 ± 8.48 at an ankle angle of DF 20° in the children, middle-aged, and older groups, respectively (p = 0.000). The shear modulus (in kPa) was 38.97 ± 7.44, 46.17 ± 8.35, and 56.80 ± 10.23 at an ankle angle of DF 30° in the children, middle-aged, and older groups, respectively (p = 0.000). The inter-class correlation coefficient ranged from 0.8853 to 0.9675 and from 0.8928 to 0.9419 for intra-observer and inter-observer measurements of the shear modulus, respectively (Table 5).
Discussion
SWE showed promise in quantifying passive muscle stiffness in the present study. By adopting a protocol of dynamic stretching, we explored the effect of age on passive muscle stiffness and measured the shear modulus of GM at a series of ankle angles in healthy participants of different ages.
Skeletal muscle becomes stiffer as it is stretched. This phenomenon has been verified by studies on healthy muscles using different methods. The shear modulus of biceps brachii has been shown to be higher when it was stretched at full extension of the elbow compared with the value obtained at 90° elbow flexion [14]. Koo and colleagues undertook elasticity measurements by SWE at tibialis anterior in 20 healthy adults with the ankle positioned from 50° PF to up to 15° DF: increased elasticity was found as the level of stretching increased [17]. An identical pattern was found in a study by Akagi and colleagues using real-time tissue elastography images on GM in 12 healthy young males [19]. Our study revealed an increase in passive muscle elasticity in the RoM we tested, which is consistent with the previous studies [14, 17, 19]. As a muscle is stretched, it reacts with increasing passive resistance. Greater recruitment of muscle fibers against stretching can lead to a longitudinal high elastic modulus [1]. In addition, the decrease in muscle cross section during stretching can induce transverse stress and increased intramuscular pressure [20, 21]. Longitudinal tension and transverse stress can contribute to increased muscle stiffness [9].
In the middle-aged group, a low ebb of shear modulus appeared at PF 20°, which might suggest it to be the optimal slack angle. PF 25° (at 90° of knee flexion) has been suggested to be the optimal slack angle of GM [22]. The set of knee angles was inconsistent with our stretching protocol, so a possible bias may exist for the optimal slack angle. However, a low ebb was not observed in the children group or older group. We considered that the optimal slack angle might have been missed owing to an increment of 10° between two measurements in our study.
In health, the response of a muscle to stretching is moderate within a normal range and is affected by the mechanical, material, and architectural properties of the muscle [23]. Structural and compositional changes, such as alterations in the composition and function of the fiber type, intramuscular fat content, capillary density, intramuscular connective tissue, or collagen [14], would affect muscle stiffness. Changes in the structure and composition of muscle persist throughout life at the physiologic level [14, 15, 24]. Age-related passive muscle stiffness has been studied by various scholars using various skeletal muscles. Brandenburg and colleagues [8] showed increasing passive stiffness with age in lateral gastrocnemius muscle at an ankle angle of DF 10°, but the result was not significant, which might due to the small sample size (20 children) and age range (2–12.6 years). Eby and collaborators quantified passive stiffness in biceps brachii of 113 adults (20–90 years) at full extension of the elbow, and concluded that the passive shear modulus increased with advancing age [14]. Alfuraih and coworkers [15] investigated the effect of aging on passive stiffness of quadriceps at static passive stretching (knee flexion of 90°) for people aged 20–94 years, and found no significant difference with age. The studies mentioned above measured passive muscle elasticity at several static stretched postures [8, 14, 15], so sufficient information on muscle characteristics could not be provided. In reality, muscles tend to be stretched dynamically at a series of stretching levels. We investigated passive muscle stiffness through dynamic stretching and obtained data of a series of stretching levels. Our results showed that passive muscle stiffness increased with age and that the differences between age groups were pronounced only after reaching a certain degree of stretching. Our results reflect the effect of muscle maturation and the aging process on passive stiffness of GM.
The cause of increased passive muscle stiffness with age may be related to the ongoing alteration of muscle structure/composition during muscle maturation and the aging process. Children are in a rapid development phase. Their muscle fiber is finer with more interstitial components than that of adults. The sparse form may serve a buffer gap to induce lower transverse stress during stretch process. Muscles mature gradually after adolescence and maintain a stable level. A gradual loss of muscle fibers begins at ~ 50 years of age and continues such that by 80 years of age, ~ 50% of fibers are lost from limb muscles [25]. The aging process causes muscle atrophy and a concomitant increase in intramuscular adipose tissue and connective tissue [24, 26]. Remodeling of skeletal muscle compromises muscle extensibility further, resulting in greater resistance at the same level of stretching. These phenomena may explain the increased passive muscle stiffness in the older group in our study.
Clinical disorders, diseases, and injury to muscles of patients of all ages would also alter the structure and composition of muscle [27], along with muscle stiffness [4, 6, 28]. For example, Duchenne muscular dystrophy and cerebral palsy occur in children, who deteriorate progressively into adulthood; stroke, Parkinson’s disease, and muscle atrophy occur in older people. Scholars have observed increased muscle stiffness in the diseases mentioned above [4,5,6, 29, 30] and illustrated that passive muscle stiffness can reflect disease severity [4, 5, 30, 31]. We measured the shear modulus of people of different ages, which could provide baseline information for assessment of muscle diseases in individuals of different ages.
Our study had two main limitations. First, the number of enrolled participants was small. Extending this study to different age groups to follow changes accurately during the entire muscle maturation process and aging process would be interesting. Second, we focused on the age-associated difference of passive stiffness of GM at a series of stretching levels. Further investigation is needed to evaluate the effect of other variables (e.g., sporting activity, muscle mass, dominant leg) on passive muscle stiffness.
Conclusions
We demonstrated that passive stiffness of GM increased with age and the difference was pronounced only after reaching a certain degree of stretching. The shear modulus of people of different ages may provide baseline information for assessment of muscle diseases of individuals of different ages.
Abbreviations
- BMI:
-
Body mass index
- DF:
-
Dorsiflexion
- GM:
-
Gastrocnemius medialis
- PF:
-
Plantarflexion
- RoM:
-
Range of motion
- SWE:
-
Shear wave elastography
References
Koo TK, Guo JY, Cohen JH, Parker KJ (2013) Relationship between shear elastic modulus and passive muscle force: an ex-vivo study. J Biomech 46:2053–2059
Liu J, Qian Z, Wang K et al (2019) Non-invasive quantitative assessment of muscle force based on ultrasonic shear wave elastography. Ultrasound Med Biol 45:440–451
Maïsetti O, Hug F, Bouillard K, Nordez A (2012) Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech 45:978–984
Lee SS, Spear S, Rymer WZ (2015) Quantifying changes in material properties of stroke-impaired muscle. Clin Biomech (Bristol, Avon) 30:269–275
Brandenburg JE, Eby SF, Song P et al (2016) Quantifying passive muscle stiffness in children with and without cerebral palsy using ultrasound shear wave elastography. Dev Med Child Neurol 58:1288–1294
Lacourpaille L, Hug F, Guével A et al (2015) Non-invasive assessment of muscle stiffness in patients with Duchenne muscular dystrophy. Muscle Nerve 51:284–286
Giambini H, Hatta T, Rezaei A, An KN (2018) Extensibility of the supraspinatus muscle can be predicted by combining shear wave elastography and magnetic resonance imaging-measured quantitative metrics of stiffness and volumetric fat infiltration: a cadaveric study. Clin Biomech (Bristol, Avon) 57:144–149
Brandenburg JE, Eby SF, Song P et al (2015) Feasibility and reliability of quantifying passive muscle stiffness in young children by using shear wave ultrasound elastography. J Ultrasound Med 34:663–670
Nordez A, Gennisson JL, Casari P, Catheline S, Cornu C (2008) Characterization of muscle belly elastic properties during passive stretching using transient elastography. J Biomech 41:2305–2311
Davis LC, Baumer TG, Bey MJ, Holsbeeck MV (2019) Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 38:2–12
Sun Y, Xiao Y, Li F et al (2020) Diagnosing muscle atrophy by use of a comprehensive method of assessing the elastic properties of muscle during passive stretching. AJR Am J Roentgenol 214:862–870
Carpenter EL, Lau HA, Kolodny EH, Adler RS (2015) Skeletal muscle in healthy subjects versus those with GNE-related myopathy: evaluation with shear-wave US—a pilot study. Radiology 277:546–554
Martínez-Payá JJ, Del Baño-Aledo ME, Ríos-Díaz J, Fornés-Ferrer V, Vázquez-Costa JF (2018) Sonoelastography for the assessment of muscle changes in amyotrophic lateral sclerosis: results of a pilot study. Ultrasound Med Biol 44:2540–2547
Eby SF, Cloud BA, Brandenburg JE et al (2015) Shear wave elastography of passive skeletal muscle stiffness influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 30:22–27
Alfuraih AM, Tan AL, O’Connor P, Emery P, Wakefield RJ (2019) The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin Exp Res 31:1755–1763
Chino K, Takahashi H (2016) Measurement of gastrocnemius muscle elasticity by shear wave elastography: association with passive ankle joint stiffness and sex differences. Eur J Appl Physiol 116:823–830
Koo TK, Guo JY, Cohen JH, Parker KJ (2014) Quantifying the passive stretching response of human tibialis anterior muscle using shear wave elastography. Clin Biomech (Bristol, Avon) 29:33–39
Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN (2013) Validation of shear wave elastography in skeletal muscle. J Biomech 46:2381–2387
Akagi R, Chino K, Dohi M, Takahashi H (2012) Relationships between muscle size and hardness of the medial gastrocnemius at different ankle joint angles in young men. Acta Radiol 53:307–311
Davis J, Kaufman KR, Lieber RL (2003) Correlation between active and passive isometric force and intramuscular pressure in the isolated rabbit tibialis anterior muscle. J Biomech 36:505–512
Sadeghi S, Johnson M, Bader DA, Cortes DH (2019) The shear modulus of lower-leg muscles correlates to intramuscular pressure. J Biomech 83:190–196
Hug F, Lacourpaille L, Maïsetti O, Nordez A (2013) Slack length of gastrocnemius medialis and Achilles tendon occurs at different ankle angles. J Biomech 46:2534–2538
Koo TK, Hug F (2015) Factors that influence muscle shear modulus during passive stretch. J Biomech 48:3539–3542
Gheller BJ, Riddle ES, Lem MR, Thalacker-Mercer AE (2016) Understanding age-related changes in skeletal muscle metabolism: differences between females and males. Annu Rev Nutr 36:129–156
Faulkner JA, Larkin LM, Claflin DR, Brooks SV (2007) Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 34:1091–1096
Sanabria SJ, Martini K, Freystätter G et al (2019) Speed of sound ultrasound: a pilot study on a novel technique to identify sarcopenia in seniors. Eur Radiol 29:3–12
Ríos-Díaz J, Del Baño-Aledo ME, Tembl-Ferrairó JI, Chumillas MJ, Vázquez-Costa JF, Martínez-Payá JJ (2019) Quantitative neuromuscular ultrasound analysis as biomarkers in amyotrophic lateral sclerosis. Eur Radiol 29:4266–4275
Gajdosik RL (2001) Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech (Bristol, Avon) 16:87–101
Kwon DR, Park GY, Lee SU, Chung I (2012) Spastic cerebral palsy in children: dynamic sonoelastographic findings of medial gastrocnemius. Radiology 263:794–801
Du LJ, He W, Cheng LG, Li S, Pan YS, Gao J (2016) Ultrasound shear wave elastography in assessment of muscle stiffness in patients with Parkinson’s disease: a primary observation. Clin Imaging 40:1075–1080
Bilgici MC, Bekci T, Ulus Y et al (2018) Quantitative assessment of muscular stiffness in children with cerebral palsy using acoustic radiation force impulse (ARFI) ultrasound elastography. J Med Ultrason (2001) 45:295–300
Acknowledgments
We thank Yang Xiao (Shenzhen Institutes of Advanced Technology, Chinese Academy of Science) for providing technical guidance.
Funding
Public Health Project of Futian District (FTWS2018049) and Shenzhen Science and Technology Project foundation (JCYJ20170817171836611).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhi-bo Wen.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Complex statistical methods were not necessary for our study.
Informed consent
Written informed consent was obtained from all study participants in this study.
Ethical approval
All procedures were approved by the Ethics Review Board of Shenzhen Hospital of Guangzhou University of Chinese Medicine (KYLS20190201) in Shenzhen, China.
Methodology
• Prospective
• Observational study
• Undertaken at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Yu, Hk., Sheng, Sy. et al. Quantitative evaluation of passive muscle stiffness by shear wave elastography in healthy individuals of different ages. Eur Radiol 31, 3187–3194 (2021). https://doi.org/10.1007/s00330-020-07367-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07367-7