Abstract
The validity of predicting peak oxygen uptake (\( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)) in sedentary participants from a perceptually regulated exercise test (PRET) is limited to two cycle ergometry studies. We assessed the validity of a treadmill-based PRET. Active (n = 49; 40.7 ± 13.8 years) and sedentary (n = 26; 33.4 ± 13.2 y) participants completed two PRETS (PRET 1 and PRET2), requiring a change in speed or incline corresponding to ratings of perceived exertion (RPE) 9, 11, 13 and 15. Extrapolation of RPE: \( \dot{V}{\text{O}}_{2} \) data to RPE 19 and 20 from the RPE 9–13 and 9–15 ranges were used to estimate \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \), and compared to \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from a graded exercise test (GXT). The \( \dot{V}{\text{O}}_{2} \) :heart rate (HR) data (≥RPE 15) from the GXT were also extrapolated to age-predicted maximal HR (HRmaxpred) to provide further estimation of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \). ANOVA revealed no significant differences between \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) predictions from the RPE 9–15 range for PRET 1 and PRET 2 when extrapolated to RPE 19 in both active (54.3 ± 7.4; 52.9 ± 8.1 ml kg−1 min−1) and sedentary participants (34.1 ± 10.2; 34.2 ± 9.6 ml kg−1 min−1) and no difference between the HRmaxpred method and measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the GXT for active (53.3 ± 10.0; 53.9 ± 7.5 ml kg−1 min−1, respectively) and sedentary participants (33.6 ± 8.4, 34.4 ± 7.0 ml kg−1 min−1, respectively). A single treadmill-based PRET using RPE 9–15 range extrapolated to RPE 19 is a valid means of predicting \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in young and middle to older-aged individuals of varying activity and fitness levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maximal oxygen uptake is commonly regarded as a gold standard measure of cardiorespiratory fitness (ACSM 2009). Due to concerns regarding the safety and cost in non-athletic, elderly or patient populations (Noonan and Dean 2000), indirect methods of estimating this value typically employ heart rate (HR) responses to submaximal exercise. However, the validity of these methods is questionable. Environmental factors (temperature and humidity; Galloway and Maughan 1997), medical conditions (e.g., hypertension; Palatini et al. 2006) or medication (e.g. beta blockade; Eston and Connolly 1996; Van Baak 1988) may affect HR and subsequently the accuracy of predicted maximal oxygen uptake. As there is a well-established relationship between the Borg 6-20 Ratings of Perceived Exertion (RPE) Scale and measures of exercise intensity (Borg 1998; Chen et al. 2002; Faulkner and Eston 2007; Whaley et al. 1997), the RPE is commonly used to complement physiological measures of exercise during graded exercise testing (GXT) and as an aid to check prescribed intensity levels in healthy and clinical populations (ACSM 2009; Buckley et al. 2009; Coquart et al. 2009; Eston and Connolly 1996; Scherer and Cassady 1999; Williams and Eston 1989). The RPE given during graded exercise testing have been shown to elicit better estimates of peak oxygen uptake (\( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)) than HR alone (Ljunggren and Johansson 1988). A few studies have explored the collective use of RPE and HR to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in sedentary and active individuals during cycling exercise (Faulkner et al. 2009; Lambrick et al. 2009), although no study has assessed the efficacy of this method in sedentary participants during treadmill exercise.
By extrapolating the RPE:work rate relationship from sub-maximal estimation trials, studies have predicted peak or maximal \( \dot{V}{\text{O}}_{2} \) (Coquart et al. 2009, 2010; Dunbar and Bursztyn 1996; Faulkner and Eston 2007; Okura and Tanaka 2001). Studies have also shown that the RPE can be used to produce target exercise intensities in a so-called ‘production’ procedure. This was first confirmed in cardiac patients (Borg and Linderholm 1970) and later supported in studies with healthy adults using treadmill (Eston et al. 1987; Smutok et al. 1980) and cycling exercise (Dunbar et al. 1992; Eston et al. 2005, 2006, 2008; Eston and Williams 1988; Faulkner et al. 2007; Glass et al. 1992; Parfitt et al. 1996). On the basis of earlier work, which used prescribed RPE levels during a graded, perceptually regulated exercise test (PRET) in cardiac patients (Eston and Thompson 1997), Eston et al. (2005) showed that a PRET procedure could be used to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in healthy adults. In their study, active men cycled for 4-min stages at five perceptually regulated intensities corresponding to RPEs 9, 11, 13, 15 and 17 on the Borg 6-20 RPE Scale. Estimates of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) were more accurate when predicted from a broader perceptual range (RPE 9–17) and following a period of practice. However, it is recognized that it would be preferable if \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) could be accurately predicted from lower submaximal RPE ranges (9–15 and 9–13) in cases where exercise intensities above RPE 15 ‘hard/heavy’ or RPE 13 ‘somewhat hard’ are not recommended. This may be possible with a treadmill-based PRET, due to the greater familiarity with this mode of exercise, especially with sedentary participants. It has been previously observed that sedentary participants become more accurate in perceptual regulation of exercise on a cycle ergometer after an initial trial (Eston et al. 2008; Faulkner et al. 2007). A potential advantage of the PRET procedure is that it permits a level of autonomy, allowing the participant to dictate the pace, intensity and increments in intensity during the exercise test. According to self-determination theory (Deci and Ryan 1985), the autonomy afforded to the individual to control exercise intensity should support the development of more intrinsically motivated exercise behaviour. Also, as the incremental stages are relatively short (3 min), the decline in affect associated with longer term exercise at moderate- to high-intensity exercise, particularly in sedentary participants, would be limited (Parfitt and Eston 1995).
Studies have confirmed the validity of the PRET methodology with variations in the temporal nature of the exercise stages for cycling ergometry in healthy active and sedentary participants (Eston et al. 2006, 2008; Faulkner et al. 2007; Morris et al. 2009), arm-crank ergometry in able-bodied and paraplegic individuals (Al-Rahamneh and Eston 2011) and treadmill running in young active students (Morris et al. 2010). However, the validity of PRET to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) during treadmill exercise for middle-aged and sedentary men and women has yet to be determined. As indicated above, walking and running are more familiar and habitual modes of exercise for the average individual. Furthermore, treadmill ergometry is a considerably more popular form of exercise testing. Stuart and Ellestad (1980) observed that of the 1,400 exercise testing facilities surveyed in the US, 71% used treadmills for testing, whereas only 17% used cycle ergometers. Consequently, it is important to determine the validity of PRET in sedentary individuals particularly as they are most likely to benefit from an accurate estimation of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from a more familiar mode of submaximal exercise intensities which are within their control. Furthermore, if it can be shown that an initial submaximal PRET can be used to provide a valid estimation of peak oxygen uptake in sedentary participants, before a maximal graded exercise test is undertaken, the potential of exercise testing using the PRET methodology is considerably increased.
Therefore, the purposes of this study were to (1) evaluate the accuracy of estimating \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the heart rate (HR):\( \dot{V}{\text{O}}_{2} \) relationship recorded within the submaximal exercise range of RPE 9–15 extrapolated to Gellish et al.’s predicted HR maximum (HRmaxpred) in active and sedentary participants, (2) assess the reliability and absolute and relative change in oxygen uptake during each stage of the two PRETs, (3) assess the validity of predicting \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the extrapolated end-points RPE 19 (commonly reported maximum) and RPE 20 (theoretical maximum) from each of the submaximal ranges (RPE 9–13 and RPE 9–15) in the active and sedentary participants, (4) compare the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) predicted from the submaximal ranges in the PRETs to the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the HRmaxpred method used in the GXT.
Materials and methods
Participants
Seventy-five volunteers (aged 18–72 years) were recruited through poster advertisement around the local community. Participants were classified as either active (n = 49, mean age = 40.7 ± 13.8 years, height = 1.71 ± 0.08 m, body mass = 66 ± 8.7 kg) or sedentary (n = 26, mean age = 33.4 ± 13.2 years, height = 1.67 ± 0.07 m, body mass = 72.5 ± 13.7 kg). The active participants were recruited from local athletics clubs and were classified as being ‘physically active’ according to self-reported activity status, performing moderate to vigorous aerobic (running) exercise on at least three structured exercise sessions (30 min per session) each week. Participants were classified as ‘sedentary’ on the basis of current physical activity guidelines (Chief Medical Officer 2004). Specifically, these guidelines recommend at least 30 min of moderate-intensity activity (e.g., activity equivalent to walking at a brisk pace, which would lead to an increase in breathing rate and an increase in heart rate, to the level where the pulse can be felt) on five or more days a week, in stages of at least 10 min; or 20 min of vigorous-intensity activity (e.g., activity which leads to feeling out of breath) on three or more days a week. To ensure participants were sedentary, and not too close to the boundaries, only participants who reported less than 30 min of moderate-intensity activity on less than 3 days per week were used.
All participants were asymptomatic of illness or disease and free from acute or chronic injury, as established by the American College of Sports Medicine (ACSM 2009) physical activity readiness questionnaire (PAR-Q). Before participating, each volunteer completed a medical history questionnaire and provided written informed consent in accordance with institutional policy. ACSM’s (2009) risk stratification procedures were undertaken for all participants prior to commencing exercise testing. For men ≥45 years and women ≥55 years written physician consent was obtained. Furthermore, basic anthropometric (height and body mass, SECA, Hamburg, Germany) and health measurements including blood pressure (Accoson, London, UK), cholesterol and non-fasting blood glucose (Cardiocheck P.A. and PTS PANELS test strips, Polymer Technology systems Inc., Indianapolis, USA) were performed. Recent smoking history (current smoker or those who had quit within the last 6 months) and family history of myocardial infarction, coronary revascularisation, or sudden death in a first degree relative under the age of 56 years was also assessed. If participants had two or more risk factors, as specified by the ACSM (2009), written physician consent was obtained prior to exercise testing. All participants provided written informed consent, and had no prior experience with perceptual scaling prior to participation. The study was approved by the ethics committee of the School of Sport and Health Sciences at the University of Exeter, where all protocols and procedures were conducted.
Procedures
Each participant performed three laboratory-based exercise sessions with at least 48 h between each session. Two sessions consisted of a sub-maximal PRET (PRET 1 and PRET 2) from which data would be used in the prediction of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \). A third session consisted of a GXT to volitional exhaustion to directly measure \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \). Submaximal HR, RPE and \( \dot{V}{\text{O}}_{2} \) data were used for the prediction of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \).
For reasons of convenience and means of attracting active runners to the study, the first session comprised the GXT, followed by PRET 1 and 2. However, for the sedentary participants, the first session comprised the PRET 1, followed by PRET 2 and GXT. This order of testing was considered to be of particular importance for the study question in relation to the sedentary group, as indicated above. We also believed that this order of testing would be considerably more acceptable to the sedentary group in this study, as the initial exposure to the study did not involve a maximal exercise test and the potentially unpleasant sensation of maximal exertion. In this regard, it is notable that there was 100% compliance from all sedentary participants recruited to the study.
All sessions were completed using a motorised treadmill (Woodway, PPS 55 sport slat-belt treadmill, Germany). The display screen of the treadmill (time, distance, running velocity) was masked from the participant throughout each test. On-line respiratory gas analysis was used in each test via a breath-by-breath automatic gas exchange system (Cortex Metalyser II, Biophysik, Leipzig, Germany). Volume and gas calibration were performed in accordance with the manufacture’s guidelines. Heart rate was continuously monitored using a wireless chest strap telemetry system (Polar Electro T31, Kempele, Finland). All physiological outputs were concealed from the participant. The RPE scale was mounted on the wall directly in front of the participants throughout the study and each participant received standardized written and verbal instructions on how they should identify and report their ‘overall’ feelings of exertion (Borg 1998). During the maximal GXT, the RPE was recorded by periodically requesting the participant to point to a numerical value that reflected how strenuous the exercise felt. During the PRET, participants adjusted and maintained their exercise intensity to correspond with the specific RPEs prescribed by the investigator.
Perceptually regulated GXT
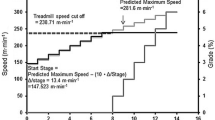
Each participant performed two PRETs on separate occasions. Each PRET consisted of four, 3-min stages of perceptually regulated exercise at intensities corresponding to RPEs of 9, 11, 13 and 15 according to the Borg 6-20 RPE Scale (Borg 1998). Following a warm-up, participants were instructed by the experimenter to exercise at a perceived effort of 9 on the RPE scale. With the treadmill gradient fixed at 1%, to parallel the suggested oxygen cost of running outside (Jones and Doust 1996), participants classed as active were free to change the speed of the treadmill to perceptually regulate to the desired RPE. Participants classed as sedentary were able to control both the speed and gradient, allowing those who did not feel capable of running to increase the gradient to perceptually regulate and achieve the higher intensity (RPE 15, hard/heavy) on the scale without having to run. At the end of the 3-min bout at RPE 9, participants adjusted the treadmill speed or gradient to a perceived effort of 11 on the RPE scale for a further 3 min. This process was repeated for RPE levels 13 and 15, respectively.
Graded exercise test (GXT) to \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)
A graded exercise test was performed by all participants to allow for the direct measurement of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \). For the active participants, the treadmill grade was set at 1% throughout all tests (Jones and Doust 1996). The test was continuous and incremental, commencing at 9.0 km h−1, increasing 1 km h−1 every 3 min. For the sedentary participants, the Balke-Ware protocol was followed. The treadmill speed was fixed at 5.3 km h−1 with the gradient commencing at 0% for the first minute and increased by 1% per minute thereafter. All tests were terminated when participants reported volitional exhaustion. In conjunction with this, a maximum effort was confirmed with a plateau in oxygen consumption, a HR within ±10 b min−1 of age-predicted maximum, an RER 1.15, or an inability to maintain the required running speed (Cooke 2009). During the final 30 s of each increment of the GXT, participants estimated their overall RPE using the Borg 6-20 RPE Scale (Borg 1998).
Data analysis
Prediction of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)
To predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \), two particular predictive methods were employed. One method, from the GXT, utilized the HR and \( \dot{V}{\text{O}}_{2} \) data recorded at the points where estimated RPEs were between 9 up to, and including 15, extrapolated to the individual’s age-predicted maximal heart rate [HRmaxpred; 206.9 – (age × 0.67), Gellish et al. 2007]. The second method was based on the individual relationship between RPE and \( \dot{V}{\text{O}}_{2} \) from the perceptually regulated graded exercise tests (PRET 1 and PRET 2). The mean \( \dot{V}{\text{O}}_{2} \) collated from the final 30 s of each RPE stage (9, 11, 13, 15) were regressed against the corresponding RPEs and extrapolated to an end-point RPE of 19 and 20 (to permit the comparison between the commonly reported maximum and the theoretical maximum).
Statistical analysis
A two-factor (group × method) repeated-measures ANOVA was used to compare actual \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) with the predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the HRmaxpred method in the GXT, in which the submaximal HR and \( \dot{V}{\text{O}}_{2} \) relationship, occurring within the 9–15 RPE range, was extrapolated to the individual’s age-predicted maximal heart rate (HRmaxpred). A three-factor [group (active, sedentary) × test (PRET 1, PRET 2) × RPE (9, 11, 13, 15)] ANOVA, with repeated measures on test and RPE was used to analyse differences in the absolute \( \dot{V}{\text{O}}_{2} \) and % \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) produced during the PRETs. A four-factor [group (active, sedentary) × test (PRET 1, PRET 2) × RPE range (9–13, 9–15) × RPE end-point (RPE 19, RPE 20)] ANOVA with repeated measures on test, range and RPE end-point, and four (PRET 1 RPE range 9–13, PRET 1 RPE range 9–15, PRET 2 RPE range 9–13, and PRET 2 RPE range 9–15) two-factor [group (active, sedentary) × method (measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \), \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from HRmaxpred, PRET RPE 19 end-point, PRET RPE 20 end-point)] ANOVA were used to examine differences in measured and predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from each of the methods used to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in the sedentary and active groups. Additionally, Bland and Altman’s (1986) 95% limits of agreement analysis (LoA) were used to quantify the agreement (bias and random error) between measured and predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) for each predictive method (PRET 1, PRET 2, HRmaxpred), group (active, sedentary), RPE range (9–13, 9–15) and extrapolation end-point (RPE 19, RPE 20). Intra-class correlation coefficients (ICC) were calculated via a two-way mixed effects model to assess reproducibility across trials and between measured and predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) values across the methods for each group (Ntoumanis 2001).
In all ANOVA, where assumptions of sphericity were violated, the critical value of F was adjusted by the Greenhouse–Geisser epsilon value from the Mauchly test of sphericity. Further, Alpha was set at 0.01 to reduce the risk of type 1 error. When necessary, statistically significant findings were followed up with pair-wise comparisons or nonorthogonal (repeated) planned comparisons to test the a priori hypotheses. All data were analyzed using the statistical package SPSS for Windows, PC software, version 17.
Results
GXT and \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) prediction by the HRmax method
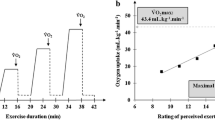
The maximal RPE reported in the GXT was similar for both active and sedentary participants (19.2 ± 0.6 cf. 19.3 ± 0.7, respectively). There was an expected significant main effect for group (F (1,73) = 101.4, p < 0.01, η2 = 0.58), with \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) significantly higher in the active participants (53.9 ± 7.5 cf. 34.4 ± 7.0 ml kg−1 min−1), and no main effect for method, indicating no difference between the measured and predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) values from the HRmaxpred method. The relationship between the measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) estimated from the heart rate relationship observed for the submaximal RPE range in the GXT is illustrated in Fig. 1a. There was no method by group interaction on \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \).
a Predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) using the HR: \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) relationship between the RPE range 9–15 in the GXT extrapolated to age-predicted maximal heart rate (HRmaxpred); b, c Scatter plot to show the positive correlations between measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the RPE range 9–15 extrapolated to RPE 19 for PRET 1 and PRET 2, respectively
Comparison of absolute and relative oxygen uptake during the perceptually regulated protocol
-
1.
\( \dot{V}{\text{O}}_{2} \): The three-factor (group × test × RPE) ANOVA with repeated measures on test and RPE resulted in a significant main effect for group (F (1,73) = 350.6, p < 0.01, η2 = 0.83) and a main effect for RPE (F (3, 116.9) = 513.1, p < 0.01, η2 = 0.88). \( \dot{V}{\text{O}}_{2} \) was higher at each RPE increment in the active participants, and pairwise comparisons revealed a significant (p < 0.01) increase in the \( \dot{V}{\text{O}}_{2} \) with each successive RPE level from 9 to 15. The \( \dot{V}{\text{O}}_{2} \) values were highly consistent between tests (F (1,73) = 0.41, p > 0.05), Table 1 presents the \( \dot{V}{\text{O}}_{2} \) values.
Table 1 Mean (±SD) oxygen uptake values (ml kg−1 min−1) at each RPE level for active (n = 49) and sedentary (n = 26) participants across both PRET trials -
2.
% \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \): Repeated-measures ANOVA revealed a significant main effect for group (F (1,73) = 86.2, p < 0.01, η2 = 0.54), a main effect for RPE (F (2.2,159.3) = 703.4, p < 0.01, η2 = 0.91) and a significant interaction for RPE × group (F (2.2,159.3) = 26.6, p < 0.01, η2 = 0.28). The percentage of the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) at which the subjects worked at each RPE increment was higher in the active group and post hoc analyses revealed a significant (p < 0.01) increase in the % \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) produced with each successive RPE level from 9 to 15. The interaction was due to the sedentary group commencing at a lower relative % \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) [mean difference (MD) >18.9%], but by RPE 15 this difference was significantly reduced (MD <8.7%). Table 1 presents the % \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) values.
Ability of the PRET to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)
A four-factor [group (active, sedentary) × test (PRET 1, PRET 2) × RPE range (9–13, 9–15) × RPE extrapolation end-point (RPE 19, RPE 20)] ANOVA revealed an expected significant main effect for group (F (1,73) = 102.2, p < 0.01, η2 = 0.58). Predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) was higher (p < 0.01) for the active group. There was also a significant main effect for RPE range (F (1,73) = 29.7, p < 0.01, η2 = 0.29) and extrapolation end-point (F (1,73) = 604.2, p < 0.01, η2 = 0.89) on predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and significant interactions for RPE group × range (F (1,73) = 29.7, p < 0.01, η2 = 0.09) and range × extrapolation end point (F (1,73) = 29.2, p < 0.01, η2 = 0.29). Values for predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) were higher in the active group, higher for the 9–15 RPE range and higher when extrapolated to an end-point RPE 20. The interactions were due to a significant difference in the predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) for the RPE 9–13 and 9–15 range in the sedentary group, but no difference in the active group’s values, and a greater difference in the predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) between the 9–13 and 9–15 range from the RPE 20 extrapolation than the RPE 19 extrapolation. Figure 1b and c shows the relationship between the measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) estimated from PRETS 1 and 2, respectively.
The two-factor (method × group) ANOVAs resulted in significant main effects for group (F (1,73) = 118.5, p < 0.01, η2 = 0.62; F (1,73) = 117.3, p < 0.01, η2 = 0.61; F (1,73) = 108, p < 0.01, η2 = 0.60; and F (1,73) = 98.7, p < 0.01, η2 = 0.58) for the PRET 1 and PRET 2 RPE range 9–13 and 9–15, respectively, with \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) higher in the active participants. Significant main effects for method (F (1.51, 110.4) = 9.37, p < 0.01, η2 = 0.11 and F (1.50, 109.7) = 5.83, p < 0.01, η2 = 0.07) were recorded for PRET 1 and PRET 2 RPE range 9–15. The significant main effects were due to higher predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in the RPE 20 extrapolation. The group × method interactions were not significant. Table 2 displays the mean differences between the direct measurement and each of the predictive methods.
Level of agreement between measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and predictive methods
Table 3 describes the correlation and LoA between measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) and each predictive technique. For the active participants, predictions of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) were superior (higher r and narrower LoA) when predicting from a RPE range 9–15 and extrapolating to RPE 19 in the PRET 1. For the sedentary participants, the predictions of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) were better when using either the estimation procedure (HRmaxpred) or the RPE range 9–15 when extrapolating to the end-point RPE 19 (see Table 3). The intraclass reliability of each of the PRET methods for predicting \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) for the active and sedentary participants is shown in Table 4.
Discussion
The study shows that the heart rates produced during a graded exercise test within the RPE range of 9–15 can be used to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in both active and sedentary individuals when the heart rate:oxygen uptake relationship is extrapolated to the age-predicted maximal heart rate. The results further confirm the usefulness of recording the RPE during graded exercise testing and provide support for an additional and valid means of estimating the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from submaximal treadmill testing when the maximum exertion is limited to an RPE 15, rather than a specific work rate or heart rate value in healthy participants.
Previous studies have yielded encouraging findings for the validity and reliability of using PRET to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) (Al-Rahamneh and Eston 2011; Eston et al. 2005, 2006, 2008; Faulkner et al. 2007, Morris et al. 2009, 2010), based on the strong linear relationship between the \( \dot{V}{\text{O}}_{2} \) produced at each perceptually regulated RPE. However, only two of the above studies included sedentary individuals (Eston et al. 2008; Faulkner et al. 2007) and both studies used cycling as the form of ergometry. Furthermore, extrapolations from the RPE 9–15 range significantly underestimated \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) with the prediction of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) being accurate only when extrapolated from intensities extending to RPE 17 (very, very hard). As indicated previously, exercise at this intensity may be problematic for sedentary individuals.
Studies have shown that familiarization through an initial PRET is important when using cycling as the mode of perceptually regulated exercise in sedentary individuals. Eston et al. (2008) observed that the accuracy of predicting \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) improved by the second trial. Similarly, Faulkner et al. (2007) observed greater variability in power output across three trials at PRET levels of RPE 13 and 15 for the sedentary participants in their cycling study. This may be partially attributable to the relative lack of familiarity with cycling as a mode of exercise and the difference in the way the exercise intensity is perceived. For cycling, localised perception of exertion around the leg muscles tends to dominate the overall perceived exertion response. Consequently, the strength of the perceptual signal of exertion appears to be greater for a given work rate for cycling compared to treadmill exercise. In this regard, studies have observed a higher RPE for a given relative exercise intensity, either as a proportion of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) (Eston and Williams 1988) or respiratory compensation threshold (Bolgar et al. 2010; Green et al. 2003).
The study shows that, using a more familiar mode of exercise, it is possible to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) in sedentary individuals from a lower perceptually regulated range of intensities on a treadmill. The similarity of the predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) scores between trials 1 and 2 show that this is true even when there has been little familiarization with the procedure. This is the first study of its kind to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) using the PRET protocol prior to application of a maximal perceptual anchor in sedentary participants. The two previous studies (Eston et al. 2008; Faulkner et al. 2007) directly measured \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) prior to predicting it from PRET. This difference in the former studies and the current study is important as it obviates the necessity to educate subjects to the degree of exertion prior to a trial that allows the prediction of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) without having to go that degree of exertion.
In line with previous findings, we observed a highly significant (p < 0.01) increase in the \( \dot{V}{\text{O}}_{2} \) during the PRET protocol with each successive RPE level from 9, 11, 13 and 15 in both the active and sedentary participants. However, contrary to expectation, the oxygen uptake values at the RPE increments during PRET 1 for the sedentary subjects were not lower than those recorded during PRET 2. This was somewhat surprising as previous research has identified the importance of protocol familiarization when using RPE in a production mode (Buckley et al. 2000; Eston et al. 2000; Eston and Williams 1988). This has also been observed in studies using PRETs to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \), with greater predictive accuracy observed following an initial perceptually regulated GXT (Eston et al. 2005, 2008; Faulkner et al. 2007; Morris et al. 2009, 2010). It appears that the sedentary participants adapted quite readily to the treadmill task and did not overestimate their effort production in the initial PRET, as previously observed. The high reliability of predicted \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) between the PRET tests is shown in Table 4. A possible explanation for the consistency of the prediction of the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) (as well as the accuracy) may be attributable to the mode of exercise employed in this study. With the exception of the study by Morris et al. (2010), all studies adopting a PRET protocol have used cycling ergometry, which is a less familiar form of exercise for some participants, compared to walking and running.
Also, as observed previously for active and sedentary participants during cycle ergometry (Eston et al. 2008; Faulkner et al. 2007), the increase in energy expenditure between the RPE-regulated increments in exercise intensity was between 1 and 2 METS for both groups of participants during treadmill exercise. This is worthy of note as it complies with the recommendations for increases in exercise intensity for standard sub-maximal graded exercise tests in healthy individuals (ACSM 2009).
Whilst the \( \dot{V}{\text{O}}_{2} \) produced at each RPE level was generally consistent across PRET trials 1 and 2 for both active and sedentary participants (p > 0.05), it is interesting to note that the perceptually regulated exercise intensity for the sedentary participants equated to a lower (p < 0.01) percentage of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \), indicating a lower perceived tolerance for a given exercise intensity. This could be due to a lack of experience and habituation to the exercise protocol, and a possible adoption of a more conservative approach. However, it is more likely attributable to the location of the ventilatory threshold, which is noted to occur at a higher proportion of the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) with training (Hill et al. 1987). They reported that although the RPE at the VT was not altered, training alters the relationship between the RPE and % \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) with trained participants reporting a lower RPE at a given % \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) compared to untrained participants. The findings from the present study are consistent with this observation.
In this study, the RPE and \( \dot{V}{\text{O}}_{2} \) values were extrapolated to RPE 19 (commonly reported maximum) and RPE 20 (the theoretical maximum). A number of studies have recognized that at volitional exhaustion, RPE 20 is infrequently reported (ACSM 2009; Eston et al. 2008; Faulkner et al. 2007; Kay et al. 2001; St Clair Gibson et al. 1999). On the basis that individual subjects reach their subjective limit of fatigue at an RPE of 18–19 (‘extremely hard’) on the Borg 6-20 scale, studies have examined the validity of using the RPE 19 as the end-point for predicting \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \). Morris et al. (2010) reported that all 18 participants reported a terminal RPE of ≈19 in their GXT, and that compared to extrapolations of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) to RPE 20, those using RPE 19 as the end-point generated smallest biases for the first two PRET trials. Faulkner et al. (2007) also reported similar observations in their third PRET trial. Indeed, in the present study, it is notable that the maximal RPE attained in the GXT was circa 19 for both groups of participants. We also noted that use of the most accurate RPE range (9–15) extrapolated to RPE 19 elicited the most accurate predictions of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) for both active and sedentary participants. It is worth noting, however, that when using the RPE range 9–13, the \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) was significantly underpredicted for the sedentary participants when extrapolated to RPE 19. Therefore, in cases where exercise intensities above RPE 13 are not advocated, extrapolation to RPE 20 is recommended to provide a closer prediction of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \). Further research on the accuracy of the extrapolation method (RPE 19, RPE 20) is warranted.
To conclude, this study has demonstrated accurate predictions of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) from the use of heart rate within the range 9–15 and from perceptually regulated GXTs in active and sedentary participants, during treadmill exercise. Specifically, use of the perceptual range of RPE 9–15 and extrapolation to RPE 19 provided the most accurate estimate of \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) for the active and sedentary participants. This suggests that the use of a PRET to predict \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) offers a practical alternative in circumstances where measures of HR are considered unreliable (i.e. environmental or medical). Furthermore, the application of a PRET protocol that terminates at lower intensity (RPE 15) permits a level of autonomy, allowing the participant to dictate the pace, intensity and increments in intensity during the exercise test with the prior knowledge that the PRET will terminate when the intensity is perceived to be ‘hard’ (RPE 15). In the present study, this equated to an exercise intensity of about 80 and 70% of the maximal aerobic capacity in the active and sedentary participants, respectively.
This study has shown that the PRET procedure is a valid means of producing consistent, acceptable and recommended increments in intensity in active and sedentary populations during treadmill exercise and further confirms that the individual linear relationship of perceived exertion and intensity can be used to predict maximal functional capacity in young and middle to older-aged individuals. The PRET method is particularly appropriate when maximal exercise testing is not recommended or desired, such as in routine assessments of fitness, or when maximal exercise testing is contraindicated or simply not desirable. We believe the PRET method has viable potential for use in the clinical populations, although this remains to be determined. Future studies are therefore recommended to assess the efficacy of the PRET method in the clinical setting.
References
Al-Rahamneh HQ, Eston RG (2011) Prediction of maximal oxygen uptake from the ratings of perceived exertion during a graded and ramp exercise test in able-bodied and persons with paraplegia. Arch Phys Med Rehabil 92:277–283
American College of Sports Medicine (2009) ACSM’s guidelines for exercise testing and prescription, 8th edn. Lippincott Williams & Wilkins, Philadelphia
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet I:307–310
Bolgar MA, Baker CE, Goss FL, Nagle E, Robertson RJ (2010) Effect of exercise intensity on differentiated and undifferentiated ratings of perceived exertion during cycling and treadmill exercise in recreationally active and trained women. J Sports Sci Med 9:557–563
Borg G (1998) Borg’s perceived exertion and pain scales. Human Kinetics, Champaign, p 15
Borg G, Linderholm H (1970) Exercise performance and perceived exertion in patients with coronary insufficiency, arterial hypertension and vasoregulatory asthenia. Acta Medica Scandinavica 187:17–26
Buckley JP, Eston RG, Sim J (2000) Ratings of perceived exertion in braille: validity and reliability in production mode. Br J Sports Med 34:297–302
Buckley J, Sim J, Eston RG (2009) Reproducibility of ratings of perceived exertion soon after myocardial infarction: responses in the stress-testing clinic and the rehabilitation gymnasium. Ergonomics 52:421–427
Chen MJ, Fan X, Moe ST (2002) Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta analysis. J Sports Sci 20:873–899
Chief Medical Officer (2004) At least five a week: evidence on the impact of physical activity and its relationship to health. Department of Health, London
Cooke CB (2009) Maximal oxygen uptake, economy and efficiency. In: Eston RG, Reilly T (eds) Kinanthropometry and exercise physiology laboratory manual: tests, procedures and data: exercise physiology, vol 2. Routledge, London, pp 147–173
Coquart JBJ, Lemaire C, Dubart AE, Douillard C, Luttenbacher DP, Wibaux F, Garcin M (2009) Prediction of peak oxygen uptake from sub-maximal ratings of perceived exertion elicited during a graded exercise test in obese women. Psychophysiology 46:1150–1153
Coquart JBJ, Eston RG, Grosbois J-M, Lemaire C, Dubart AE, Luttenbacher DP, Garcin M (2010) Prediction of peak oxygen uptake from age and power output at RPE 15 in obese women. Eur J Appl Physiol 110:645–649
Deci EL, Ryan RM (1985) Intrinsic motivation and self-determination in human behavior. Plenum, New York
Dunbar CC, Bursztyn DA (1996) The slope method for prescribing exercise with ratings of perceived exertion (RPE). Percept Mot Skills 83:91–97
Dunbar CC, Robertson RJ, Baun R, Blandin MF, Metz RK, Burdett R, Goss FL (1992) Validity and regulating exercise intensity by ratings of perceived exertion. Med Sci Sports Exerc 24:94–99
Eston RG, Connolly D (1996) Use of ratings of perceived exertion for exercise prescription in patients receiving beta-blocker therapy. Sports Med 21:176–190
Eston RG, Thompson M (1997) Use of ratings of perceived exertion for prediction of maximal exercise levels and exercise prescription in patients receiving atenolol. Br J Sports Med 31:114–119
Eston R, Williams JG (1988) Reliability of ratings of perceived exertion for regulation of exercise intensity. Br J Sports Med 22:153–154
Eston RG, Davies BL, Williams JG (1987) Use of perceived effort ratings to control exercise intensity in young healthy adults. Eur J Appl Physiol 56:222–224
Eston RG, Parfitt CG, Campbell L, Lamb KL (2000) Reliability of effort perception for regulating exercise intensity in children using the Cart and Load Effort Rating (CALER) Scale. Pediatr Exerc Sci 12:388–397
Eston RG, Lamb KL, Parfitt G, King N (2005) The validity of predicting maximal oxygen uptake from a perceptually-regulated graded exercise test. Eur J Appl Physiol 94:221–227
Eston RG, Faulkner JA, Mason E, Parfitt G (2006) The validity of predicting maximal oxygen uptake from perceptually-regulated exercise tests of different durations. Eur J Appl Physiol 97:535–541
Eston R, Lambrick D, Sheppard K, Parfitt G (2008) Prediction of maximal oxygen uptake in sedentary males and from a perceptually regulated, sub-maximal graded exercise test. J Sports Sci 26:131–139
Faulkner J, Eston R (2007) Overall and peripheral ratings of perceived exertion during a graded exercise test to volitional exhaustion in individuals of high and low fitness. Eur J Appl Physiol 101:613–620
Faulkner J, Parfitt G, Eston R (2007) Prediction of maximal oxygen uptake from the ratings of perceived exertion and heart rate during a perceptually-regulated sub-maximal exercise test in active and sedentary participants. Eur J Appl Physiol 101:397–407
Faulkner J, Lambrick D, Parfitt G, Rowlands A, Eston R (2009) Prediction of maximal oxygen uptake from the Astrand Rhyming nomogram and ratings of perceived exertion. In: Reilly T, Atkinson G (eds) Contemporary sport. Leisure and ergonomics, Routledge, pp 197–214
Galloway SDR, Maughan RJ (1997) Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29:1240–1249
Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK (2007) Longitudinal modelling of the relationship between age and maximal heart rate. Med Sci Sports Exerc 39:822–829
Glass SC, Knowlton RG, Becque MD (1992) Accuracy of RPE from graded exercise to establish exercise training intensity. Med Sci Sports Exerc 24:1303–1307
Green JM, Crews TR, Bosak AM, Peveler WW (2003) Overall and differentiated ratings of perceived exertion at the respiratory compensation threshold: effects of gender and mode. Eur J Appl Physiol 89:445–450
Hill DW, Cureton KJ, Grisham SC, Collins MA (1987) Effect of training on the rating of perceived exertion at the ventilatory threshold. Eur J Appl Physiol Occup Physiol 56:206–211
Jones AM, Doust JH (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14(4):321–327
Kay D, Marino FE, Cannon J, St Clair Gibson A, Lambert MI, Noakes TD (2001) Evidence for neuromuscular fatigue during cycling in warm humid conditions. Eur J Appl Physiol 84:115–121
Lambrick DM, Faulkner JA, Rowlands AV, Eston RG (2009) Prediction of maximal oxygen uptake from submaximal ratings of perceived exertion and heart rate during a continuous exercise test: the efficacy of RPE 13. Eur J Appl Physiol 107:1–9
Ljunggren G, Johansson SE (1988) Use of submaximal measures of perceived exertion during bicycle ergometer exercise as predictors of maximal work capacity. J Sports Sci 6:189–203
Morris M, Lamb KL, Cotterrell D, Buckley J (2009) Predicting maximal oxygen uptake via a perceptually regulated exercise test (PRET). J Exerc Sci Fit 7:122–128
Morris M, Lamb KL, Hayton J, Cotterrell D, Buckley J (2010) The validity and reliability of predicting maximal oxygen uptake from a treadmill-based sub-maximal perceptually regulated exercise test. Eur J Appl Physiol 109:983–988
Noonan V, Dean E (2000) Submaximal exercise testing: clinical application and interpretation. Phys Ther 80:782–807
Ntoumanis N (2001) A step by step guide to SPSS for sport and exercise studies. Routledge, London pp 146–149
Okura T, Tanaka K (2001) A unique method for predicting cardiorespiratory fitness using rating of perceived exertion. J Physiol Anthropol Appl Hum Sci 20:255–261
Palatini P, Benetos A, Julius S (2006) Impact of increased heart rate on clinical outcomes in hypertension: implications for antihypertensive drug therapy. Drugs 66:133–144
Parfitt G, Eston RG (1995) Changes in ratings of perceived exertion and psychological affect in the early stages of exercise. Percept Mot Skills 80:259–266
Parfitt G, Eston R, Connolly D (1996) Psychological affect at different ratings of perceived exertion in high- and low-active women: a study using a production protocol. Percept Mot Skills 82:1035–1042
Scherer S, Cassady SL (1999) Rating of perceived exertion: development and clinical applications for physical therapy exercise testing and prescription. Cardiopulm Phys Ther J 10:143–147
Smutok MA, Skrinar GS, Pandolf KB (1980) Exercise intensity: subjective regulation by perceived exertion. Arch Phys Med Rehab 61:569–574
St Clair Gibson A, Lambert MI, Hawley JA, Broomhead SA, Noakes TD (1999) Measurement of maximal oxygen uptake from two different laboratory protocols in runners and squash players. Med Sci Sports Exerc 31:1226–1229
Stuart RJ, Ellestad MH (1980) National survey of exercise stress testing facilities. Chest 77:94–97
Van Baak MA (1988) Beta-adrenoreceptor blockade and exercise: an update. Sports Med 5:209–225
Whaley MH, Brubaker PH, Kaminski LA, Miller CR (1997) Validity of rating of perceived exertion during graded exercise testing in apparently healthy adults and cardiac patients. J Cardiopulm Rehabil 17:261–267
Williams JG, Eston RG (1989) Determination of the intensity dimension in vigorous exercise programmes with particular reference to the use of the rating of perceived exertion. Sports Med 8:177–189
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David C. Poole.
Rights and permissions
About this article
Cite this article
Eston, R., Evans, H., Faulkner, J. et al. A perceptually regulated, graded exercise test predicts peak oxygen uptake during treadmill exercise in active and sedentary participants. Eur J Appl Physiol 112, 3459–3468 (2012). https://doi.org/10.1007/s00421-012-2326-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2326-8