Abstract
Postmenopausal osteoporosis is associated with high level of adipogenesis within the bone marrow at the expense of osteoblast population. The mechanical effect on β-catenin through phosphorylation of glycogen synthase kinase-3β (GSK-3β) is critical for inhibition of adipogenesis in mesenchymal stem cells in vitro. In present study, we hypothesized that treadmill training could regulate the β-catenin signaling through phosphorylation of GSK-3β in the lumbar vertebrae of ovariectomized (OVX) rats. 3-month-old female Sprague–Dawley rats were divided randomly into the following four groups: (a) Sham, (b) OVX, (c) OVX exercised (EX), and (d) OVX estrogen replacement (E2). At the end of the experiment, the serum levels of estradiol (E2) and luteinizing hormone (LH), the ultimate lumbar vertebra strength, as well as the protein expression for peroxisome proliferators-activated receptor γ (PPARγ), β-catenin, P-GSK-3β, and osterix (Osx) in lumbar vertebrae were analyzed. Moreover, the protein expression for β-catenin and P-GSK-3β were also examined in the uterus. The EX group had lower protein level of PPARγ, higher ultimate lumbar vertebral strength, and higher protein levels of β-catenin, and P-GSK-3β in lumbar vertebral bodies compared with sedentary OVX group. The effects of EX treatment on the protein levels of β-catenin and P-GSK-3β in bones were not reproducible in the uterus. Moreover, exercise treatment produced no estrogenic effect as evidenced by serum level of LH. In conclusion, this study suggested that treadmill training could activate the GSK-3β/β-catenin signaling and inhibit the production of PPARγ in lumbar vertebrae of OVX rats, which may contribute to the prevention of bone loss in OVX rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Menopause is an important physical transitional period for women, and osteoporosis is a serious healthy problem faced by them after menopause (Sipos et al. 2009). Currently available treatment of postmenopausal osteoporosis is largely dependent on pharmaceutical therapy; however, the undesired adverse effects of drug treatment have already led patients to refuse such medication (Sewerynek 2011). In comparison, physical exercise, one of the natural, effective, non-pharmacological procedures, has received increasing attention and ratification in treating postmenopausal osteoporosis over the recent years. However, the exact mechanism by which physical exercise prevent osteoporosis is incompletely known (Pigozzi et al. 2009; Schmitt et al. 2009).
Recently, it has been reported that osteoporosis in postmenopausal women (Syed et al. 2008) or ovariectomized (OVX) rats (Chen et al. 2011; Kurabayashi et al. 2001; Siddiqui et al. 2010) is associated with increased bone marrow adipose tissue, leading to the formation of adipocytes at the expense of osteoblasts. Osteoblasts and bone marrow adipocytes are derived from common bone multipotential mesenchymal stem cell (BMSC) progenitors (Rosen et al. 2009). And it has been reported that mechanical loading could down-regulate peroxisome proliferators-activated receptor gamma (PPARγ) in BMSCs and favor osteoblastogenesis at the expense of adipogenesis (David et al. 2007). β-Catenin is known to regulate adipogenesis at multiple loci, including attenuation of PPARγ expression (Ross et al. 2000). Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable β-catenin signal (Sen et al. 2008). Furthermore, mechanical load is reported to activate β-catenin signal through inhibition of GSK3β, an enzyme that is inhibited via phosphorylation and that limits β-catenin degradation (Sen et al. 2009), providing further evidence that mechanical load could utilize GSK-3β/β-catenin signaling in vitro.
It has been reported that osterix (Osx, or Sp7), a novel zinc finger-containing transcription factor, is required for osteoblast differentiation and bone formation (Nakashima et al. 2002), functioning as a molecular link between mechanostressing and osteogenic differentiation (Fan et al. 2007). In vitro study has indicated that mechanical strain increased Osx expression in an immortalized pre-osteoblast cell by nearly two-folds as measured by RT-PCR (Fan et al. 2006). However, whether the anabolic effect of treadmill training on trabecular bone of OVX rats is related to the Osx expression has not been studied.
Our previous paper has indicated that treadmill training inhibited marrow adipogenesis of trabecular bone in OVX rats by down-regulating PPARγ (Chen et al. 2011). Herein, we hypothesized that treadmill training may regulate the β-catenin signaling through inhibition of GSK-3β in the lumbar vertebrae of OVX rats. Therefore, the present paper studied the effects of treadmill exercise on the protein expression of PPARγ, β-catenin, phosphorylated glycogen synthase kinase-3β (P-GSK-3β), and Osx in lumbar vertebral bodies of OVX rats and compared them to the effects of estrogen replacement. In addition, we also investigated the effects of treadmill training on the serum level of luteinizing hormone, (LH) as well as the protein expression of β-catenin and P-GSK-3β in the uterus as to role out the estrogenic effects of treadmill training in OVX rats (Seidlova-Wuttke et al. 2010).
Materials and methods
Animals and treatments
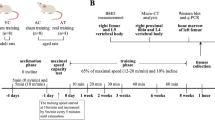
12-week-old female Sprague–Dawley rats weighing 235 ± 8 g were supplied by Animal Department of Peking University, and were given regular rat chow and water ad libitum. The housing unit was maintained at 23 ± 2°C with a reversed 12/12 h light–dark cycle. Animal procedures were approved by the Capital Institute of Physical Education and were reviewed by an animal Ethics Review Board that followed the appropriate guidelines as outlined by the European Journal of Applied Physiology. Rats were either sham or ovariectomy operated at 7 days after their arrival and randomly assigned to four groups according to their body weight: (a) sham operated (Sham, n = 10), (b) OVX control (OVX, n = 10), (c) OVX given 17β-estradiol (E2, n = 10), and (d) OVX undergoing treadmill exercise training (EX, n = 10).
14 days after the surgery operation, rats in E2 group were subcutaneously injected with 17β-estradiol (Sigma Chemical, St Louis, MO) at a dose of 25 μg/kg body weight 3 days per week for 14 weeks. 7 days after the surgery operation, rats in EX group were firstly accustomed to exercise by running at low exercise intensity (15 m/min at 0% grade for 15–40 min/day) for 1 week and then were regularly trained at a speed of 18 m/min for 40 min 4 days per week for 14 weeks. The experimental protocol is seen in our previous paper (Chen et al. 2011). Body weight was measured once per week and the injection dose of E2 was adjusted according to the body weight. The rats were treated at the same time of day.
Tissue collection
Rats were killed 24–36 h after the last exercise or E2 treatment. The food was removed from the animals’ cage 12 h before killing. After having anesthetized with a solution of 10% chloral hydrate at a dose of 3 ml/kg body weight, blood samples were collected from the abdominal aorta and serum was separated by centrifugation at 1,500g for 20 min at 4°C. Aliquots of serum were frozen and kept at −20°C for later analyses. Immediately after completion of blood collection, the uterus and viscera fat of each rat were isolated and weighed. The left uterine horns were fixed in 4% formaldehyde in phosphate buffer (pH 7.4) for immunohistochemistry and the right uterine horns were frozen in liquid and stored in −80°C for protein exaction. Then the lumbar vertebrae were excised and cleared of soft tissues. The fifth lumbar vertebrae (L5) were wrapped in saline-moistened gauze and frozen up to −70°C for bone mineral density (BMD) and biomechanics measurement. The third lumbar vertebrae (L3) were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4 overnight at room temperature for immunohistochemistry. The fourth lumbar vertebrae (L4) were frozen in liquid nitrogen for protein exaction.
Serum E2 and LH
Serum E2 and LH concentrations were assayed by radioimmunoassay (RIA). The kit for E2 was offered and manufactured by China Institute of Atomic Energy (Beijing, China). The kit for LH was offered and manufactured by North Biotechnology Research Institute (Beijing, China). All samples were run in the same assay. The intra- and inter-assay coefficients of variation for E2 were 4.8 and 10.0%, respectively. The intra- and inter-assay coefficients of variation for LH were 5.4 and 10.2%, respectively.
BMD measurement
Bone mineral density measurement of isolated L5 was performed by dual energy X-ray absorptiometry (DEXA) (XR-36, Norland Company, USA) using manufacture-provided high-resolution software for small animals as in our previous study (Chen et al. 2011).
Mechanical properties
Mechanical properties of L5 were determined after BMD measurement. Lumbar vertebral bodies were tested in axial compression with a Materials Testing System QT-10 (MTS Crop. Minneapolis, MN). Before testing, the cephalic and caudal endplates were removed using a diamond saw to provide flat and parallel surfaces for loading. During testing, force and displacement measurements were collected every 0.02 s from which ultimate force was derived.
Immunohistochemistry
Immunohistochemistry was performed according to our previous report (Chen et al. 2011). In brief, L3 were deparaffinized, rehydrated, and rinsed with PBS. Then they were treated with 0.1% trypsin for 15 min at 37°C for antigen retrieval. After washing in PBS containing 0.1% BSA, non-specific binding was blocked in 10% normal animal serum in PBS for 1 h. Then the sections were incubated with a diluted mouse monoclonal β-catenin antibody (SC-7963; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit polyclonal osterix antibody (ab22552, Abcam) overnight at 4°C in a humidified chamber. The antibodies were diluted 1:100 in PBS. After incubation with the primary antibodies, the tissues were washed with PBS and were subsequently incubated with biotin-conjugated secondary antibodies (Vector, 1:200 dilution in PBS) for 1 h at 37°C. The sections were washed in PBS and incubated with horseradish peroxidase streptavidin for another 1 h at 37°C. After rinsing in PBS, the primary antibody binding was visualized with a diaminobenzidine (DAB) kit (Vector) according to the manufacturer’s instructions. The tissues were then washed with water. Counterstaining was done with hematoxylin. To ensure antigenic specificity, parallel experiments were performed using animal preimmune IgG as a negative control.
Protein extraction
Protein lysates of L4 and uterine horns were prepared using Mammalian Cell Lysis Kit (MCL-1, Sigma, USA) as described previously (Narayana Murthy et al. 2006). In brief, two or three lumbar bodies or uterine horns obtained from same group were homogenized on ice in RIPA buffer containing 50 mM Tris–HCL, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholic acid and 1% lgepal along with protease inhibitor cocktail (P8340; Sigma) provided with the kit. The tissue homogenates were placed on ice for 60 min and centrifuged at 12,000×g at 4°C for 30 min. The supernatants were collected and protein content of the tissue lysates was estimated by BCA methods.
Western blot analysis
Western blot was also performed according to our previous report (Chen et al. 2011). In brief, about 20 μg proteins from each sample were loaded onto 10% SDS-PAGE and transferred onto a PVDF membrane (Millipore). Membranes were then incubated in 5% non-fat dry milk in TBS for 1 h. The primary antibody for β-catenin (similar with immunohistochemistry), P-GSK-3β (#9336S, Cell signaling Technology), PPARγ antibody (SC-7273, Santa Cruz, CA, USA), osterix (similar with immunohistochemistry), and β-actin (SC-1616R, Santa Cruz, CA, USA) were incubated with the membrane overnight at 4°C. The secondary antibody, alkaline phosphatase–conjugated IgG (Vector), was diluted to 1:1,000 and incubated with the membrane for 2 h at room temperature. After the last washing step, NBT–BCIP (Zymed, USA) detection was carried out according to the manufacturer’s instructions.
Statistical analysis
All data were presented as mean ± standard deviation (SD). The statistical analysis was performed using one-way analysis of variance (ANOVA), and significant differences among groups were defined by a P < 0.05. Three independent experiments were performed for calculating the intensities of the western blot bands.
Results
Body weight and viscera fat weight
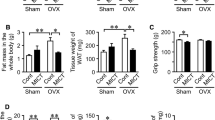
At the end of the week 1 post-OVX, there was no significant difference in body weight among the four groups. From week 2 post-OVX to the end of the experiment, the body weight in the OVX group was significantly higher than that of in the Sham group, as expected. From week 4 or week 3 post-OVX to the end of the experiment, the body weight in the EX group or E2 group was significantly decreased compared to the OVX group, respectively (Fig. 1a).
In agreement with the body-weight changes, an increase in visceral fat weight and the ratio of visceral weight to body weight was observed in OVX animals compared with Sham rats, which was suppressed by intervention of exercise training or estrogen replacement (Fig. 1b, c).
The serum levels of E2 and LH
The serum level of E2 was lower in rats of OVX group than in rats of the other three groups (Fig 2a). The serum level of LH was higher in rats of OVX group than in rats of Sham and E2 group. Exercise treatment had no effect on serum level of LH (Fig 2b).
Biomechanics
As shown in Fig. 3, the ultimate L5 strength was significantly lower in OVX group compared with the other three groups.
PPARγ protein expression in lumbar vertebrae
Western blot results showed that the value of PPARγ/β-actin in lumbar vertebrae of OVX group was increased significantly compared with Sham group. This change was reversed by both EX treatment and E2 treatment (Fig. 4).
Exercise and E2 induced changes in PPARγ protein expression in lumbar vertebrae samples detected by western blot. a The western blotting results. β-Actin was used as loading control. b Quantitative analysis of PPARγ protein expression in four groups. Values are means ± SD. OVX significantly increased the protein expression of PPARγ and this change could be reversed by either exercise treatment or E2 replacement treatment. *P < 0.05, **P < 0.01 versus Sham group; # P < 0.05, ## P < 0.01 versus OVX group
β-Catenin and P-GSK-3β protein expression in lumbar vertebrae
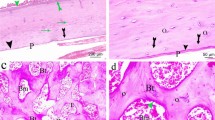
Immunohistochemistry results indicated that β-catenin positive staining was mainly in nucleus and cytoplasm of bone marrow cells and osteocytes in Sham group. OVX treatment inhibited the brown staining for β-catenin and this inhibition could be activated by both EX treatment and E2 treatment. No immunoreactivity staining of β-catenin was visible in negative control without primary antibodies (Fig. 5). Western blot results showed the same change tendency as observed by immunohistochemistry (Fig. 6a, b).
The protein expression of β-catenin in lumbar vertebrae detected by immunohistochemistry. Specimens were observed at magnification ×100. Positive staining for β-catenin was mainly localized in bone marrow cells and osteocytes. No staining for β-catenin was found in negative control without primary antibodies
Exercise and E2-induced changes of β-catenin and P-GSK-3β protein expression in lumbar vertebrae samples detected by western blot. a The western blotting results. β-Actin was used as loading control. b The relative levels of β-catenin and P-GSK-3β protein expression in four groups. Values are means ± SD. OVX significantly decreased the protein expression of β-catenin and P-GSK-3β and these changes could be reversed by both exercise treatment and E2 replacement treatment. *P < 0.05 versus Sham group; # P < 0.05, ## P < 0.01 versus OVX group
In order to test whether the activation of β-catenin was associated with inhibition of GSK-3β through GSK-3β phosphorylation, we further examined the effect of EX treatment on protein expression of P-GSK-3β by western blot. The results indicated that the value of P-GSK-3β/β-actin in lumbar vertebrae of OVX group was decreased significantly compared with Sham group. This change was reversed by both EX treatment and E2 treatment (Fig. 6a, c).
Osx protein expression in lumbar vertebrae
There was no difference in the protein expression of Osx in lumbar vertebrae among the four groups tested by both immunohistochemistry and western blot results (Fig. 7).
The protein expression of osterix in lumbar vertebrae detected by both immunohistochemistry and western blotting. a Specimens were observed at magnification ×100. Positive staining for osterix was mainly localized in bone marrow cells and osteocytes. No staining for osterix was found in negative control without primary antibodies. b The western blotting results. β-Actin was used as loading control. c The relative levels of osterix protein expression in four groups. Values are means ± SD
GSK3β/β-catenin signaling pathway protein expression in the uterus
In the uterus, western blot results showed that the lower protein levels of β-catenin and P-GSK-3β induced by OVX could be reversed by E2 replacement, but not by EX treatment (Fig. 8).
Exercise and E2 induced changes of β-catenin and P-GSK-3β protein expression in uterus samples detected by western blot. a The western blotting results. β-actin was used as loading control. b The relative levels of β-catenin and P-GSK-3β protein expression in four groups. Values are means ± SD. OVX significantly decreased the protein expression of β-catenin and P-GSK-3β and these changes only could be reversed by E2 replacement treatment, but not by exercise treatment. *P < 0.05 versus Sham group; # P < 0.05, ## P < 0.01 versus OVX group
Discussion
The major findings of the present investigation emphasized that regular treadmill exercise could activate Wnt/β-catenin signaling pathway and inhibit the PPARγ protein expression in the lumbar vertebrae of OVX rats. In present study, we found that OVX rats showed dramatically decreased ultimate lumbar vertebra strength as well as the protein expression for β-catenin and P-GSK-3β. All these OVX-induced changes were significantly reversed by treadmill exercise. In addition, exercise treatment did not produce estrogenic effects on serum level of LH and the protein expression for β-catenin and P-GSK-3β in the uterus.
Bone mineral density is a good predictor for osteoporosis diagnosis, whereas the bone strength is an important predictor for fracture risk (Raisz 2005). Our results showed that the ultimate L5 strength was significantly decreased after OVX compared to the Sham group. This reduction was prevented by the treatment with exercise. These results are consistent with the previous results reported by Simões et al. (2008) who suggested that treadmill training had an important role to prevent the osteopenia in lumbar vertebrae.
Bone mass homeostasis is regulated by an interaction of various factors, including growth factors, systemic hormones, and mechanical loading (Liedert et al. 2010). Among the factors, mechanical loading is the origin of the mechanism by which bone cells adjust bone architecture to maintain bone strength (Armstrong et al. 2007). It is known that some signaling pathways and genes are involved in this response. Among the signaling pathways, Wnt/beta-catenin signaling is not only a normal physiological response of bone to mechanical loading (Bonewald and Johnson 2008; Robinson et al. 2006), but also is a component of osteoblastic bone cell’s early responses to load-bearing (Armstrong et al. 2007). As the level of β-catenin is an indication of the Wnt/β-catenin signaling activation (Case et al. 2008), we firstly analyzed the protein expression of β-catenin in lumbar vertebrae of OVX rats after treatment with exercise training. We showed that exercise treatment had a stimulation effect on β-catenin expression in both lumbar vertebra sections and lysates, suggesting an involvement of the Wnt/β-catenin signaling pathway in the exercise suppression of bone loss in OVX rats. Recent evidence has shown that mechanical strain inhibited adipogenesis in mesenchymal stem cells by stimulating a durable β-catenin signal (Sen et al. 2008). Whether the mechanical stimulation of β-catenin is critical for mechanical suppression of bone marrow adipogenesis in bones of OVX rats (Chen et al. 2011) still needs to be identified by further study.
GSK-3β, a component of the canonical Wnt signaling pathway, is implicated in regulation of bone mass. Inactivation of GSK-3β leads to stabilization, accumulation, and translocation of β-catenin into the nucleus to activate downstream Wnt target genes (Kulkarni et al. 2006). Previous studies have indicated that mechanical preservation of the cellular β-catenin level depends at least partially on the ability of mechanical strain to inactivate GSK-3β (Sen et al. 2009; Armstrong et al. 2007). In present study, treadmill training increased the phosphorylation of GSK-3β in the lumbar vertebrae of OVX rats, suggesting that mechanical activation of β-catenin signaling in bones of OVX rats also likely depends, at least in part, on the inhibition of GSK-3β activity.
Osx is a transcription factor necessary for osteoblast formation (Lau et al. 2011). In present study, the protein expression of Osx did not change after treatment by both exercise and E2. In addition, our previous study (Chen et al. 2011) has indicated that treadmill exercise had no promotion effect on the protein expression of Runx2, another osteoblast-associated marker, in bones of OVX rats. These in vivo results are similar with the previous in vitro study reported by Lau et al. (2011) who showed that a low-magnitude, high-frequency (LMHF) vibration did not enhance the mRNA level of Osx and Runx2 in BMSCs. Taken together, our present and previous results suggested that mechanical prevention of bone loss may not be through the enhancement of the osteogenic factors such as Runx2 and Osx in OVX rats.
PPARγ is a ligand-activated transcription factor that belongs to the nuclear hormone receptor superfamily and functions as a heterodimer with a retinoid X receptor by binding to PPAR responsive elements (Viccica et al. 2010). The functional interaction between PPARγ and β-catenin has been demonstrated in some tissues and cells including BMSCs (Takada et al. 2009). PPARγ insufficiency enhanced osteogenesis through osteoblast formation from bone marrow progenitors (Akune et al. 2004). In contrast, increased PPARγ could enhance adipogenesis by inducing degradation of β-catenin (Sen et al. 2008). These results suggested that preventing degradation of β-catenin could bias stem cell differentiation away from formation of adipocytes with the sum effect being stimulated towards osteogenesis. In the present study, treadmill training not only inhibited the protein expression of PPARγ, but also stimulated the protein expression of β-catenin. It is suggested that the effect of treadmill training on decreasing fat mass and preventing bone loss might function through the regulation of BMSC differentiation via inhibiting PPARγ and preventing degradation of β-catenin.
Estrogen is often used to treat estrogen-deficiency osteoporosis, such as during menopause or ovariectomy. However, ever since the publication of increased risk of cancer in estrogen target tissues, including mammary gland and endometrium following long-term E2 therapy, intensive searches for alternative treatments have began (Wang et al. 2009). Our previous study (Chen et al. 2011) has indicated that exercise treatment produced no estrogenic effects on estrogen target tissue such as uteri. Besides uterine weight, serum LH level is another sensitive parameter to monitor estrogenicity (Seidlova-Wuttke et al. 2010). The previous study demonstrated that the increased serum LH level in OVX animals was due to the lacking of negative feedback of E2 and LH was consequently decreased by estrogenic compounds (McNeilly et al. 2003). Our present study also showed similar results in E2 group. Importantly, our present study firstly showed that exercise treatment produced no estrogenic effect on serum LH in OVX rats. Interestingly, we asked why the increased serum level of E2 induced by exercise treatment in OVX rats did not suppress the serum level of LH in the present study. One possibility is that exercise treatment may exert selective estrogen receptor modulator-like activity, since exercise treatment in present study differentially affected different estrogen-dependent organs.
Ovaries are the primary source of estrogen. In OVX rats, the production of estrogen is shifted from ovary to a number of extragonadal sites, including the mesenchymal cells of adipose tissue, osteoblasts and chondrocytes of bone, the vascular endothelium and aortic smooth muscle cells, and numerous sites in the brain (Simpson and Davis 2001; Simpson 2003). It has been reported that both the subcutaneous abdominal adipose tissues and the liver tissues contributed to the extragonadal aromatization to promote the circulating E2 levels in the rats along with the time after ovariectomy (Zhao et al. 2005). Similar with our previous report (Hao et al. 2010), the present paper also indicated that exercise treatment could increase serum E2 levels in OVX rats. Although the regulation mechanism of treadmill exercise on serum E2 levels needs to be further elucidated, we speculated that treadmill exercise may affect the metabolic pathway regulating estrogen release from extra-ovarian sources such as the subcutaneous abdominal adipose tissues and the bone tissues.
Estrogen-induced alterations in the uterus, especially in shape of glands and endometrium, are probably associated with changes in regulating systems that maintain general composition of the uterus. Wnt/β-catenin pathway may be regarded as one of such systems. It is documented that some components of Wnt/β-catenin pathway are involved in the regulation of cyclic changes of the endometrium and in procession of endometrial cancer (Wang et al. 2010). In present study, E2 treatment exhibited ability to increase both β-catenin and P-GSK-3β levels in the uterus of OVX rats, but exercise treatment produced no such stimulating effects, suggesting that the positive effects of EX treatment on the protein levels of β-catenin and P-GSK-3β in the lumbar vertebrae of OVX rats were not reproducible in the uterus.
In summary, our present data suggest that mechanical strain may activate β-catenin signaling through inactivation of GSK-3β activity in OVX rats. Thus, β-catenin may serve as the link between osteoblast and adipogenesis in the response of bone tissues to treadmill training in OVX rats. Moreover, the effects of EX treatment on the activation of Wnt/β-catenin signaling in bones of OVX rats were not reproducible in the uterus.
References
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H (2004) PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113(6):846–855
Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE (2007) Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem 282(28):20715–20727
Bonewald LF, Johnson Ml (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J (2008) Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem 283(43):29196–29205
Chen Y, Wang S, Bu S, Wang Y, Duan Y, Yang S (2011) Treadmill training prevents bone loss by inhibition of PPARγ expression but not promoting of Runx2 expression in ovariectomized rats. Eur J Appl Physiol 111(8):1759–1767
David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A (2007) Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148(5):2553–2562
Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J (2006) Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol 207(2):454–460
Fan D, Chen Z, Wang D, Guo Z, Qiang Q, Shang Y (2007) Osterix is a key target for mechanical signals in human thoracic ligament flavum cells. J Cell Physiol 211(3):577–584
Hao L, Wang Y, Duan Y, Bu S (2010) Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. Eur J Appl Physiol 109(5):879–886
Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, Engler T, Wei T, Kriauciunas A, Martin TJ, Sato M, Bryant HU, Ma YL (2006) Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res 21(6):910–920
Kurabayashi T, Tomita M, Matsushita H, Honda A, Takakuwa K, Tanaka K (2001) Effects of a beta 3 adrenergic receptor agonist on bone and bone marrow adipocytes in the tibia and lumbar spine of the ovariectomized rats. Calcif Tissue Int 68(4):248–254
Lau E, Lee WD, Li J, Xiao A, Davies JE, Wu Q, Wang L, You L (2011) Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells. J Orthop Res 29(7):1075–1080. doi:10.1002/jor.21334
Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A (2010) Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun 394(3):755–759
McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR (2003) The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl 61:463–476
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29
Narayana Murthy PS, Sengupta S, Sharma S, Singh MM (2006) Effect of ormeloxifene on ovariectomy-induced bone resorption, osteoclast differentiation and apoptosis and TGF beta-3 expression. J Steroid Biochem Mol Biol 100(4–5):117–128
Pigozzi F, Rizzo M, Giombini A, Parisi A, Fagnani F, Borrione P (2009) Bone mineral density and sport: effect of physical activity. J Sports Med Phys Fitness 49(2):177–183
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115(12):3318–3325
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ (2006) Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281(42):31720–31728
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19(2):109–124
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA (2000) Inhibition of adipogenesis by Wnt signaling. Science 289(5481):950–953
Schmitt NM, Schmitt J, Dören M (2009) The role of physical activity in the prevention of osteoporosis in postmenopausal women-An update. Maturitas 63(1):34–38
Seidlova-Wuttke D, Christel D, Kapur P, Nguyen BT, Jarry H, Wuttke W (2010) Beta-ecdysone has bone protective but not estrogenic effects in ovariectomized rats. Physitomedicine 17(11):884–889
Sen B, Xie Z, Case N, Ma M, Rubin CT, Rubin J (2008) Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 149(12):6065–6075
Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J (2009) Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem 284(50):34607–34617
Sewerynek E (2011) Current indications for prevention and therapy of steroid-iduced osteoporosis in men and women. Endokrynol Pol 62(1):38–44
Siddiqui JA, Swarnkar G, Sharan K, Chakravarti B, Sharma G, Rawat P, Kumar M, Khan FM, Pierroz D, Maurya R, Chattopadhyay N (2010) 8,8’’-Biapigeninyl stimulates osteoblast functions and inhibits osteoclast and adipocyte functions: osteoprotective action of 8,8’’-biapigeninyl in ovariectomized mice. Mol Cell Endocrinol 323(2):256–267
Simões PA, Zamarioli A, Blóes P, Mazzocato FC, Pereira LH, Volpon JB, Shimano AC (2008) Effect of treadmill exercise on lumbar vertebrae in ovariectomized rats: anthropometrical and mechanical analyses. Acta Bioeng Biomech 10(2):39–41
Simpson ER (2003) Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86(3–5):225–230
Simpson ER, Davis SR (2001) Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology 142(11):4589–4594
Sipos W, Pietschmann P, Rauner M, Kerschan-Schindl K, Patsch J (2009) Pathophysiology of osteoporosis. Wien Med Wochenschr 159(9–10):230–234
Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S (2008) Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 19(9):1323–1330
Takada I, Kouzmenko AP, Kato S (2009) Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5(8):442–447
Viccica G, Francucci CM, Marcocci C (2010) The role of PPARγ for the osteoblastic differentiation. J Endocrinol Invest 33(7 Suppl):9–12
Wang L, Wang YD, Wang WJ, Li DJ (2009) Differential regulation of dehydroepiandrosterone and estrogen on bone and uterus in ovariectomized mice. Osteoporosis Int 20:79–92
Wang Y, van der Zee M, Fodde R, Blok LJ (2010) Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget 1(7):674–684
Zhao H, Tian Z, Hao J, Chen B (2005) Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol 3:6
Acknowledgments
This work was supported by the National Scientific Foundation of China (Grant No. 30771046) and the Beijing Candidate of Millions of Talents Project in the New Century.
Conflict of interest
No conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
Rights and permissions
About this article
Cite this article
Bu, S., Chen, Y., Wang, S. et al. Treadmill training regulates β-catenin signaling through phosphorylation of GSK-3β in lumbar vertebrae of ovariectomized rats. Eur J Appl Physiol 112, 3295–3304 (2012). https://doi.org/10.1007/s00421-011-2306-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2306-4