Abstract
Osteoporosis increases bone fragility and fractures. Preptin hormone is regulated by moderate exercise training and increases bone formation. Therefore, this study was conducted to see how estradiol administration and moderate exercise training affected osteoporotic changes in ovariectomized (OVX) rats. To achieve this aim, 36 healthy adult female Wistar albino rats were randomized into Sham, OVX, ovariectomized estradiol-treated (OVX + E) (OVX + E rats were treated using subcutaneous estradiol benzoate 2.5 μg/kg body weight/day), ovariectomized practicing moderate exercise training, ovariectomized estradiol-treated and practiced a moderate exercise training, and ovariectomized alendronate-treated (OVX + Alen) (OVX + Alen rats were treated orally with alendronate 3 mg/kg body weight/week) groups. Alendronate was used as a standard anti-osteoporotic drug. Moderate exercise training, including therapy with estradiol and alendronate for OVX rats began on the fourth week and lasted for six weeks. Results showed that OVX rats had estrogen and preptin deficiency in serum. These deficiencies were associated with a significant increase in bone resorption biomarkers (urinary deoxypyridinoline and hydroxyproline), and bone formation biomarkers (serum osteocalcin and bone-specific alkaline phosphatase). Also, serum pro-inflammatory cytokines (tumor necrosis factor alpha and interleukin-6) were increased, while bone osteopontin (OPN) expression was decreased. Subsequently, the osteoporotic alterations were verified based on histopathological changes. From the results, estradiol therapy and moderate exercise training significantly improved these findings to the same extent as that of the standard alendronate treatment. Therefore, through their anti-inflammatory properties, increasing bone OPN expression, and regulating serum preptin; estradiol therapy and moderate exercise training can reduce osteoporotic alterations in OVX rats. Thus, combined estradiol therapy and moderate exercise training could be a promising potential therapeutic protocol to reduce postmenopausal osteoporosis. Also, targeting serum preptin and bone osteopontin regulation could have a critical role in the treatment of postmenopausal osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone remodeling, a regulated balance between osteoblastic bone production and osteoclastic bone resorption, is essential for maintaining skeletal mechanical integrity (Ducy et al. 2000). Bone loss occurs when bone resorption outpaces bone production, thereby resulting in osteoporosis. Osteoporosis is a metabolic bone disease linked with increased bone fragility and fractures (Byun and Lee 2010). Osteoporosis is more frequent in women owing to menopausal ovarian dysfunction (Stachenfeld 2014) that is manifested by an estrogen shortage, a decrease in bone mineral content, and bone microarchitecture disarrangement (Abu Taleb et al. 2020). Therefore, because of increased life expectancy, postmenopausal osteoporosis is becoming more common (Heilmeier et al. 2016). Although estrogen therapy is widely used as a prophylactic and therapeutic method for postmenopausal osteoporosis (Da Paz et al. 2001), yet, as a drug, there is evidence for adverse effects of its use as venous thrombosis (Bergendal et al. 2016). Thus, it will be interesting to find an alternative to estrogen therapy with minimal hazards, or at least a combination of such alternative with estrogen treatment that can minimize its hazards and get use of its benefits. Exercise is considered an inexpensive and safe non-drug treatment strategy to protect musculoskeletal health and prevent fractures (Daly et al. 2019). Sun et al. (2015) stated that exercise has positive effects on bone morphology, mass, and strength. Chen et al. (2008) noticed that osteoclasts and osteoblasts respond to mechanical stimuli with increased alkaline phosphatase release by osteoblasts. Suzuki et al. (2008) reported that exercise inhibits osteoclast differentiation, and bone resorption. Müller et al. (2018) reported that ovariectomy-induced estrogen deficiency decreased movement drive that is ameliorated with estrogen treatment. Also, Lee et al. (2003) detected that disturbance of estrogen signaling impaired skeletal response to mechanical strain. In addition, Yarrow et al. (2012) observed that ovariectomy decreased exercise-induced bone remodeling during recovery from disuse. Alendronate is a bisphosphonate that inhibits bone loss by inhibiting osteoclastic bone resorption (Kanis et al. 1995). Preptin is a 34-amino-acid peptide hormone that is produced from the pancreas, kidneys, breast tissues, and salivary glands (Aydin et al. 2013). A study showed that preptin has an anabolic impact on bone growth (Xiao et al. 2019). In another study, preptin was also shown to possess in vitro osteoblastic proliferative and in vivo bone mineralization effects (Cornish et al. 2007). Furthermore, serum preptin levels were shown to be low in individuals with osteoporosis and osteopenia (Li et al. 2013). In contrast, Tomeleri et al. (2016) found that moderate exercise training raised preptin serum levels. Nazari Soltan Aahmad et al. (2018) also reported a positive correlation between blood preptin levels and bone formation indicators. Mierzwicka et al. (2018) reported a significant increase in serum preptin level in women with polycystic ovary syndrome, without a significant correlation between serum levels of both preptin and estradiol. On the other hand, no study has investigated changes in serum preptin levels in ovariectomized (OVX) rats. Thus, this study was designed to investigate changes in serum level of preptin with ovariectomy and to identify the effect of estradiol treatment and moderate exercise training on osteoporotic changes in OVX rats. Results were then compared with alendronate as a standard treatment of osteoporosis.
Materials and methods
Thirty-six healthy, adult female Wistar albino rats weighing 150–190 g, were obtained from the Animal House, Faculty of Veterinary Medicine, Zagazig University. They were kept under hygienic conditions in steel wire cages (50 × 30 × 20 cm), six rats per cage, and acclimatized on an ordinary diet obtained from the Zagazig College of Agriculture for two weeks before starting the experiment. During this period, all animals had free access to water, and maintained at a comfortable temperature (20 °C–24 °C) under normal light–dark cycles. In addition, the rats were monitored for estrous cyclicity by daily vaginal cytology, and only rats with at least two consecutive regular four-day estrous cycles were used. The Physiology Department Committee and the Institutional Animal Care and Use Committee, Zagazig University (ZU-IACUC/3/F/16/2021) approved the experimental protocol used for this study.
The experimental design
Following an accommodation phase, 36 female rats were randomized by simple random sampling (Taherdoost 2016) into six equal groups (n = 6). Group 1 was the sham-operated (Sham) group. Group 2 was OVX. Group 3 was OVX estradiol-treated (OVX + E), in which OVX rats were treated using subcutaneous injections of estradiol benzoate (2.5 μg/kg body weight/day) dissolved in sesame oil (Heather and Karen 2002). Group 4 was OVX practicing moderate exercise training (OVX + Ex). Group 5 was OVX estradiol-treated (as in the OVX + E group) that practiced moderate exercise training (as in the OVX + Ex group) (OVX + E + Ex). Group 6 was OVX alendronate-treated (OVX + Alen), in which OVX rats were treated orally with the standard anti-osteoporotic drug; alendronate, using single weekly oral gavage (3 mg/kg body weight) (Pytlik et al. 2004) dissolved in saline (Salazar et al. 2015). Exercise training and therapy with estradiol and alendronate for OVX rats began on the fourth week and lasted for six weeks. Rats in the Sham, OVX, OVX + Ex and OVX + Alen groups were injected subcutaneously with 0.1 ml sesame oil/rat/day for six weeks starting from the fourth week. Also, animals in the Sham, OVX, OVX + E, OVX + Ex and OVX + E + Ex groups were given saline (1 ml/rat/week) orally for the last six weeks. To ensure that most of the sex hormone residues were cleared, OVX rats were kept for three weeks after surgery (Mustafa et al. 2018). Estradiol benzoate was purchased from Sigma-Aldrich, USA (catalog No. 1251000). Alendronate (Fosamax®, Merck Sharp and Dohme Company, Italy) was obtained as tablets, each containing 70 mg alendronate sodium Table 1.
The ovariectomy procedure
After overnight fasting, rats were anesthetized with urethane (1200 mg/kg intramuscularly) (Miller and Wiegman 1977) and attached to an operating table. Then, bilateral ovariectomy was done by making two dorsolateral incisions with sharp dissecting scissors. Skin and dorsal muscles were also sliced, thereby allowing access to the peritoneal cavity. Subsequently, the uterine horn was removed in addition to the fatty tissue around the ovary. Next, artery forceps were used to clamp the link between the fallopian tube and uterine horn; then, an incision was conducted under the clamped region to remove the ovary (Lasota and Danowska-Klonowska 1995). Finally, the viscera was restored, and the abdominal wall was reconstructed with a catgut thread, followed by sterile silk sutures to seal the skin incisions. Tincture iodine antiseptic solution was also applied locally on the skin at both sites of operation. Moreover, gentamicin (intramuscularly 5 mg/kg for five days) for reducing the risk of skin infection after suturing was applied as well (Popović et al. 2016). Similarly, a sham operation was performed where the ovaries were exposed but not removed. After surgery, rats were housed individually for some hours to allow recovery, then re-grouped in their home cages. Then, hormonal status was assessed after ovariectomy by daily vaginal smears obtained at ~ 8 am. Rats whose vaginal smears indicated that the rat exhibited constant leukocytes confirmed the success of the ovariectomy procedure (Turkozan et al. 2009).
Moderate swimming exercise program
In the exercised groups (OVX + Ex and OVX + E + Ex), for the last six weeks, rats were subjected to swimming at a moderate intensity in a cylindrical tank (80-cm high and 45-cm diameter). This tank was filled with 32 °C–35 °C temperature of water at a depth of 45-cm deep. The swimming duration was for one hour daily, five days per week (Silva et al. 2012). The swimming period for rats was originally 15 min daily, then we gradually extended the duration until the rats exercised for one hour per day, which they did at the end of the first week of training (Feng et al. 2019). Therefore, the exercise was practiced between 9.00–10.00 am. At the end of each exercise session, rats were kept to dry in a warm environment (Röhling et al. 2016).
24-h urine collection
Each rat was housed in a unique metallic cage with a perforated plate form for 24 h before scarification to calculate urine production from 8 to 8 am the next day. For the urine collection, a glass funnel was attached to the region of each cage’s plate form, after which urine was channeled through it to a collecting bottle. Urine samples were collected in dry test tubes and maintained at − 20 °C until analysis for hydroxyproline and deoxypyridinoline (DPD) levels (Gallo et al. 2005).
Collection of samples
All rats were weighed at the end of the experiment to determine their final body mass indices (BMIs) in g/cm2 according to Novelli et al. (2007). Then, venous blood samples were collected under thiopental (50 mg/kg) anesthesia (Kanjana et al. 2013) from the rat’s tail vein (Abu Taleb et al. 2020). The blood samples were incubated at 37 °C for 10 min to clot. Then, they were centrifuged at 12,000 rpm for 15 min to separate the serum. The separated serum was then kept frozen at – 80 °C until biochemical analysis. Subsequently, the rats were decapitated, and their uterine horns were dissected and weighed. Also, femur bones were dissected and processed for histological inspection.
Determination of bone turnover biomarkers
These biomarkers are considered useful tools for estimating the rate of osteoporosis that is related to bone loss. In addition, they monitor the effectiveness of anti-resorptive therapies in osteoporosis. They are also used due to their high specificity and sensitivity during clinical tests (Stavropoulou et al. 2005). Bone formation was investigated by measuring serum osteocalcin and bone-specific alkaline phosphatase (BsALP), whereas bone resorption was assessed by measuring urinary hydroxyproline and DPD levels. DPD is a cross-linked collagen in the bone that is excreted in urine with collagen by osteoclastic activity (Byun and Lee 2010). Urinary creatinine levels were also used to adjust DPD values for variations in urine volumes. Results are expressed in nanomoles of DPD per millimole of creatinine to avoid the possible influence of glomerular filtration rates (Byun and Lee 2010). From Mybiosource.com (USA), commercial kits were purchased to assess serum BsALP (Catalog No. MBS700986), serum interleukin-6 (IL-6, Catalog No. MBS726707), serum estradiol (Catalog No. MBS843353), urinary DPD (Catalog No. MBS043935), and urinary hydroxyproline (Catalog No. MBS017427). Also, commercial ELISA kits were used to measure serum levels of osteocalcin (Novus Biologicals, LLC, USA, Catalog No. NBP2-68,153), TNFa (abcam.com, UK, Catalog No. ab46070), and preptin (antibodies-online, USA, Catalog No. ABIN5696558).
Femur histopathology
After the rats were sacrificed, all femurs were immediately sampled, cut and fixed in 10% formol saline solution. Then, the upper parts and shafts of the bones were decalcified using EDTA. The samples embedded in paraffin were subsequently sliced into 6-μm sections and processed for staining as follows:
-
Hematoxylin–Eosin (H&E) according to Bancroft and Stevens (2013). To demonstrate basic structure.
-
Immunohistochemical osteopontin (OPN, bone remodeling biomarker) staining of femur specimens: Bone sections were incubated in a rabbit anti-OPN polyclonal antibody (Calbiochem, San Diego, CA, USA). OPN immunoreactivity appeared positive in cement lines, osteoblasts, osteocytes, and the bone matrix. Next, primary antibodies were replaced with a buffer solution to get negative control sections, whereas the osteosarcoma specimen was used as a positive control (Singh et al. 2018).
Bone histomorphometry
Using an image analyzer (Leica Qwin 500 image analyzer computer system) at the Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, each section that was stained with H&E, including the cortical thickness (µm) and trabecular bone thickness (µm), was measured using a quantitative image analysis system in random microscopic areas under 100 high-power fields. Ten readings were obtained for each specimen, five on the lateral and five on the medial cortical bone according to Kim et al. (2003). Then, to measure the mean thickness of the outer cortical bone of the middle shaft of the femur, perpendicular lines were drawn from the periosteum to the endosteum at many sites (Surve et al. 2001). Subsequently, the area percentage of the positive OPN immune-staining was obtained from OPN immune-stained slides.
Statistical analysis
The obtained data were analyzed by computer using IBM Statistical Package for Social Sciences (SPSS) Statistics, Version 25 Software for Windows (Ibm 2017). The Shapiro–Wilk test was used to examine the variance homogeneity and distribution properties of variables. Data were described using mean and standard deviation (SD) as they were normally distributed. One-way analysis of variance (ANOVA) and Tukey HSD for post hoc multiple comparisons were employed to test differences between groups, as the variances were equal. P value ≤ 0.05 was considered significant.
Results
Final body weight (g) in different studied groups
A significant (P < 0.005) increase was observed in the final body weight values of the OVX, OVX + E, OVX + Ex, OVX + E + Ex, and OVX + Alen groups compared with the Sham group. However, compared with the OVX group, a significant (P < 0.005) decrease was detected in the final body weight values in the OVX + E, OVX + Ex, and OVX + E + Ex groups, with a significant increase (P < 0.005) in that of OVX + Alen group. Also, in comparison to the OVX + Ex group, a significant (P < 0.005) decrease was observed in the final body weight values of the OVX + E + Ex group, with a significant increase (P < 0.005) in that of OVX + Alen group. In addition, a significant (P < 0.005) increase was observed in the final body weight values of the OVX + Alen group when compared with the OVX + E + Ex group Table 2.
Final BMI changes among the different groups
A significant (P < 0.005) increase was observed in the final BMI values of the OVX group compared with the Sham group. Also, there was a significant increase (P < 0.005) in final BMI values of OVX + Alen group in comparison to that of all groups. However, compared with the OVX group, a significant (P < 0.005) decrease was detected in the final BMI values in the OVX + E, OVX + Ex, and OVX + E + Ex group Table 2.
Relative uterine weight changes among the different groups
A significant (P < 0.005) decrease was observed in the relative uterine weights of all OVX groups compared with the Sham group. However, compared with the OVX group, a significant (P < 0.005) increase was detected in their relative uterine weights in the OVX + E, OVX + Ex, OVX + E + Ex, and OVX + Alen groups. Furthermore, rats in the OVX + E + Ex group showed significantly (P < 0.005) increased relative uterine weights compared with both OVX + E and OVX + Ex groups. As reported, a significant (P < 0.005) increase in the relative uterine weight of the OVX + Alen group compared with the OVX + E + Ex group was observed Table 2.
Changes in bone biomarkers [serum (osteocalcin & BsALP) and urine (DPD & hydroxyproline)] among the different groups
A significant (P < 0.005) increase was observed in the bone biomarkers of all OVX groups compared with the Sham group. In contrast, compared with the OVX group, a significant (P < 0.005) decrease was detected in the bone biomarkers in the OVX + E, OVX + Ex, OVX + E + Ex, and OVX + Alen groups. Results also showed that OVX + E + Ex significantly (P < 0.005) decreased serum BsALP levels compared with both OVX + E and OVX + Ex. Moreover, a significant (P < 0.005) increase was observed as well in the serum BsALP levels of the OVX + Alen group compared with the OVX + E + Ex group Table 2.
Changes in serum pro-inflammatory cytokines (TNFa and IL-6) among the different groups
A significant (P < 0.005) increase was observed in TNFa and IL-6 serum levels in all OVX groups compared with the Sham group. However, compared with the OVX group, a significant (P < 0.005) decrease was detected in TNFa and IL-6 serum levels of the OVX + E, OVX + Ex, OVX + E + Ex, and OVX + Alen groups. Furthermore, OVX + E + Ex significantly (P < 0.005) decreased TNFa and IL-6 serum levels compared with OVX + E and OVX + Ex. Moreover, a significant (P < 0.005) increase was observed in the TNFa and IL-6 serum levels in the OVX + Alen group compared with that of the OVX + E + Ex group Table 2.
Changes in estradiol and preptin serum levels among the different groups
A significant (P < 0.005) decrease was observed in the estradiol and preptin serum levels of OVX group compared with the Sham group. However, compared with the OVX group, a significant (P < 0.005) increase was detected in estradiol serum levels of the OVX + E, and OVX + E + Ex groups.
Also, a significant increase (P < 0.005) in serum preptin level was observed in OVX + E, OVX + Ex, OVX + E + Ex, and OVX + Alen groups in comparison to OVX group Table 2.
Histopathological results
H&E
Sections of the sham group showed normal cortical femur bone appearance as the outer layer formed with the periosteum, and the appearance of a subperiosteal bone formation. Furthermore, the compact bone revealed circumferential lamella, including osteocytes within their lacunae. Also, the endosteum was lined with osteoblasts (Fig. 1a, b). The bone trabeculae appeared thick with the bone marrow between them which is lined by endosteum with cuboidal osteoblast. In contrast, the bone matrix appeared homogenous with osteocytes inside their lacunae (Fig. 1c, d). The cortical bone in the femur of the OVX group also showed a decrease in cortical bone thickness with the appearance of osteoporotic cavities having granulation tissues inside them, and multinucleated osteoclasts having acidophilic cytoplasm near the cavities. Furthermore, the periosteum appeared thick and irregular (Fig. 2a, b). The bone trabeculae also appeared thin and interrupted with wide bone marrow cavities between them as well with faint stained matrix, bone marrow lined by endosteum with few osteoblast cells and shows dense fat deposition (Fig. 2c, d). Additionally, the femur shaft of the OVX + E group appeared to show a thin fibrous periosteum with thin bone depositions under its surface. However, the cortical bone exhibited small osteoporotic cavities and many large and empty lacunae. Osteocytes also appeared inside their lacunae with deposition of cement around them (Fig. 3a, b). As shown, the OVX + E group also revealed restoration of bone trabeculae with many osteocytes within their lacunae, and wide bone marrow cavities between the bone trabeculae (Fig. 3c, d). Longitudinal sections of the femur shaft of the OVX + EX group exhibited thin fibrous periosteum as well, with irregular bone depositions under its surface. Furthermore, the cortical bone showed many osteoporotic cavities, with a deposition of cement around the lacunae of osteocytes (Fig. 4a, b). A transverse section from rat femurs of the same group had thin bone trabeculae with wide bone marrow cavities between them. Additionally, bone trabeculae showed few osteoblasts lined the endosteum (Fig. 4c, d). Most of those changes were lesser in the OVX + E + EX group, as the longitudinal section of rat femurs showed thinning of the outer layer of the periosteum with basophilic subperiosteal depositions. Moreover, the compact bone that appeared had circumferential lamellae and osteocytes that were inside their lacunae, with cement deposition arranged around them (Fig. 5a, b). Besides, bone trabeculae of the OVX + E + EX group restored their thickness (Fig. 5c, d). Likewise, the OVX + Alen group femur showed some osteoporotic cavities within its cortical bone, with few osteoblasts lining the endosteum (Fig. 6a, b). Transverse sections from rat femur of the OVX + Alen group exhibited well-formed bone trabeculae (Fig. 6c, d).
Photomicrograph of a rat femur. a, b from the sham group femur cortex appears with outer periosteal layer (P), subperiosteal basophilic line (arrowhead), circumferential lamella (tailed arrow) with osteocytes (O) inside their lacunae (green arrow) and inner lamellae (green tailed arrow) also appear.endosteum appears lined with osteoblast (green arrowhead). c, d sham group femur trabeculae appear with thick bone trabeculae (Bt), bone marrow (Bm) is lined with endosteum (E) with cuboidal osteoblast (green arrowhead) (H&E ×100&400)
Photomicrograph of a rat femur. a, b OVX group femur cortex, shows periosteum (P) with periosteal irregularities (thick arrow) and some empty lacunae (zigzag arrow). Some osteoporotic cavities (arrow) appear having granulation tissues inside them, and multinucleated osteoclast (blue arrow in inset magnification × 1000) having acidophilic cytoplasm appears near the cavities. c, d OVX femur trabeculae (Bt) appeared thin and interrupted with wide bone marrow (Bm) cavities between them as well with faint stained matrix (blue tailed arrow), bone marrow is lined by endosteum (E) with few osteoblast cells (green arrowhead) and shows dense fat deposition (blue asterisk) (H&E ×100,400,1000)
Photomicrograph of a rat femur. a, b from the OVX + E group femur cortex, shows thin fibrous periosteum (P) with thin subperiosteal bone deposition (arrowhead).the cortical bone exhibits small osteoporotic cavities (asterisk), empty lacunae (arrow), some osteocytes(O) appear within their lacunae with cement around them (green arrow). c, d OVX + E group femur trabeculae show restoration of bone trabeculae (Bt) and bone marrow (Bm) is lined with endosteum (E) with osteoblasts (green arrowhead) (H&E ×100 &400)
Photomicrograph of a rat femur a, b the OVX + Ex group femur cortex, shows fibrous periosteum (P) with irregular subperiosteal bone deposition (arrowhead). Small osteoporotic cavities (asterisk) appear within cortical bone and osteocytes (O) within their lacunae with cement around them (green arrow). c, d OVX + Ex femur trabeculae show thin bone trabeculae (Bt), with wide bone marrow cavities (Bm) between them. Additionally, bone trabeculae showed few osteoblasts (green arrowhead) lined the endosteum (E) and have multiple fat cells (blue asterisk) within bone marrow cavities (H&E × 100,400)
Photomicrograph of a rat femur. a, b from the OVX + E + EX group femur cortex shows thinning of the outer layer of the periosteum (P) with basophilic subperiosteal depositions (arrowhead). Moreover, the compact bone has circumferential lamellae (tailed arrow) and osteocytes (O) that were inside their lacunae, with cement deposition arranged around them (green arrow) and few small osteoporotic cavities (asterisk) appear. c, d OVX + E + EX group femur trabeculae, appear with thick bone trabeculae (Bt), bone marrow (Bm) is lined with endosteum (E) with cuboidal osteoblast (green arrowhead) (H&E ×100&400)
Photomicrograph of a rat femur a, b OVX + Alen group femur cortex, group femur cortex shows thin outer periosteum (P) with basophilic subperiosteal depositions (arrowhead). Moreover, the compact bone that appeared had circumferential lamellae (tailed arrow) and osteocytes (O) that were inside their lacunae, with cement deposition arranged around them (green arrow) and few small osteoporotic cavities (asterisk) appear. c, d OVX + Alen femur trabeculae, appear with thick bone trabeculae (Bt), bone marrow (Bm) is lined with endosteum (E) with cuboidal osteoblast (green arrowhead) (H&E ×100&400)
Immunohistochemical results
From the sham group, results revealed marked OPN expression in the bone matrix (Fig. 7a), whereas the OVX group showed a marked decreased OPN expression with the appearance of an osteoporotic cavity (Fig. 7b). Furthermore, the OVX + E group showed moderate OPN expression (Fig. 7c). However, although the OVX + Ex group showed a mild increase in OPN expression (Fig. 8a), OPN expression showed a marked increase within the cortical bone of the OVX + E + Ex group (Fig. 8b). In contrast, the OVX + Alen group showed moderate OPN expression (Fig. 8c).
Morphometric results
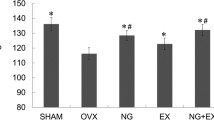
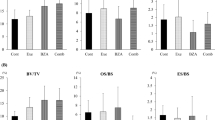
Results showed a significant decrease in the thickness of both cortical and trabecular bones of the OVX group compared with that of the sham group. Moreover, compared with the OVX group, including OVX + E, OVX + Ex, OVX + E + Ex, and OVX + Alen, results only showed a significant increase in cortical bone thickness. Furthermore, the increased thickness of the trabecular bone in both OVX + E and OVX + Ex groups was non-significant compared with that of the OVX group (Table 3). The area percentage of OPN expression within the bone matrix of the femur cortical bone also showed a significant decrease in the OVX group compared with that of the sham group. However, compared with the OVX group, including OVX + E, OVX + Ex, OVX + E + Ex, and the OVX + Alen groups, results showed a significant increase in the area percentage of OPN expression within the bone matrix of the femur cortical bone. Likewise, OPN expression of OVX + E + EX showed a significant increase than that of OVX + E and OVX + EX and was non-significant compared with OVX + Alen (Table 3).
Discussion
In this study, OVX rat models were used to simulate postmenopausal changes in females. Results declared that combined estradiol treatments and exercise training significantly improved the osteoporotic changes observed in OVX rats as seen in chemical and histopathological changes. These improvements were comparable to those of the standard anti-osteoporotic treatments observed using alendronate. We propose that these changes are partly owed to the corresponding variations in both serum preptin levels, and bone OPN expression. In the OVX group, rats experienced a significant increase in both final BMI levels, and skeletal remodeling with uterine atrophy. These changes can be explained by estrogen deficiency that was supported by Abdel-Sater and Mansour (2013) who added that estrogen deficiency is associated with fat deposition. The skeletal remodeling observed in OVX group is reflected by the associated increase in both bone resorption biomarkers (urinary DPD and hydroxyproline), and bone formation biomarkers (serum osteocalcin and BsALP). Altered bone histopathologic findings and morphometric parameters confirmed the development of osteoporosis in OVX rats. These results were in agreement with Abdel-Sater and Mansour (2013), Abuohashish et al. (2015), Osman et al. (2018) and Baloğlu and Özkorkmaz (2019) who owed the associated increase in bone formation and resorption biomarkers in OVX rats to the compensatory bone formation that accompanied osteoporotic changes in this case. Abdel-Sater and Mansour (2013) attributed the osteoporotic changes observed in OVX rats to estrogen deprivation which enhanced osteoclast production and activities. Bone sections of OVX rats revealed periosteal thickening and irregularity, reduced cortical thickness, and several osteocytes. These bone features had the appearance of multiple resorption cavities, osteoclasts, many empty lacunae, and thinned bone trabeculae separated by wide medullary cavities. These findings were in line with Abuohashish et al. (2015), Osman et al. (2018), and Sharaf et al. (2015). The reduction in bone cortical and trabecular thickness depicted in this study reflects unbalanced osteoclast-mediated bone resorption. Osterhoff et al. (2016) stated that the proximal femur has a unique cortical structure, and any loss in cortical thickness can increase the chance of sustaining a hip fracture. Felsenberg and Boonen (2005) reported that the trabecular bone microarchitecture deterioration contributes to bone fragility in severe osteopenia. Additionally, the bone sections of OVX rats have revealed a significant decrease in OPN expression in the bone matrix. This observation reflects osteoporosis occurrence, as OPN is a bone formation indicator that is formed by osteoblasts and accumulates in the mineralized bone matrix (Baloğlu and Özkorkmaz 2019; El-Haroun et al. 2020). This result was supported by Osterhoff et al. (2016) who stated that changes in the matrix composition of the OPN changed bone biomechanical characteristics because it functions as a glue that keeps mineralized collagen fibers together. In addition, Tanaka et al. (2011) reported OPN deficiency in the femoral head in patients with a hip fracture. In contrast, Fodor et al. (2013) noted increased serum OPN levels in postmenopausal women with osteoporosis, which was proposed to be due to species differences. In this study, OVX rats had a significant increase in serum pro-inflammatory cytokines (TNF-α and IL-6), which had a strong stimulatory action on bone resorption as declared by Weitzmann and Pacifici (2006), thereby causing osteoporosis. Also, OVX rats had a significant reduction in serum preptin levels, which was in agreement with the studies of Li et al. (2013), Ozkan et al. (2013), and Bebars et al. (2019), who recorded lower preptin levels in patients with osteoporosis. These results, with the significant increase in serum preptin observed in OVX + E group in comparison to OVX group, could indicate that estradiol was among the regulators of serum preptin, and that preptin can be used as a biomarker for osteoporotic changes with ovariectomy, which was in line with the studies of Ozkan et al. (2013), Bebars et al. (2019), and Mohammad Rahimi et al. (2020). The use of preptin as a biomarker for osteoporosis in this case may be superior on the use of estradiol as some factors may improve osteoporosis without affecting estradiol serum level as observed in the current study with both moderate exercise training and alendronate treatment of OVX rats. After estradiol treatment of OVX rats, the previously mentioned biochemical changes in this study were ameliorated. Also, histopathological studies showed that estradiol therapy slightly improved osteoporotic changes, as few osteoporotic cavities were observed with some empty lacunae, including an increase in the thickness of cortical and trabecular bones. These results were supported by Sharaf et al. (2015) who reported that examination of bone sections of OVX rats treated with estrogen showed a slight increase in the thickness of the cortical bone of the shaft. Estrogen also can arrest osteoporosis progression by inhibiting the secretion of IL-6 and TNF-α [cytokines increased osteoclast development (Weitzmann & Pacifici 2006)], and increasing serum preptin levels. Kitaura et al. (2020) stated that estrogen stimulates transforming growth factor-β production to increase osteoclast apoptosis. Exercise training improved osteoporotic changes in OVX rats in a similar way as that of the estradiol treatment in the OVX + E group. This improvement occurred in part through increasing both serum preptin levels and OPN bone expression. This was in agreement with Cornish et al. (2007), Li et al. (2013), Tomeleri et al. (2016), and Xiao et al. (2019) who reported that preptin has bone anabolic effects through increasing osteogenesis and suppressing osteoblastic apoptosis. Combined estradiol therapy and moderate exercise training in OVX rats (OVX + E + Ex group) showed a significant improvement compared with either OVX + E or OVX + Ex rats regarding biochemical, histopathological, and immunohistochemical results. These results were supported by Sun et al. (2015) who stated that exercise has positive effects on bone morphology, mass, and strength. Also, Chen et al. (2008) noticed that osteoclasts and osteoblasts respond to mechanical stimuli with increased alkaline phosphatase release by osteoblasts. Suzuki et al. (2008) reported that exercise inhibits osteoclast differentiation, and bone resorption. In addition, Tomeleri et al. (2016) found that moderate exercise training raised preptin serum levels. Nazari Soltan Aahmad et al. (2018) also reported a positive correlation between blood preptin levels and bone formation indicators. In line with these studies, our results declared that serum preptin is significantly increased in OVX + E + Ex, OVX + E, and OVX + Ex groups in comparison to OVX rats, but still significantly decreased in comparison to that in sham group which indicates that moderate exercise and estradiol are regulators of preptin secretion, but still there are other regulators. In agreement with our results, Yoon et al. (2012) reported higher plasma osteocalcin in OVX rats than in sham-operated animals and they owed this to estrogen deficiency and indicated increased bone turnover. Also, Honjo et al. (1989) found that serum osteocalcin is higher in postmenopausal women in comparison to premenopausal women. Ahn and Kim (2016) found a significant increase in osteocalcin serum levels in the osteoporotic animals after 12 weeks of exercise in comparison to that in control group. The improvements observed in OVX + E + Ex group were comparable with those of the OVX + Alen rats. Therefore, this result confirmed the potential synergistic effects of combined estradiol treatments and exercise training to minimize osteoporotic changes in OVX rats. Results of this study also declared a significant elevation in final BMI in OVX + Alen group when compared with that of OVX group which was in agreement with Chen et al. (2014), Abdel-Sater and Mansour (2012), and Notomi et al. (2003) who owed that to the neo-formation of bone tissue. In addition, OVX + Alen group showed a significant decline in the bone turnover in comparison to what was reported in the OVX rats which was in line with Özşahin et al. (2017). Previous studies explained the anti-osteoporotic effects of alendronate treatment on the basis that it induces osteoclast apoptosis Xiong et al. (2010), affects formation of wrinkle boundaries of osteoclast Halasy-Nagy et al. (2001), and increases strength of bone by influencing bone quality and bone mass Kolios et al. (2010). The study’s limitations include the fact that it was done on rats, and the results are proposed to differ from those seen in humans. Also, the study only employed a few rats. Additionally, preptin itself was not used as a treatment to assess its effects on OVX rats. Moreover, the mechanism by which both estradiol and moderate exercise training upregulates serum preptin levels in OVX rats requires further assessment. In addition, the beneficial effects of the combined estradiol therapy and moderate exercise training on other body systems were not assessed.
Conclusion
Through their anti-inflammatory properties, increasing bone OPN expression, and regulating serum preptin, estradiol therapy and moderate exercise training can reduce osteoporotic alterations in OVX rats. Thus, combined estradiol therapy and moderate exercise training could be a promising potential therapeutic protocol to reduce postmenopausal osteoporosis. Also, targeting serum preptin and bone osteopontin regulation could have a critical role in the treatment of postmenopausal osteoporosis.
References
Abdel-Sater K, Mansour H (2012) Effects of leptin on metabolic bone turnover in ovariectomy rats. Acta Endocrinol 1841–0987:8
Abdel-Sater KA, Mansour H (2013) Bone biomarkers of ovariectomised rats after leptin therapy. Bratisl Lek Listy 114:303–307
Abu Taleb OM, Wissa MY, Abou El Nour RK, Awad HA, Moussa NM (2020) Potential effectiveness of exenatide in experimentally-induced osteoporosis. Egypt Rheumatol 42:57–62
Abuohashish HM, Ahmed MM, Al-Rejaie SS, Eltahir KEH (2015) The antidepressant bupropion exerts alleviating properties in an ovariectomized osteoporotic rat model. Acta Pharmacol Sin 36:209–220
Ahn N, Kim K (2016) Effects of 12-week exercise training on osteocalcin, high-sensitivity C-reactive protein concentrations, and insulin resistance in elderly females with osteoporosis. J Phys Ther Sci 28:2227–2231
Aydin S, Celik O, Gurates B et al (2013) Concentrations of preptin, salusins and hepcidins in plasma and milk of lactating women with or without gestational diabetes mellitus. Peptides 49:123–130
Baloğlu M, Özkorkmaz EG (2019) Biochemical and immunohistochemical investigations on bone formation and remodelling in ovariectomised rats with tamoxifen citrate administration. Folia Morphol 78:789–797
Bancroft J, Stevens A (2013) Theories and practice of histological techniques.) New York, London and Madrid: Churchil Livingstone
Bebars GM, Sallam SA, Gaber SS, Abdelaziz AH (2019) Assessment of Preptin peptide level in the sera of rachitic children and in breast milk of their mothers. Ital J Pediatr 45:34
Bergendal A, Kieler H, Sundström A, Hirschberg AL & Kocoska-Maras L (2016) Risk of venous thromboembolism associated with local and systemic use of hormone therapy in peri- and postmenopausal women and in relation to type and route of administration. Menopause (New York, N.Y.) 23, 593–9.
Byun JS, Lee SS (2010) Effect of soybeans and sword beans on bone metabolism in a rat model of osteoporosis. Ann Nutr Metab 56:106–112
Chen Y-J, Huang C-H, Lee I-C, Lee Y-T, Chen M-H, Young T-H (2008) Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res 49:7–14
Chen S-Y, Yu H-T, Kao J-P et al (2014) An NMR metabolomic study on the effect of alendronate in ovariectomized mice. PLoS One 9:e106559
Cornish J, Callon KE, Bava U et al (2007) Preptin, another peptide product of the pancreatic beta-cell, is osteogenic in vitro and in vivo. American journal of physiology. Endocrinol Metab 292:E117–E122
Da Paz L, De Falco V, Teng N, Dos Reis L, Pereira R, Jorgetti V (2001) Effect of 17ß-estradiol or alendronate on the bone densitometry, bone histomorphometry and bone metabolism of ovariectomized rats. Braz J Med Biol Res 34:1015–1022
Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW (2019) Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 23:170–180
Ducy P, Amling M, Takeda S et al (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
El-Haroun H, Soliman M, El-Gawad A (2020) Comparative study on the possible protective effect of lepidium sativum versus teriparatide in induced osteoporosis in adult male Guinea pigs. Egypt J Histol 43:931–947
Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27:1–11
Feng R, Wang L, Li Z et al (2019) A systematic comparison of exercise training protocols on animal models of cardiovascular capacity. Life Sci 217:128–140
Fodor D, Bondor C, Albu A, Simon S-P, Craciun A, Muntean L (2013) The value of osteopontin in the assessment of bone mineral density status in postmenopausal women. J Investig Med 61:15–21
Gallo D, Zannoni GF, Apollonio P, et al. (2005) Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: effects on bone, uterus, and lipid profile. Menopause (New York, N.Y.) 12, 589–600.
Halasy-Nagy J, Rodan G, Reszka A (2001) Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 29:553–559
Heather B, Karen J (2002) Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas 41:157–165
Heilmeier U, Youm J, Torabi S, Link TM (2016) Osteoporosis imaging in the geriatric patient. Curr Radiol Rep 4:18
Honjo S, Mizunuma H, Soda M, Igarashi M (1989) Effect of estrogen replacement therapy on the serum osteocalcin level in the postmenopausal and castrated women. Nihon Sanka Fujinka Gakkai Zasshi 41:1571–1576
Ibm II (2017) Statistics for Windows, Version 25.0. Armonk, NY: IBM Corporation.
Kanis JA, Gertz B, Singer F, Ortolani S (1995) Rationale for the use of alendronate in osteoporosis. Osteoporos Int 5:1–13
Kanjana K, Haygarth KS, Wu W, Bartels DM (2013) Laboratory studies in search of the critical hydrogen concentration. Radiat Phys Chem 82:25–34
Kim BT, Mosekilde L, Duan Y et al (2003) The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Mineral Res 18:150–155
Kitaura H, Marahleh A, Ohori F et al (2020) Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci 21:5169
Kolios L, Hoerster AK, Sehmisch S et al (2010) Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis prophylaxis? Calcif Tissue Int 86:23–32
Lasota A, Danowska-Klonowska D (1995) (2004) Experimental osteoporosis–different methods of ovariectomy in female white rats. Rocz Akad Med Bialymst 49(Suppl 1):129–131
Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L (2003) Bone adaptation requires oestrogen receptor-α. Nature 424:389–389
Li N, Zheng YB, Han J et al (2013) Lower circulating preptin levels in male patients with osteoporosis are correlated with bone mineral density and bone formation. BMC Musculoskelet Disord 14:49
Mierzwicka A, Kuliczkowska-Plaksej J, Kolačkov K, Bolanowski M (2018) Preptin in women with polycystic ovary syndrome. Gynecol Endocrinol 34:470–475
Miller FN, Wiegman DL (1977) Anesthesia-induced alteration of small vessel response to norepinephrine. Eur J Pharmacol 44:331–337
Mohammad Rahimi GR, Bijeh N, Rashidlamir A (2020) Effects of exercise training on serum preptin, undercarboxylated osteocalcin and high molecular weight adiponectin in adults with metabolic syndrome. Exp Physiol 105:449–459
Müller ST, Keiler AM, Kräker K, Zierau O, Bernhardt R (2018) Influence of estrogen on individual exercise motivation and bone protection in ovariectomized rats. Lab Anim 52:479–489
Mustafa RA, NaA A, Hijazi HH, Header EA, Azzeh FS (2018) Biological effect of calcium and vitamin D dietary supplements against osteoporosis in ovariectomized rats. Prog Nutr 20:86–93
Nazari Soltan Aahmad S, Nourollahi S, Kazerouni F et al (2018) Investigation of the relation between bone mass density and serum preptin levels in pre- and postmenopausal women. J Bone Miner Metab 36:710–715
Notomi T, Okimoto N, Okazaki Y, Nakamura T, Suzuki M (2003) Tower climbing exercise started 3 months after ovariectomy recovers bone strength of the femur and lumbar vertebrae in aged osteopenic rats. J Bone Mineral Res 18:140–149
Novelli E, Diniz Y, Galhardi C et al (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41:111–119
Osman AS, Aly Labib DA, Omar AI (2018) Do acid suppressive drugs (pantoprazole and ranitidine) attenuate the protective effect of alendronate in estrogen-deficient osteoporotic rats? Egypt Rheumatol 40:99–106
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, Mcnamara LM, Augat P (2016) Bone mechanical properties and changes with osteoporosis. Injury 47:S11–S20
Ozkan Y, Timurkan ES, Aydin S et al (2013) Acylated and desacylated ghrelin, preptin, leptin, and nesfatin-1 Peptide changes related to the body mass index. Int J Endocrinol 2013:236085–236085
Özşahin ETT, Çam B, Dere F et al (2017) The effect of alendronate sodium on trabecular bone structure in an osteoporotic rat model. Turkish J Phys Med Rehabil 63:165
Popović T, Šrbić R, Matavulj M, Obradović Z & Sibinčić S (2016) Experimental model of osteoporosis on 14 weeks old ovariectomised rats: biochemical, histological and biomechanical study. Biologia Serbica 38.
Pytlik M, Kaczmarczyk-Sedlak I, Sliwiński L, Janiec W, Rymkiewicz I (2004) Effect of concurrent administration of alendronate sodium and retinol on development of changes in histomorphometric parameters of bones induced by ovariectomy in rats. Pol J Pharmacol 56:571–579
Röhling M, Herder C, Stemper T & Müssig K (2016) Influence of acute and chronic exercise on glucose uptake. J Diabet Res
Salazar M, Hernandes L, Ramos AL et al (2015) Effect of alendronate sodium on tooth movement in ovariectomized rats. Arch Oral Biol 60:776–781
Sharaf H, Shaffie N, Morsy F, Badawi M, Abbas N (2015) Role of some phytoestrogens in recovering bone loss: histological results from experimental ovariectomized rat models. J Arab Soc Med Res 10:65–75
Silva J, Fernandes T, Soci UP, Monteiro AW, Phillips MI, Em DEO (2012) Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc 44:1453–62
Singh A, Gill G, Kaur H, Amhmed M, Jakhu H (2018) Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog Orthod 19:1–8
Stachenfeld NS (2014) Hormonal changes during menopause and the impact on fluid regulation. Reproduct Sci (Thousand Oaks, Calif.) 21: 555–561
Stavropoulou A, Christopoulou GE, Anastassopoulos G et al (2005) Alteration in serum leptin correlates with alterations in serum N-telopeptide of collagen type I and serum osteocalcin during the progression of osteoporosis in ovariectomized rats. Clin Chem Lab Med 43:1359–1365
Sun X, Fengbo L, Xinlong M et al (2015) The effects of combined treatment with naringin and treadmill exercise on osteoporosis in ovariectomized rats. Sci Rep 5:1–9
Surve VV, Andersson N, Lehto-Axtelius D, Håkanson R (2001) Comparison of osteopenia after gastrectomy, ovariectomy and prednisolone treatment in the young female rat. Acta Orthop Scand 72:525–532
Suzuki N, Yoshimura Y, Deyama Y, Suzuki K, Kitagawa Y (2008) Mechanical stress directly suppresses osteoclast differentiation in RAW264. 7 cells. Int J Mol Med 21:291–296
Taherdoost H (2016) Sampling methods in research methodology; how to choose a sampling technique for research. How to Choose a Sampling Technique for Research
Tanaka S, Narusawa K, Onishi H et al (2011) Lower osteocalcin and osteopontin contents of the femoral head in hip fracture patients than osteoarthritis patients. Osteoporos Int 22:587–597
Tomeleri CM, Ribeiro AS, Souza MF et al (2016) Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp Gerontol 84:80–87
Turkozan N, Ulusoy A, Balcioglu H, Bal F, Ozel S (2009) The effects of ovariectomy and naproxen treatment on the strength of femoral midshaft and molar alveolar region in rats. Int J Morphol 27:659–666
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Investig 116:1186–1194
Xiao C, Li W, Lu T, Wang J, Han J (2019) Preptin promotes proliferation and osteogenesis of MC3T3-E1 cells by upregulating β-catenin expression. IUBMB Life 71:854–862
Xiong H, Wei L, Hu Y, Zhang C, Peng B (2010) Effect of alendronate on alveolar bone resorption and angiogenesis in rats with experimental periapical lesions. Int Endod J 43:485–491
Yarrow JF, Mccoy SC, Ferreira JA et al (2012) A rehabilitation exercise program induces severe bone mineral deficits in estrogen deficient rats following extended disuse. Menopause (New York, NY) 19:1267
Yoon K-H, Cho D-C, Yu S-H, Kim K-T, Jeon Y, Sung J-K (2012) The change of bone metabolism in ovariectomized rats : analyses of microct scan and biochemical markers of bone turnover. J Korean Neurosurg Soc 51:323–327
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed in research design and interpretation of the studies. KAA, HOM, and NAM conducted experiments and performed data analysis. KAA, RRAA, RSR, and NAM contributed to review and writing of the manuscript. All individuals contributed in this research are included in the list of authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelfattah Abulfadle, K., Refaat Abdelkader Atia, R., Osama Mohammed, H. et al. The potential anti-osteoporotic effect of exercise—induced increased preptin level in ovariectomized rats. Anat Sci Int 98, 22–35 (2023). https://doi.org/10.1007/s12565-022-00666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-022-00666-7