Abstract
Skeletal muscles fatigue after exercise, and reductions in maximal force appear. A difference in training status between the legs was introduced by unilateral immobilization of the calf muscles for 2 weeks in young men, who were randomly assigned to two groups, either a RUN group (n = 8) that was exposed to prolonged exercise (1-h running: individual pace) or a REST group (n = 12) that did no exercise after immobilization. Cross-sectional area (CSA) of the triceps-surae muscles was calculated by magnetic resonance imaging (MRI), and maximal voluntary contraction (MVC) force of the plantar flexors was measured before and after immobilization and after the running protocol. The CSA of triceps-surae muscles decreased significantly with a 7% reduction in both groups. A significant drop in the MVC of the triceps-surae muscle (10%; P < 0.05) was observed in response to immobilization. When subjected to running exercise immediately after immobilization, the muscle strength of the triceps-surae muscles dropped even further, but just in the immobilized leg (41%; P < 0.05). The present study highlights the importance of determining the muscle endurance when evaluating the effect of immobilization on muscle parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle demonstrates enormous plasticity, and increases and decreases in activity level influence strength and endurance of the muscle. While strength training will increase maximal voluntary contraction (MVC) and endurance training will increase the oxygen uptake of muscles (Alway et al. 1988), inactivity or unloading will decrease both strength and endurance of the muscle (Appell 1990).
Studies on immobilization and unloading have underlined the fact that skeletal muscle tissue responds quickly to unloading with a lowering of muscle strength by approximately 10–22% after 2 weeks (Alfredson et al. 1998; Berg and Tesch 1996; Urso et al. 2006), and that longer periods of unloading decrease the muscle strength and cross-sectional area (CSA) even further (Christensen et al. 2008; Stevens et al. 2006). In addition to the muscle strength, neuromuscular functions have also shown impairment by immobilization (Clark et al. 2006). Numerous studies have measured MVC before and immediately after immobilization, or in response to a recovery period with reloading (Christensen et al. 2008; Vandenborne et al. 1998; White et al. 1984). But the question is whether measurements of MVC or neuromuscular control are sufficient to describe the changes taking place within the muscle tissue in response to immobilization and inactivity. Fatigue can be defined as any exercise-induced reduction in the ability to generate muscle force or power (Gandevia 2001), and subdivided into central fatigue, described as a failure in the neural drive, and peripheral fatigue related to the impairment of the force-generating capacity of the muscle (Maclaren et al. 1989). Numerous studies of fatigue have shown that both peripheral and central fatigue impair exercise performance (Nybo and Nielsen 2001; Saldanha et al. 2008; Bigland-Ritchie et al. 1986), and that the risk of injuries increases when athletes are in a fatigued state (Thorlund et al. 2008; Ostenberg and Roos 2000; Hawkins et al. 2001). One explanation could be that untrained muscles experience higher levels of load and stress than do trained muscles, and that when the load exceeds the capacity of the tissue, injuries might result.
In the present study design, immobilization is utilized as a model to weaken the muscle, which provides the contrast of a paired untrained and trained legs. When subjected to the same absolute load (running), this design allowed the authors to determine the differentiated response based on training status. Thus, the aim of the present study is to determine whether a reduction in muscle strength as a result of previous immobilization influences the fatigue state of the muscle. The authors hypothesized that the immobilized leg would respond with a further drop in muscle strength when subjected to a workload. This would help clarify whether untrained tissue has a higher injury risk as compared to well-trained tissue.
Materials and methods
Twenty young healthy non-smoking men (age 24 ± 3 years, height 184 ± 7 cm, weight 84 ± 12 kg, BMI 25 ± 3 kg m−2) were recruited for this study, which evaluated the effects of 2 weeks of immobilization with plaster of Paris from below the knee to the toes. The ankle joint was immobilized at a 90° angle, leaving the tendon in a neutral position. This study randomized whether it was the non-dominant or the dominant leg that was immobilized. The subjects used crutches, since no weight bearing was allowed on the immobilized leg through the whole protocol. None of the subjects suffered from any present or previous muscle-tendon problems or injuries in the lower extremity. All subjects gave, after receiving oral and written information, written informed consent to participate in the study, in adherence to the declaration of Helsinki. The study was approved by the local Human Subject Ethics Committee of Copenhagen and Frederiksberg [(H KF) 319605].
The subjects were all immobilized in one leg and then randomly assigned to either a control group (REST; n = 12) or a group that was exposed to 1-h acute running exercise immediately after the immobilization period was complete (RUN; n = 8). After 2 weeks of immobilization, the cast was checked for signs of load bearing (which would have resulted in exclusion from the study) and then removed, and then an magnetic resonance imaging (MRI) scan was taken. Immediately after removal of the plaster casts, the subjects of the REST group were strength tested, while the subjects of the RUN group were remobilized on a treadmill for 1 h at individual pace. The subjects were told to keep running at all times and as close to 70–80% of maximum heart rate as possible. However, the subjects were allowed to lower the pace if the exercise became uncomfortable. During the run, the subjects’ heart rates were monitored. Following the running exercise, a subsequent strength test of the calf muscles was made.
MVC force
The subjects were seated in a custom-made rigid steel frame as previously described (Magnusson et al. 2003). One leg at a time was tested, and the right leg was always tested first. The testing position of the leg was with knees fully extended, the foot resting in a neutral position on the middle of the steel plate, and the hip in 90° of flexion. The position of the heel was adjusted to ensure that the mechanical axis of rotation corresponded to the lateral malleolus. Plantar flexion force was measured with a strain gauge load cell attached between the footplate and the steel frame. Two sets of five contractions were performed (three force ramp contractions and two maximum isometric plantar flexions). The first set was used as a warm-up and the second set sampled at 50 Hz by an analog-to-digital converter. Each contraction lasted 5 s, and there was a resting period of 1 min between each contraction. Data were analyzed for maximum isometric plantar flexion force defined as the highest effort out of the two maximum isometric plantar flexions.
Muscle morphology
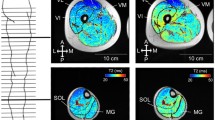
Axial plane images of the calf muscle with the ankle joint in 90° were obtained by MRI. CSA of the triceps-surae muscle was measured at 10 cut points using a lower extremity coil (General Electric Sigma Horizon LX, 1.5 T, (T1)-weighed spin echo). The scanning protocol for the triceps surae was repetition time to echo time 400:6; field of view 28; matrix 320 × 320; slice thickness 6 mm; spacing 25 mm. To avoid differences in scanning procedure, one person scanned all images using a standardized protocol. CSA from triceps-surae muscle was manually outlined using the software (http://www.sim.hcuge.ch/osiris; National Institutes of Health color scale mode). To account for the superimposed magnetic gradient field, the software program Osiris 4.19 was used to adjust the color intensity of each image (Couppe et al. 2008). The intensity was increased until the first pixel turned white in the tibia bone. Further, the image was shifted back to the gray-scale image display for analyzing CSA. All images were manually analyzed three times, single blinded, and the mean of the three measurements was calculated for further analysis.
Statistics
To analyze the interactions between the two groups (REST, RUN), between the two time points (pre- and post-immobilization) and between the two legs (control and immobilized legs), a three-way ANOVA and a Tukey’s HSD post hoc test were applied using the computer software SAS 9.1. Paired t tests were used to analyze differences between pre- and post-immobilization and between the control and the immobilized legs. Graph Pad PRISM 4.0 was used for all subsequent statistical analyses. In all statistical analyses, the level of significance was set to P < 0.05. All results are expressed as mean ± SD.
Results
Immobilization
In none of the subjects could any indications of loading of the immobilized leg be detected.
Muscle strength
A significant interaction between the two groups (REST, RUN), between the two time points (pre- and post-immobilization), and between the two legs (control and immobilized legs) has been observed (P ≤ 0.05). Furthermore, significant interactions between the legs (control and immobilized legs) and the time points (pre- and post-immobilization) were observed (P ≤ 0.05). In additional, significant differences were observed between the groups (REST and RUN) and time points (pre- and post-immobilization) (P ≤ 0.05). No difference in MVC of the triceps-surae muscle could be detected between the legs involved in this study prior to immobilization (P > 0.05). Furthermore, both the interactions between the leg and time and between groups and time were significant (P < 0.05 and P < 0.05, respectively).
Immobilization of the triceps-surae muscle resulted in a significant drop in MVC of 10% [REST: 233 ± 31 to 207 ± 22 N m (mean ± SD); P < 0.05] (Fig. 1). When exposed to exercise (1-h running), a significant drop of 41% in strength was determined (RUN: 225 ± 72 to 129 ± 66 N m; P < 0.05) (Fig. 2). This drop in strength due to the combination of immobilization and exercise was significantly larger than that due to the immobilization alone. However, there was not a significant reduction in strength (7%) resulting from the exercise alone (RUN: 210 ± 66 to 192 ± 72 N m; n.s.) (Fig. 2).
Muscle strength of REST group. Maximal voluntary contraction (MVC) force of the control and the immobilized legs, pre- and post-immobilization of the REST group. A significant interaction between the two groups (REST, RUN), between the two time points (pre- and post-immobilization) and between the two legs (control and immobilized legs) has been observed (P ≤ 0.05). The immobilized leg weakened significantly in response to immobilization (P ≤ 0.05), while no significant changes took place in the control leg. Further analysis showed a significant difference between the control leg and the immobilized leg post-immobilization (P ≤ 0.05). All values are shown as mean ± SD
Muscle strength of RUN group. Maximal voluntary contraction (MVC) force of the control and the immobilized legs, pre- and post-immobilization of the RUN group. A significant interaction between the two groups (REST, RUN), between the two time points (pre- and post-immobilization) and between the two legs (control and immobilized legs) has been observed (P ≤ 0.05). The immobilized leg weakened significantly in response to immobilization (P ≤ 0.05), while no significant changes took place in the control leg. Further analysis showed a significant difference between the control leg and the immobilized leg post-immobilization (+running exercise) (P ≤ 0.05). All values are shown as mean ± SD
Cross-sectional area of the triceps-surae muscle
An equal and significant decrease of CSA in the triceps-surae muscle was observed in both groups in response to 2 weeks of immobilization in the immobilized leg [REST (6%): 5,413 ± 908 to 5,077 ± 824 mm2 (mean ± SD); RUN (7%): 5,226 ± 794 to 4,844 ± 675 mm2 (mean ± SD); P < 0.05] (Fig. 3). The non-immobilized legs of both the REST and RUN groups showed no significant changes in CSA of the triceps-surae muscle over the 2 weeks of the experiment (P > 0.05) (Fig. 3).
CSA of the triceps-surae muscle. CSA of the triceps-surae muscle of the control and the immobilized legs, of both the REST and the RUN groups, pre- and post-immobilization. There was no significant difference between the REST and the RUN groups, but a significant decrease of CSA was observed in the immobilized leg post-immobilization in both REST (P < 0.05) and RUN (P < 0.05) groups, which is a reduction of approximately 7%. All values are shown as mean ± SD
Discussion
The major finding of the present study is that the muscular strength of the calf muscles drops dramatically (−41%) in response to a period of 2 weeks of immobilization followed immediately by a prolonged run. This decrease is more pronounced than the drop in force due to immobilization per se (10%). This paper is the first to demonstrate such a very large reduction in muscle strength due to the combination of immobilization and endurance exercise.
However, it has been demonstrated previously that 5 weeks of unilateral lower limb suspension increased the vulnerability to eccentric exercise-induced dysfunction and muscle injury, even at relatively light loads (Ploutz-Snyder et al. 1996). These results strongly support our findings.
Measurements of MVC, whether isometric, eccentric or concentric, have often been used to monitor the loss in muscle functionality and recovery in response to surgery, immobilization and rehabilitation interventions (Alfredson et al. 1998; Christensen et al. 2008; Berg and Tesch 1996). In many cases, only a single MVC measurement has been made to determine the changes in muscle performance (Christensen et al. 2008). The present data, however, underline that a single measurement of MVC after immobilization leads to an underestimation of the strength deficit. This is very important for many clinical situations, where the strength recovery of an immobilized or injured extremity, measured as MVC, is used to determine the return of the athlete/patient to sport or to labor (Maaløe and Poulsen 2003). This could potentially predispose the athlete to overloading and thus injury in the muscular-tendinous unit. A spaceflight study supports this suggestion. It has been observed that 17 days of spaceflight resulted in significant changes in muscle function during the recovery phase and not in microgravity (Narici et al. 2003). Furthermore, unloading has been shown to increase the vulnerability to injury from ECC exercise. The authors of this study concluded that dysfunction and injury during reloading were sufficient to prolong recovery (Ploutz-Snyder et al. 1996).
Full recovery of human tissue after immobilization is known to be a slow process (Alfredson et al. 1998). Often, both muscle strength (Alfredson et al. 1998) and neuromuscular functions are strongly impaired in response to unloading (Karladani et al. 2001). Thus, a 5-year follow-up study on patients surgically treated for Achilles tendinosis followed by immobilization showed the persistence of a significant side-to-side difference in strength at 1- and 5-year follow-up after the surgery (Ohberg et al. 2001).
It is well documented that hypertrophy, determined as increased muscle CSA, is a result of resistance training (Cureton et al. 1988; Brown et al. 1990), and that muscle CSA correlates with muscle strength (Mayhew et al. 1993). Furthermore, it is well known that immobilization leads to muscle atrophy (Stevens et al. 2006; Stehno-Bittel et al. 1998; Veitch et al. 2006). In the present study, we observed a dramatic reduction in MVC strength of triceps-surae muscle following the immediate remobilization of the immobilized leg, although no difference in CSA of the triceps-surae muscle was detected between the REST and RUN groups. In both groups, there was a reduction in triceps-surae muscle CSA of approximately 7% after 2 weeks of immobilization. The present data indicate that the decrease in MVC in response to running exercise after immobilization might be caused by multiple physiological and biochemical factors, rather than only the loss of muscle contractile tissue as determined by CSA.
Fatigue is defined as a reduction in maximal force or power output that occurs with exercise (Gandevia 2001). Fatigue can arise from peripheral changes at the level of the skeletal cells (peripheral fatigue) and/or due to a decreased activation of the central nervous system (central fatigue) (Gandevia 2001; Allen et al. 2008). Central fatigue can be caused by a decline in the supraspinal drive of motorneurons and by direct inhibition of motorneurons due to altered input from muscle receptors (Gandevia 2001). One study showed a central activation failure in response to exercise; a marked reduction was observed in voluntary force development during prolonged maximal isometric contractions, despite the fact that total muscle force was not affected (Nybo and Nielsen 2001). In a recent study, it was found that 2 h of treadmill running caused a reduction in MVC (17%) of plantar muscle strength which was mainly related to central fatigue (Saldanha et al. 2008).
Fatigued and weak muscles have been shown to exhibit marked neural impairments. Two weeks of ankle joint immobilization not only led to a decrease in MVC, but was also accompanied by an increase in the H-reflex excitability (Lundbye-Jensen and Nielsen 2008).
Furthermore, unloading has been shown to cause a decrease in the central activation. Neural factors in central activation have been shown to explain 48% of the variability in strength loss, while muscular factors, such as sarcolemma function, explained 39% of the variability (Clark et al. 2006).
The present study observed also a reduction in MVC due to the 1-h run (7%) which fits nicely with the data in the literature. The additional drop in MVC of 30–40%, as determined in the present study in response to exercise, had not previously been reported in the literature. This indicates that peripheral fatigue had a great impact on the present subjects and this leads to the suggestion that fatigue in untrained muscles in response to endurance exercise primarily is related to peripheral factors. Our observations are in agreement with the finding of an accelerated development of peripheral fatigue following 8 weeks of bed rest (Mulder et al. 2007). The results do not exclude the importance of central fatigue. Most studies are done in healthy and trained subjects, a fact which could cause overestimation of the peripheral fatigue as compared with untrained individuals. Our results indicate that untrained and weak muscles might react differently to load than trained muscles, and that peripheral fatigue might have a relatively greater impact on performance in weak muscles than central fatigue.
The subjects of the present study were exposed to only a single bout of running exercise, so unfortunately it was not possible to investigate whether running performance was affected by the 2 weeks of immobilization. This would be an interesting issue for future studies. In conclusion, the present data underline that untrained and weak muscles react differently to load than do trained muscles, and indicate that peripheral fatigue might have an impact on performance in previously immobilized and weak muscles.
Both exercise and immobilization are known to impair muscle strength (Christensen et al. 2008; Petersen et al. 2007). While exercise causes fatigue (Westerblad and Allen 2002), immobilization leads to weakness of the tissue (Christensen et al. 2008; Berg and Tesch 1996; Pathare et al. 2006). Fatigue has been defined as any exercise-induced reduction in the maximal power capacity to generate force or power output (Vollestad 1997), whereas weakness describes a maintained low maximal force, which persists over longer periods and appears independent of exercise (Vollestad 1997). Both conditions might be of interest in relation to development of musculo-skeletal injuries. It is well established that the risk for acute injuries increases with fatigued state (Ostenberg and Roos 2000; Hawkins et al. 2001), as muscle fatigue alters the neuromuscular system, leads to impaired muscle performance, and modifies both peripheral proprioceptive system and the central processing of sensory inputs (Gandevia 2001; Taylor et al. 2000). Such fatigue causes impaired balance, clumsiness and decreased precision of motor control (Salavati et al. 2007; Corbeil et al. 2003; Sharpe and Miles 1993). While the coherence between fatigue and acute injuries is well documented, it is still unclear whether weak and untrained muscles or muscles with increased fatigability, such as those following immobilization, play a role in the development of overuse injuries as well. Achilles tendinopathy is a very common injury among long distance runners (Schepsis et al. 2002). Training errors, such as running excessive distances and a great running intensity, have been reported to be major causes of tendon overuse injuries in runners (Clement et al. 1984; Kannus 1997). It now seems obvious to give consideration as to whether overuse injuries might be due to weakness or fatigue of the musculature, or alternatively to other extrinsic factors. It has been suggested previously that a weak or fatigued muscle, as in the present case, is unable to protect the tendon from strain injury since the energy-absorbing capacity of the whole muscle-tendon unit is reduced (Kannus 1997). Thus, fatigue or general muscle weakness might result in repeated tissue overload and lead to the development of overuse injuries.
The present study combines factors of weakness and fatigue by exercising a strong and a weak muscle simultaneously with the same relative load during 1-h running exercise. This “model” allows for the investigation of how a weak muscle-tendon unit reacts on reloading or overloading. This model may be used in an attempt to understand the etiology of contraction-induced overuse injuries in the Achilles tendon. The optimal treatment to prevent and/or rehabilitate injuries is a central problem facing both workers and athletes, and more evidence is needed to solve these problems.
Given the design of this present study, it is not possible to determine whether the fatigue following the run is due to peripheral fatigue. The application of electrical stimulation before and after the running exercise might be able to demonstrate the appearance or absence of central fatigue. Another limitation is the lack of postural control measurements, which could have underscored a greater risk of injury due to weak muscles, e.g. loaded very early after immobilization.
Conclusion
The present findings show a significant difference in MVC in the immobilized leg in response to 1-h running exercise, while only a minor drop in MVC was observed in response to immobilization per se. The drop in force-generating capacity indicates that the present model can be used as a way to investigate the divergent response to the same absolute workload in trained, weak and fatigued muscles. In addition, the present study design may also be used in an attempt to understand the etiology of contraction-induced overuse injuries in the muscle-tendon unit.
References
Alfredson H, Pietila T, Ohberg L, Lorentzon R (1998) Achilles tendinosis and calf muscle strength. The effect of short-term immobilization after surgical treatment. Am J Sports Med 26:166–171
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332. doi:10.1152/physrev.00015.2007
Alway SE, MacDougall JD, Sale DG, Sutton JR, McComas AJ (1988) Functional and structural adaptations in skeletal muscle of trained athletes. J Appl Physiol 64:1114–1120
Appell HJ (1990) Muscular atrophy following immobilisation. A review. Sports Med 10:42–58. doi:10.2165/00007256-199010010-00005
Berg HE, Tesch PA (1996) Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand 157:63–70. doi:10.1046/j.1365-201X.1996.476217000.x
Bigland-Ritchie B, Furbush F, Woods JJ (1986) Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol 61:421–429
Brown AB, McCartney N, Sale DG (1990) Positive adaptations to weight-lifting training in the elderly. J Appl Physiol 69:1725–1733
Christensen B, Dyrberg E, Aagaard P, Enehjelm S, Krogsgaard M, Kjaer M, Langberg H (2008) Effects of long-term immobilization and recovery on human triceps surae and collagen turnover in the Achilles tendon in patients with healing ankle fracture. J Appl Physiol 105:420–426. doi:10.1152/japplphysiol.00201.2008
Clark BC, Manini TM, Bolanowski SJ, Ploutz-Snyder LL (2006) Adaptations in human neuromuscular function following prolonged unweighting. II. Neurological properties and motor imagery efficacy. J Appl Physiol 101:264–272. doi:10.1152/japplphysiol.01404.2005
Clement DB, Taunton JE, Smart GW (1984) Achilles tendinitis and peritendinitis: etiology and treatment. Am J Sports Med 12:179–184. doi:10.1177/036354658401200301
Corbeil P, Blouin JS, Begin F, Nougier V, Teasdale N (2003) Perturbation of the postural control system induced by muscular fatigue. Gait Posture 18:92–100. doi:10.1016/S0966-6362(02)00198-4
Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP (2008) Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 105:805–810. doi:10.1152/japplphysiol.90361.2008
Cureton KJ, Collins MA, Hill DW, McElhannon FM Jr (1988) Muscle hypertrophy in men and women. Med Sci Sports Exerc 20:338–344. doi:10.1249/00005768-198808000-00003
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Hawkins RD, Hulse MA, Wilkinson C, Hodson A, Gibson M (2001) The association football medical research programme: an audit of injuries in professional football. Br J Sports Med 35:43–47. doi:10.1136/bjsm.35.1.43
Kannus P (1997) Etiology and pathophysiology of chronic tendon disorders in sports. Scand J Med Sci Sports 7:78–85
Karladani AH, Svantesson U, Granhed H, Styf J (2001) Postural control and torque of the knee joint after healed tibial shaft fracture. Injury 32:57–60. doi:10.1016/S0020-1383(00)00112-1
Lundbye-Jensen J, Nielsen JB (2008) Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans. J Physiol 586:4121–4135. doi:10.1113/jphysiol.2008.156547
Maaløe L, Poulsen I (2003) Inaktivitet, immobilitet og sygepleje (inactivity, immobility and nursing), 2nd edn. Munksgaard, Denmark, pp 28–46
Maclaren DP, Gibson H, Parry-Billings M, Edwards RH (1989) A review of metabolic and physiological factors in fatigue. Exerc Sport Sci Rev 17:29–66
Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M (2003) Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci 58:123–127
Mayhew JL, Piper FC, Ware JS (1993) Anthropometric correlates with strength performance among resistance trained athletes. J Sports Med Phys Fitness 33:159–165
Mulder ER, Kuebler WM, Gerrits KH, Rittweger J, Felsenberg D, Stegeman DF, de Haan A (2007) Knee extensor fatigability after bedrest for 8 weeks with and without countermeasure. Muscle Nerve 36:798–806. doi:10.1002/mus.20870
Narici M, Kayser B, Barattini P, Cerretelli P (2003) Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol 90:275–282. doi:10.1007/s00421-003-0955-7
Nybo L, Nielsen B (2001) Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 91:1055–1060
Ohberg L, Lorentzon R, Alfredson H (2001) Good clinical results but persisting side-to-side differences in calf muscle strength after surgical treatment of chronic Achilles tendinosis: a 5-year follow-up. Scand J Med Sci Sports 11:207–212. doi:10.1034/j.1600-0838.2001.110403.x
Ostenberg A, Roos H (2000) Injury risk factors in female European football. A prospective study of 123 players during one season. Scand J Med Sci Sports 10:279–285. doi:10.1034/j.1600-0838.2000.010005279.x
Pathare NC, Stevens JE, Walter GA, Shah P, Jayaraman A, Tillman SM, Scarborough MT, Parker GC, Vandenborne K (2006) Deficit in human muscle strength with cast immobilization: contribution of inorganic phosphate. Eur J Appl Physiol 98:71–78. doi:10.1007/s00421-006-0244-3
Petersen K, Hansen CB, Aagaard P, Madsen K (2007) Muscle mechanical characteristics in fatigue and recovery from a marathon race in highly trained runners. Eur J Appl Physiol 101:385–396. doi:10.1007/s00421-007-0504-x
Ploutz-Snyder LL, Tesch PA, Hather BM, Dudley GA (1996) Vulnerability to dysfunction and muscle injury after unloading. Arch Phys Med Rehabil 77:773–777. doi:10.1016/S0003-9993(96)90255-5
Salavati M, Moghadam M, Ebrahimi I, Arab AM (2007) Changes in postural stability with fatigue of lower extremity frontal and sagittal plane movers. Gait Posture 26:214–218. doi:10.1016/j.gaitpost.2006.09.001
Saldanha A, Nordlund Ekblom MM, Thorstensson A (2008) Central fatigue affects plantar flexor strength after prolonged running. Scand J Med Sci Sports 18:383–388
Schepsis AA, Jones H, Haas AL (2002) Achilles tendon disorders in athletes. Am J Sports Med 30:287–305
Sharpe MH, Miles TS (1993) Position sense at the elbow after fatiguing contractions. Exp Brain Res 94:179–182. doi:10.1007/BF00230480
Stehno-Bittel L, Reddy GK, Gum S, Enwemeka CS (1998) Biochemistry and biomechanics of healing tendon. Part I. Effects of rigid plaster casts and functional casts. Med Sci Sports Exerc 30:788–793. doi:10.1097/00005768-199806000-00002
Stevens JE, Pathare NC, Tillman SM, Scarborough MT, Gibbs CP, Shah P, Jayaraman A, Walter GA, Vandenborne K (2006) Relative contributions of muscle activation and muscle size to plantarflexor torque during rehabilitation after immobilization. J Orthop Res 24:1729–1736. doi:10.1002/jor.20153
Taylor JL, Butler JE, Gandevia SC (2000) Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. Eur J Appl Physiol 83:106–115. doi:10.1007/s004210000269
Thorlund JB, Michalsik LB, Madsen K, Aagaard P (2008) Acute fatigue-induced changes in muscle mechanical properties and neuromuscular activity in elite handball players following a handball match. Scand J Med Sci Sports 18:462–472
Urso ML, Clarkson PM, Price TB (2006) Immobilization effects in young and older adults. Eur J Appl Physiol 96:564–571. doi:10.1007/s00421-005-0109-1
Vandenborne K, Elliott MA, Walter GA, Abdus S, Okereke E, Shaffer M, Tahernia D, Esterhai JL (1998) Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve 21:1006–1012. doi:10.1002/(SICI)1097-4598(199808)21:8<1006::AID-MUS4>3.0.CO;2-C
Veitch SW, Findlay SC, Hamer AJ, Blumsohn A, Eastell R, Ingle BM (2006) Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int 17:364–372. doi:10.1007/s00198-005-2025-y
Vollestad NK (1997) Measurement of human muscle fatigue. J Neurosci Methods 74:219–227. doi:10.1016/S0165-0270(97)02251-6
Westerblad H, Allen DG (2002) Recent advances in the understanding of skeletal muscle fatigue. Curr Opin Rheumatol 14:648–652. doi:10.1097/00002281-200211000-00003
White MJ, Davies CT, Brooksby P (1984) The effects of short-term voluntary immobilization on the contractile properties of the human triceps surae. Q J Exp Physiol 69:685–691
Acknowledgments
We thank Peter Butty for his great support by MRI scanning all subjects and Burt Farber for his generous contribution in correcting the language and the grammar of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pingel, J., Moerch, L., Kjaer, M. et al. The influence of training status on the drop in muscle strength after acute exercise. Eur J Appl Physiol 106, 605–611 (2009). https://doi.org/10.1007/s00421-009-1055-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1055-0