Abstract
Heart rate recovery is an indirect marker of autonomic function and changes therein may offer a practical way of quantifying the physiological effects of training. We assessed whether per cent heart rate recovery (HRr%) after a standardized sub-maximal running (Heart rate Interval Monitoring System: HIMS) test, changed with acute changes in training load. A total of 28 men and women (mean age 30 ± 5 years) trained ad libitum for 2 weeks during which their heart rate (HR) was recorded. Training load was quantified using Training Impulse (TRIMPs). The participants were grouped based on whether they increased (Group I, n = 9), decreased (Group D, n = 8) or kept their training load constant (Group S, n = 11) from week 1 to week 2. Each week, the subjects completed a HIMS test. Changes between weeks in HR at the end of the test and HRr% were compared between groups. Mean per cent change in TRIMPs from week 1 to week 2 was significantly different among the groups (Group I, 55 ± 21% vs Group S, −6 ± 6% vs Group D, −42 ± 16%; P < 0.05). Group I had a slower HRr% and Group D tended to have a slightly faster HRr% after HIMS 2 than after HIMS 1 (mean per cent change 5.6 ± 8.7 vs −2.6 ± 3.9; P = 0.03). Thus a negative effect on HRr was observed with increases in training load. Sub-maximal HR was not affected by acute changes in training load. Whereas HR during exercise measures cardiac load, HRr may reflect the state of the autonomic nervous system, indicating the body’s capacity to respond to exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing demand for improvements in elite sports performances and the desire for more rapid achievement of personal fitness/sports goals require a more precise, evidence-based method of prescribing personalized training programmes. An optimal programme would prevent undertraining, overtraining and injury (Meeusen et al. 2006), and produce favourable physiological adaptations towards desired outcomes at specific times (Lambert and Borresen 2006). In order to achieve this, workloads need to be manipulated over time to correctly balance the stress stimulus of exercise with periods of recovery, thereby inducing positive haemodynamic adaptations (Charlton and Crawford 1997) that contribute to improvements in performance. The ability to measure and monitor these positive and negative training effects would thus make a valuable contribution to the design of effective training programmes.
Endurance training induces numerous physiological adaptations, many of which have been proposed as markers of overtraining (Meeusen et al. 2004; Smith and Roberts 1994; Urhausen et al. 1995; Urhausen and Kindermann 2002). However, no single measure has been identified that quantitatively assesses how an athlete is responding to training. Ideally, this marker should be sensitive to change, predictive, easy to quantify and require methods of measurement that are non-invasive, easy to administer and inexpensive. The nervous system, particularly the sympathetic nervous system and adrenal glands, is pivotal to the body’s response to acute training stimuli and adaptation (Mazzeo 1991) and has a direct effect on heart rate. Carter et al. (2003a) reviewed studies investigating changes in autonomic control of heart rate with endurance training and concluded that parasympathetic activity to the heart is increased and sympathetic activity is reduced at rest and during sub-maximal exercise following endurance training. Changes in sleeping (O’Connor et al. 1993), resting (Bonaduce et al. 1998; Uusitalo et al. 1998; Wilmore et al. 1996, 2001), sub-maximal (Skinner et al. 2003; Swaine et al. 1994; Uusitalo et al. 1998; Wilmore et al. 2001) and maximal heart rate (Zavorsky 2000) have been investigated for their potential as markers of training status. However, conflicting results regarding the effects of training on these indices exist, making the usefulness of these measures to monitor training status inconclusive. Heart rate variability (HRV) has been recognized as a means with which to evaluate the autonomic control of heart rate (Aubert et al. 2003). However, the interpretation of heart rate variability indices remains inconclusive due to inconsistent methodologies used in their measurement (Achten and Jeukendrup 2003; al Ani et al. 1996; Aubert et al. 2003; Tulppo et al. 2003). In addition, age (Carter et al. 2003b; Levy et al. 1998; Tulppo et al. 1998), respiration (al Ani et al. 1996; Tulppo et al. 2003) and temperature (Carter et al. 2003b) may influence measurements of heart rate variability. Consensus about the effects of training on heart rate variability has also not been reached, as the disparity in training protocols used in various studies makes the comparison of results difficult (al Ani et al. 1996; Bonaduce et al. 1998; Carter et al. 2003a; Iwasaki et al. 2003; Loimaala et al. 2000; Melanson and Freedson 2001; Tulppo et al. 2003). The use of heart rate variability in the assessment of training status may thus be impractical at this stage, until standardized methods are adopted.
Few studies have investigated the effects of training on the autonomic control of heart rate after the cessation of exercise (Bunc et al. 1988; Michael et al. 1972; Short and Sedlock 1997; Sugawara et al. 2001; Yamamoto et al. 2001). Heart rate recovery is the rate at which heart rate decreases, usually in the first minute or two, after moderate to heavy exercise and is a consequence of parasympathetic re-activation and sympathetic withdrawal (Kannankeril et al. 2004; Pierpont and Voth 2004; Savin et al. 1982; Shetler et al. 2001). Cross-sectional studies have shown that trained athletes have a faster heart rate recovery after exercise at similar absolute intensities than untrained subjects (Bunc et al. 1988; Short and Sedlock 1997). Bunc et al. (1988) studied highly trained rowers and Short and Sedlock (1997) compared untrained subjects (that had participated in 2 h of walking or low-impact aerobics per week for 4–6 months) with trained runners, cyclists and swimmers who had been participating in more than 5 h of training per week for 4–6 months before the study began. Otsuki et al. (2007) showed that both strength- and endurance-trained athletes have improved heart rate recovery after 8 min of steady-state exercise at 40% of maximal oxygen uptake compared to untrained controls. In this study, all athletes had been competitive for at least 2 years. The endurance-trained athletes had been participating in about 5.7 sessions per week, each lasting about 2.6 h at an RPE of 15–17. The strength-trained athletes had participated in about 5.2 sessions per week, each lasting about 3.1 h at an average RPE of 15. Strength training comprised weight training, throwing, sprint and plyometric and skills training (Otsuki et al. 2007). Longitudinal studies have found that heart rate recovery improves significantly after moderate intensity training (Yamamoto et al. 2001) and that this enhanced post-exercise vagal re-activation is reversed after subsequent detraining (Michael et al. 1972; Sugawara et al. 2001). Yamamoto et al. (2001) suggested that endurance training-induced changes in cardio autonomic regulation, partly contributes to the decrease in heart rate during post-exercise recovery and that adaptations to cardiac autonomic control occur sooner in immediate post-exercise periods than at rest. These results suggest that there may be potential in using heart rate recovery to distinguish trained from untrained individuals and to establish an athlete’s state of training by assessing the changes in heart rate after exercise (Bunc et al. 1988).
Previous studies have therefore shown that there are long-term training effects on heart rate recovery. What has not been as thoroughly investigated is whether heart rate recovery is sensitive to small acute changes in training workload. This is important within the framework of optimizing training programmes, as the sensitivity of the measure would be a pre-requisite for it to be used frequently to monitor the physiological response to exercise. Since heart rate recovery is governed by the autonomic nervous system (Kannankeril et al. 2004; Pierpont and Voth 2004; Savin et al. 1982), it is likely that the changes observed in heart rate recovery may provide some information on the current condition of the autonomic nervous system. The autonomic nervous system interacts with all other physiological systems; therefore, monitoring changes in the autonomic nervous system function in response to an exercise stimulus may reflect the body’s current capacity to respond to such a stress. The use of heart rate recovery to monitor training status is appealing, as it is a non-invasive measure that can be quantified easily and frequently with little inconvenience to the athlete.
Being relatively “unchartered” territory, a number of questions arise about what heart rate recovery represents and how it should be interpreted: Are the changes that have been observed in heart rate recovery with endurance training an indication of an athlete’s chronic, cumulative state of fitness and/or fatigue (adaptation)? If so, could changes in heart rate recovery detect or predict overtraining or is it merely another symptom of the syndrome? Further, does heart rate recovery also change acutely in response to changes in the training stimulus (load)? If so, could changes in heart rate recovery be a marker of overreaching, a more acute physiological response to increased training load, or even indicate if an athlete is undertraining? To begin to answer these complex questions, one first needs to establish whether heart rate recovery remains stable if training load is maintained at a level to which the body has already adapted, and whether it changes with acute decreases or increases in training load. It was therefore the aim of this study to assess whether acute changes in training load were reflected in heart rate recovery after a standardized sub-maximal exercise test.
Methods
Study design and subjects
A total of 34 physically active participants were recruited for a 2-week trial. This prospective observational cohort study was approved by the University of Cape Town Ethics and Research Committee and the participants gave their written informed consent after the testing protocol was explained to them. Before the start of the trial body composition, maximal oxygen consumption and maximal heart rate of the participants were measured. Participants were asked to maintain during the trial the same type and quantity of ad libitum training that they had been doing for 3 weeks prior to the trial. The ad libitum training of the subjects during the 2-week trial was quantified. Participants were excluded from the trial if they were injured or ill or had other reasons which prevented them from training to their normal capacity during the study (n = 6). Thus the data from 28 participants (12 men, 16 women; mean age 30 ± 5 years) were analyzed. There are no gender differences in heart rate recovery; therefore, we believed it appropriate to use both men and women in the study (MacMillan et al. 2006; Mahon et al. 2003).
The 28 participants selected for this study exercised regularly, but varied in the average amount of training they did each week. For example, the subject that performed the least amount of training in the study did 2–3 sessions per week, with each session ranging between 20 and 30 min at an RPE of 5–7, using the Borg CR 0–10 scale (Williams and Eston 1989). Whereas, a highly trained athlete who performed the most training in the study participated in 7–8 sessions per week that ranged in duration (30–190 min) and intensity (RPE 2–8). Exercise modes included running, cycling and/or interval-type cardiovascular gym training, which combined stepping, elliptical machines, super circuit and rowing machines. The heterogeneity of the participants’ training strengthens the study design as it reduces the risk of sample bias and enabled us to evaluate the effects of relative, rather than absolute, changes in training volume.
The sample size was determined using the data of Lamberts et al. (2004), which showed that the day-to-day variability of sub-maximal heart rate and recovery heart rate after exercise was about 5–6 beats min−1 and 7–8 beats min−1, respectively (defined as the 95% confidence intervals of within subject range), with a standard deviation of 3 beats min−1. In the study of Lamberts et al. (2004) the subjects maintained their training load over the period of the study, whereas in the current study changes in training load were observed over the 2-week trial. Therefore, in the current study the smallest meaningful difference was taken to be 9 beats min−1 and a standard deviation of 6 beats min−1 was estimated to accommodate the possible effects of variations in training load on heart rate recovery. The sample size required for this study, in order to achieve a statistical power of 80% and a significance level of 5% was therefore n = 7 for each group (Altman 1991).
Methodology
Body composition and physiological measurements
Body fat per cent was assessed with a near infrared (NIR) measurement on the right bicep using the Futrex-6100A/ZL (Kett Electric Laboratory, Futrex Inc., Gaithersburg, MD, USA). Body mass was recorded on a calibrated scale (Seca model 708, Germany) and recorded to the nearest 100 g. The stature of each athlete was recorded to the nearest millimetre using a stadiometer (Seca model 708, Germany). Resting heart rate, recorded immediately after waking in the morning with a Vantage XL heart rate monitor (Polar Electro, Kempele, Finland), was taken at least three times during the trial.
An incremental exercise test to volitional exhaustion was conducted on a motor driven treadmill to determine the subject’s maximal oxygen uptake \( {\left( {\dot{V}{\text{O}}_{{\text{2}}} \max } \right)}, \) peak treadmill running speed (PTRS) and maximum heart rate (HRmax) according to the protocol of Noakes et al. (1990). A face mask was secured over the athlete’s nose and mouth to collect expired O2 and CO2 and the Oxycon Alpha (Jaeger/Mijnhardt, Groningen, Netherlands) was used to measure \( \dot{V}{\text{O}}_{{\text{2}}} \) during the test. A 5-min warm-up at 8–10 km h−1 and 0% gradient preceded the start of the test. The subject began the test running at 10–12 km h−1, after which the speed was increased by 0.5 km h−1 every 30 s until the subject was unable to maintain the pace. The test was terminated at volitional exhaustion, and maximal achievement was accepted if the subject reached two of the following: (1) a rating of 8–10 on the RPE scale; (2) an estimated maximal age-predicted heart rate; (3) an RER of >1.15. \( \dot{V}{\text{O}}_{{\text{2}}} \max \) (ml kg−1 min−1), PTRS (km h−1) and HRmax (beats min−1) were defined as the highest respective measurements recorded during the last full 30 s interval completed.
Protocol
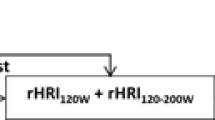
An outline of the 2-week protocol and the timing of the measurements taken during the 2-week period are shown in Fig. 1.
Schematic representation of 2-week study protocol and measurements taken during this period. Training load calculated using TRIMPs (Eq. 1). HIMS, sub-maximal shuttle running test; HRr%, 1-min recovery heart rate expressed as a per cent of the heart rate reached at the end of the fourth running stage of the HIMS test (Eq. 3). HRS4%, heart rate at the end of the fourth running stage of the HIMS test expressed as a per cent of maximal heart rate (Eq. 4 )
Quantification of training load
During the 2 weeks of the trial, each participant wore a heart rate monitor for each exercise training session to record the heart rate (beats min−1) and exercise duration (min). Average heart rate during each training session (HRex), resting heart rate (HRrest) and maximal heart rate (HRmax) were used in the Training Impulse (TRIMPs) equation to quantify the training load for each exercise session performed during the trial (Eq. 1). Session training loads were added for each subject to calculate the total training load for each week.
where e = 2.712,
(Banister 1991). Participants were then divided into three groups based on whether they increased (Group I, n = 9), decreased (Group D, n = 8) or kept their training load the same (Group S, n = 11) in week 2 compared to week 1 of the trial. This was done by calculating the per cent difference in weekly TRIMPs (Eq. 2). A variation of 20% was selected as a reasonable range with which to account for normal intra-individual variation. As such, those subjects who kept their training load within a 20% range were regarded as having kept their training constant over the 2 weeks.
Quantification of per cent heart rate recovery
Heart rate recovery was assessed during the first minute after a standardized sub-maximal shuttle test at the end of each week of the trial. The sub-maximal heart rate shuttle test (Heart rate Interval Monitoring System: HIMS) developed in this laboratory consists of four running stages of increasing intensity interspersed with recovery periods (Lamberts et al. 2004). The subjects ran on a rubberized indoor floor between two lines drawn 20 m apart. The ambient air temperature was maintained between 17 and 21°C. The pace of each of the four running stages (8.4, 9.6, 10.8 and 12.0 km h−1, respectively) was set by a pre-recorded auditory signal recorded onto compact disk. Each running stage lasted 2 min and was separated by 1 min rest periods in which the subjects stood upright and motionless with their hands by their sides. The subjects rested for 2 min after the fourth stage. Therefore, the total duration of the test is 13 min and the intensity of the test is controlled and constant for each test. The HIMS test was designed to be sub-maximal and non-invasive for athletes so that it can be administered frequently during different phases of training. Furthermore, the test is quick, easy to administer to a number of athletes simultaneously and may form part of a warm-up before a training session. In a previous study, the intra-class correlation coefficient of the heart rate on a day-to-day basis during the four stages and recovery periods ranged between R = 0.94 and 0.99 in a group of subjects who maintained a constant training load (Lamberts et al. 2004). Heart rate was recorded at 5 s intervals during the HIMS test and heart rate recovery was assessed by expressing the heart rate 1 min after the fourth running stage as a percentage of the heart rate attained at the end of the fourth running stage of the test (HRr%; Eq. 3).
In addition, the heart rate reached at the end of the fourth running stage of the HIMS test was expressed as a per cent of maximal heart rate (HRS4%, Eq. 4) determined in the treadmill test to exhaustion.
Statistical analyses
A Pearson’s product moment correlation assessed the relationship between the first and second week’s training load (TRIMPs), HRr% and HRS4%. The 95% confidence intervals around the correlation coefficient were calculated using a spreadsheet for this purpose downloaded from http://www.newstats.org (accessed 3 December 2006). An analysis of variance with repeated measures was used to determine whether there were differences between heart rate at the end of the sub-maximal test and recovery at week 1 compared to week 2 within groups. A Kruskal–Wallis test was used to compare changes in TRIMPs, HRr% and HRS4% from week 1 to week 2 between the three groups (Group I, D and S). The use of non-parametric statistics was required for these comparisons as the data had unequal variance and were not always normally distributed (Kolmogorov-Smirnov one-sample test for normality). Statistical analysis was performed using STATISTICA 7.0 data analysis software system (StatSoft, Inc. Tulsa, OK, USA). All data are expressed as the mean ± standard deviation and statistical significance was accepted at P < 0.05.
Results
Subject characteristics
The 28 participants (mean age 30 ± 5 years) selected for this study trained regularly, but varied in the amount of training they habitually did each week (range 82–747 min week−1). Baseline measurements and selected physiological measurements calculated during the trial are shown in Table 1.
Relationship between TRIMPs week 1 and TRIMPs week 2
Figure 2a depicts the relationship between training load (TRIMPs) in week 1 and TRIMPs in week 2 for all subjects. Figure 2b illustrates how each of the groups differed with respect to the per cent change in weekly TRIMPs from week 1 to week 2. Group D significantly decreased their TRIMPs compared to Group S (P = 0.04) and Group I significantly increased their TRIMPs compared to Group S (P = 0.02), who maintained their training load within a 20% range (Fig. 2b). The mean per cent change in TRIMPs was also different between Group D and I (P < 0.05; Fig. 2b).
a Relationship between TRIMPs calculated for week 1 and week 2; b per cent change in weekly TRIMPs (mean ± SD) from week 1 to week 2 for Group I (n = 9), Group S (n = 11) and Group D (n = 8). *Group S significantly different from Group I and Group D, and Group I significantly different from Group D (P < 0.05)
Changes in per cent heart rate recovery from HIMS 1 to HIMS 2
Table 2 includes the absolute heart rate at the end of the sub-maximal test and after 1 min of recovery (week 1 and week 2), and the mean per cent heart rate recovery (HRr%) after HIMS 1 and HIMS 2 for all groups. There were no differences between groups for heart rate measured at either the end of the sub-maximal test (week 1 vs. week 2) or after recovery (week 1 vs. week 2). The heart rate in Group I was significantly higher after recovery week 2 vs. week 1 (121 ± 23 vs. 112 ± 34 beats min−1; P = 0.017).
Although HRr% was not significantly different from week 1 to week 2, within each group there was a tendency for the mean per cent heart rate recovery in Group I (the group that increased their training load) to be slower after HIMS 2 than after HIMS 1 (Table 2). Conversely, there was a tendency for heart rate recovery in Group D (who decreased their training load during week 2) to be slightly faster after HIMS 2 than after HIMS 1 (Table 2). The mean change in HRr% in Group I (which showed an increase) was significantly different from the decrease in HRr% in Group D (P = 0.03, Fig. 3a). Mean heart rate recovery in Group S remained the same after each HIMS test (Fig. 3a).
Per cent change in: a heart rate recovery (HRr%); b stage 4 heart rate (HRS4%; mean ± SD) from HIMS 1 (end of week 1) to HIMS 2 (end of week 2) in Group S, Group I and Group D. HRr%, 1-min recovery heart rate expressed as a per cent of the heart rate reached at the end of the fourth running stage of the HIMS test (Eq. 3). HRS4%, heart rate at the end of the fourth running stage of the HIMS test expressed as a per cent of maximal heart rate (Eq. 4). *Group D significantly different from Group I (P < 0.05)
Repeatability of HIMS Stage 4 heart rate
There were no differences between groups on comparing changes from HIMS 1 to HIMS 2 in the heart rate attained at the end of Stage 4 (Fig. 3b). All groups showed strong correlations between heart rate attained at the end of Stage 4 of HIMS 1 and HIMS 2: Group S, r = 0.88 (95% CI = 0.62–0.97); Group I, r = 0.95 (95% CI = 0.74–0.99); and Group D, r = 0.96 (95% CI = 0.79–0.99).
Discussion
Changes in per cent heart rate recovery from HIMS 1 to HIMS 2
The first finding of this study was that the heart rate recovery was slightly slower after the second week of the trial in subjects who increased their training load. There was no change in heart rate recovery for those subjects who maintained their training load or the subjects who decreased their training load through the trial. A decrease in heart rate recovery, as observed in Group I in this study, may represent the negative training response to an increase in training load/exercise stimulus. The basis for training is the manipulation of workloads over time to induce positive haemodynamic adaptations (Charlton and Crawford 1997) that contribute to improvements in performance. The aim of this manipulation is to produce the correct balance between stress (exercise) stimuli and rest (recovery) periods, since an imbalance in these factors could lead to an overtrained state (Uusitalo 2001). We propose that the use of heart rate recovery may contribute to a better understanding of these positive and negative training effects.
Other studies have investigated the effect of training, detraining and taper on heart rate recovery specifically. Sugawara et al. (2001) reported that 8 weeks of training in previously untrained men improved 30 s heart rate recovery. Two weeks of subsequent detraining maintained the improved heart rate recovery; however, by the fourth week of detraining, heart rate recovery had returned to baseline levels (Sugawara et al. 2001). The heart rate recovery of ten track and field high school girls, who had been training daily for 3–4 months, was recorded before and after 3 weeks of detraining. In this case, heart rate recovery was assessed as a “score” consisting of the sum of the heart rates at 1, 2 and 3 min of recovery. Using this method, Michael et al. (1972) reported a slower heart rate recovery after 3 weeks of detraining. Results from these studies suggest that extended periods of detraining reverse training-induced improvements in heart rate recovery. Since the current study did not investigate the long-term effects of training, nor the effects of complete cessation of exercise (detraining) on heart rate recovery, we cannot compare our results with those reported in the above studies.
In a study by Brynteson and Sinning (1973), 21 men were trained for 5 weeks (5 days per week, 30 min exercise session per day), which resulted in an improvement in the 5 min heart rate recovery (Brynteson and Sinning 1973). Subsequently, training was decreased to 1, 2, 3 or 4 days per week for a further 5 weeks. This study found that the larger decrease in training from 5 to 1–2 days per week (perhaps comparable with detraining) reversed the training-induced improvement in heart rate recovery, whereas the smaller reduction in training to 3–4 days per week (possibly comparable to tapering) improved heart rate recovery slightly more (Brynteson and Sinning 1973). This slight improvement in heart rate recovery after a relatively small decrease in training load is similar to our results in which there was a tendency for improved heart rate recovery in Group D, who had decreased their training load by 42%.
In contrast, Houmard et al. (1989) found that the 1 and 2 min heart rate recovery was slower after sub-maximal treadmill running in five highly trained runners after a 10-day taper that followed 3 months of intensive training. Mujika et al. (2004) suggested that the content of pre-taper training might have an effect on heart rate indices during the taper period. For example, 2 weeks of high intensity training before a 2-week taper produced increases in sub-maximal and maximal heart rate and decreases in resting heart rate post-taper compared to pre-taper (Jeukendrup et al. 1992). However, post-taper values were comparable to baseline measurements before the start of the high-intensity training period (Jeukendrup et al. 1992). These changes in heart rate indices during taper contradict other studies involving tapering, but are credited by Mujika et al. (2004) to a reversal of the physiological effects of the high-intensity training immediately prior to the taper. This may be the case as well in the study by Houmard et al. (1989) where subjects had participated in 3 months of high-intensity training before the taper. In our study and in the study of Brynteson and Sinning (1973), the subjects were more likely in an adapted state before the decrease in training and subsequent tendency for improvement in heart rate recovery. However, this suggestion remains inconclusive due to limitations such as insufficient data being available or the use of many different methods of training, tapering and measuring heart rate recovery. Participants in our study who maintained their training load (i.e. Group S) were probably already adapted to the amount of training they performed each week, which may have been represented by the stability of their heart rate recovery during the trial. To our knowledge, no studies have specifically examined the effects of small acute increases in training load (as occurred in Group I in our study) on heart rate recovery. We are therefore not able to compare our results to previous research.
Repeatability of HIMS Stage 4 heart rate
The HIMS test, designed in this laboratory, is a sub-maximal shuttle test that is easily administered and provides a controlled workload, which precedes the measurement of heart rate recovery data (Lamberts et al. 2004). In this study, there were no differences between or within groups when comparing changes in the heart rate attained at the end of Stage 4 from HIMS 1 to HIMS 2. The intra-individual heart rate responses to the test have been shown to be repeatable over 5 days in subjects who kept their training constant, with a variation of about six beats occurring in the sub-maximal heart rate attained at the end of the fourth stage of the test (Lamberts et al. 2004). The repeatability of the heart rate measurement at this sub-maximal stage has been supported by results in the current study, even with acute changes in training load. A comprehensive review by Mujika et al. (2004), of the physiological changes occurring during pre-event taper, includes studies that vary in taper duration from 6 to 35 days. The authors concluded that resting, maximal and sub-maximal heart rates do not change during taper, whereas increases in blood and red cell volume, haemoglobin and haematocrit indicate a positive red cell balance during this phase (Mujika et al. 2004). Similarly, the stability of the heart rate reached at the end of the HIMS test in our study suggests that sub-maximal heart rate represents something different from that being represented by heart rate recovery, which did change with increases and decreases in training load. Whereas heart rate during exercise may be a measure of cardiac load, heart rate recovery may represent the adaptive state or the ability of the autonomic nervous system to respond to that cardiac load. If the HIMS test is administered frequently, a profile of changes in heart rate recovery after the test can be established for each individual, thereby monitoring the athlete’s physiological capacity to respond positively to subsequent training. While the heart rate recovery test on its own may not be diagnostic, if the data are interpreted in conjunction with other data it may provide useful information with which to design and prescribe optimal and personalized training programmes.
In conclusion the current study shows that heart rate recovery responds to acute changes in training load. Heart rate recovery slowed slightly after increases in training load, whereas heart rate recovery tended to improve in subjects who decreased their training load. There was no change in heart rate recovery for those subjects who maintained their training load through the 2-week trial. Since heart rate recovery is governed by the autonomic nervous system (Kannankeril et al. 2004; Pierpont and Voth 2004; Savin et al. 1982), it is likely that the changes observed in heart rate recovery may represent negative and positive responses of the autonomic nervous system to training/exercise stress. Further, since the autonomic nervous system interacts with all other physiological systems, the condition of this system may give a global indication of the physiological state of the whole body. So, while changes in sub-maximal heart rate may be a measure of physiological stress during exercise, heart rate recovery recorded under standardized conditions may be interpreted as a practical, reliable and quantifiable measure of the body’s current capacity to respond to stress. Prospective studies that include controlled increases and decreases in weekly training load, along with analysis of the relationship between parasympathetic and sympathetic systems during exercise and recovery, are required to confirm these suggestions and develop a better understanding of what the changes in heart rate recovery represent, and whether it can be used practically to monitor and prescribe training.
References
Achten J, Jeukendrup AE (2003) Heart rate monitoring: applications and limitations. Sports Med 33:517–538
al Ani M, Munir SM, White M, Townend J, Coote JH (1996) Changes in R–R variability before and after endurance training measured by power spectral analysis and by the effect of isometric muscle contraction. Eur J Appl Physiol Occup Physiol 74:397–403
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London
Aubert AE, Seps B, Beckers F (2003) Heart rate variability in athletes. Sports Med 33:889–919
Banister EW (1991) Modeling elite athletic performance. In: MacDougall JD, Wenger HA, Green HJ (eds) Physiological testing of the high-performance athlete. campaign (IL) Human Kinetics Books, pp 403–425
Bonaduce D, Petretta M, Cavallaro V, Apicella C, Ianniciello A, Romano M, Breglio R, Marciano F (1998) Intensive training and cardiac autonomic control in high level athletes. Med Sci Sports Exerc 30:691–696
Brynteson P, Sinning WE (1973) The effects of training frequencies on the retention of cardiovascular fitness. Med Sci Sports 5:29–33
Bunc V, Heller J, Leso J (1988) Kinetics of heart rate responses to exercise. J Sports Sci 6:39–48
Carter JB, Banister EW, Blaber AP (2003a) Effect of endurance exercise on autonomic control of heart rate. Sports Med 33:33–46
Carter JB, Banister EW, Blaber AP (2003b) The effect of age and gender on heart rate variability after endurance training. Med Sci Sports Exerc 35:1333–1340
Charlton GA, Crawford MH (1997) Physiologic consequences of training. Cardiol Clin 15:345–354
Houmard JA, Kirwan JP, Flynn MG, Mitchell JB (1989) Effects of reduced training on submaximal and maximal running responses. Int J Sports Med 10:30–33
Iwasaki K, Zhang R, Zuckerman JH, Levine BD (2003) Dose–response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol 95:1575–1583
Jeukendrup AE, Hesselink MK, Snyder AC, Kuipers H, Keizer HA (1992) Physiological changes in male competitive cyclists after 2 weeks of intensified training. Int J Sports Med 13:534–541
Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ (2004) Parasympathetic effects on heart rate recovery after exercise. J Investig Med 52:394–401
Lambert MI, Borresen J (2006) A theoretical basis of monitoring fatigue: a practical approach for coaches. Int J Sports Sci Coach 1:371–388
Lamberts RP, Lemmink KAPM, Durandt JJ, Lambert MI (2004) Variation in heart rate during submaximal exercise: implications for monitoring training. J Strength Cond Res 18:103–107
Levy WC, Cerqueira MD, Harp GD, Johannessen KA, Abrass IB, Schwartz RS, Stratton JR (1998) Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol 82:1236–1241
Loimaala A, Huikuri H, Oja P, Pasanen M, Vuori I (2000) Controlled 5-mo aerobic training improves heart rate but not heart rate variability or baroreflex sensitivity. J Appl Physiol 89:1825–1829
MacMillan JS, Davis LL, Durham CF, Matteson ES (2006) Exercise and heart rate recovery. Heart Lung 35:383–390
Mahon AD, Anderson CS, Hipp MJ, Hunt KA (2003) Heart rate recovery from submaximal exercise in boys and girls. Med Sci Sports Exerc 35:2093–2097
Mazzeo RS (1991) Catecholamine responses to acute and chronic exercise. Med Sci Sports Exerc 23:839–845
Meeusen R, Duclos M, Gleeson M, Rietjens G, Steinacker J, Urhausen A (2006) Prevention, diagnosis and treatment of the overtraining syndrome. Eur J Sports Sci 6:1–14
Meeusen R, Piacentini MF, Busschaert B, Buyse L, De Schutter G, Stray-Gundersen J (2004) Hormonal responses in athletes: the use of a two-bout exercise protocol to detect subtle differences in (over)training status. Eur J Appl Physiol 91:140–146
Melanson EL, Freedson PS (2001) The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol 85:442–449
Michael E, Evert J, Jeffers K (1972) Physiological changes of teenage girls during five months of detraining. Med Sci Sports 4:214–218
Mujika I, Padilla S, Pyne D, Busso T (2004) Physiological changes associated with the pre-event taper in athletes. Sports Med 34:891–927
Noakes TD, Myburgh KH, Schall R (1990) Peak treadmill running velocity during the VO2 max test predicts running performance. J Sports Sci 8:35–45
O’Connor PJ, Crowley MA, Gardner AW, Skinner JS (1993) Influence of training on sleeping heart rate following daytime exercise. Eur J Appl Physiol Occup Physiol 67:39–42
Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Sugawara J, Ajisaka R, Miyauchi T (2007) Postexercise heart rate recovery accelerates in strength-trained athletes. Med Sci Sports Exerc 39:365–370
Pierpont GL, Voth EJ (2004) Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. Am J Cardiol 94:64–68
Savin WM, Davidson DM, Haskell WL (1982) Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol 53:1572–1575
Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J (2001) Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 38:1980–1987
Short KR, Sedlock DA (1997) Excess postexercise oxygen consumption and recovery rate in trained and untrained subjects. J Appl Physiol 83:153–159
Skinner JS, Gaskill SE, Rankinen T, Leon AS, Rao DC, Wilmore JH, Bouchard C (2003) Heart rate versus %VO2max: age, sex, race, initial fitness, and training response—HERITAGE. Med Sci Sports Exerc 35:1908–1913
Smith DJ, Roberts D (1994) Effects of high volume and/or intense exercise on selected blood chemistry parameters. Clin Biochem 27:435–440
Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M (2001) Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur J Appl Physiol 85:259–263
Swaine IL, Linden RJ, Mary DA (1994) Loss of exercise training-induced bradycardia with continued improvement in fitness. J Sports Sci 12:477–481
Tulppo MP, Hautala AJ, Makikallio TH, Laukkanen RT, Nissila S, Hughson RL, Huikuri HV (2003) Effects of aerobic training on heart rate dynamics in sedentary subjects. J Appl Physiol 95:364–372
Tulppo MP, Makikallio TH, Seppanen T, Laukkanen RT, Huikuri HV (1998) Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol 274:H424–H429
Urhausen A, Gabriel H, Kindermann W (1995) Blood hormones as markers of training stress and overtraining. Sports Med 20:251–276
Urhausen A, Kindermann W (2002) Diagnosis of overtraining: what tools do we have? Sports Med 32:95–102
Uusitalo AL, Uusitalo AJ, Rusko HK (1998) Exhaustive endurance training for 6–9 weeks did not induce changes in intrinsic heart rate and cardiac autonomic modulation in female athletes. Int J Sports Med 19:532–540
Uusitalo ALT (2001) Overtraining: making a difficult diagnosis and implementing targeted treatment. Phys Sportsmed 29:35–50
Williams JG, Eston RG (1989) Determination of the intensity dimension in vigorous exercise programmes with particular reference to the use of the rating of perceived exertion. Sports Med 8:177–189
Wilmore JH, Stanforth PR, Gagnon J, Leon AS, Rao DC, Skinner JS, Bouchard C (1996) Endurance exercise training has a minimal effect on resting heart rate: the HERITAGE Study. Med Sci Sports Exerc 28:829–835
Wilmore JH, Stanforth PR, Gagnon J, Rice T, Mandel S, Leon AS, Rao DC, Skinner JS, Bouchard C (2001) Heart rate and blood pressure changes with endurance training: the HERITAGE family study. Med Sci Sports Exerc 33:107–116
Yamamoto K, Miyachi M, Saitoh T, Yoshioka A, Onodera S (2001) Effects of endurance training on resting and post-exercise cardiac autonomic control. Med Sci Sports Exerc 33:1496–1502
Zavorsky GS (2000) Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med 29:13–26
Acknowledgments
The research undertaken in this study was funded in part by the University of Cape Town, Discovery Health, National Research Foundation, Ernst & Ethel Eriksen Foundation and Deutscher Akademischer Austausch Dienst.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borresen, J., Lambert, M.I. Changes in heart rate recovery in response to acute changes in training load. Eur J Appl Physiol 101, 503–511 (2007). https://doi.org/10.1007/s00421-007-0516-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0516-6