Abstract
Purpose
This study aimed at examining the autonomic cardiovascular modulation in well-trained masters and young cyclists following high-intensity interval training (HIT).
Methods
Nine masters (age 55.6 ± 5.0 years) and eight young cyclists (age 25.9 ± 3.0 years) completed a HIT protocol of 6 x 30 sec at 175% of peak power output, with 4.5-min’ rest between efforts. Immediately following HIT, heart rate and R–R intervals were monitored for 30-min during passive supine recovery. Autonomic modulation was examined by i) heart rate recovery in the first 60-sec of recovery (HRR60); ii) the time constant of the 30-min heart rate recovery curve (HRRτ); iii) the time course of the root mean square for successive 30-sec R–R interval (RMSSD30); and iv) time and frequency domain analyses of subsequent 5-min R–R interval segments.
Results
No significant between-group differences were observed for HRR60 (P = 0.096) or HRRτ (P = 0.617). However, a significant interaction effect was found for RMSSD30 (P = 0.021), with the master cyclists showing higher RMSSD30 values following HIT. Similar results were observed in the time and frequency domain analyses with significant interaction effects found for the natural logarithm of the RMSSD (P = 0.008), normalised low-frequency power (P = 0.016) and natural logarithm of high-frequency power (P = 0.012).
Conclusion
Following high-intensity interval training, master cyclists demonstrated greater post-exercise parasympathetic reactivation compared to young cyclists, indicating that physical training at older ages has significant effects on autonomic function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart rate (HR) indices of recovery following exercise such as heart rate recovery (HRR), and heart rate variability (HRV) have historically been used as non-invasive measures of autonomic modulation [4, 5]. Specifically, autonomic modulation during recovery is characterised by a withdrawal of sympathetic activity and parasympathetic reactivation, which results in a decreasing heart rate [4]. Moreover, parasympathetic activity has previously been reported to play a cardio-protective role through enhanced cardiac electrical stability [2]. Therefore, a delay in parasympathetic reactivation monitored via the HRR and HRV responses during recovery may indicate an autonomic imbalance and has been suggested to be a strong predictor of all-cause mortality [15] and coronary artery disease [13], as well as suggesting a delay in physical recovery following exercise in athletes [18]. HRR and HRV responses to exercise are known to be negatively influenced by age [33], low physical fitness [30] and high exercise intensity [22]. Despite this, physical activity and training in older adults has been shown to improve resting autonomic modulation through increased parasympathetic responsiveness [26, 31], which suggests that physical training into older age can have a positive influence on these autonomic functions.
Masters athletes are defined as older adults who have continued participation in structured physical activity and competitive sport into older age [20]. As a result, masters athletes have demonstrated the ability to attenuate declines in some physical and physiological characteristics into older age [3]. Consequently, there has been increasing research interest in masters athletes as their physically active lifestyle has been suggested to offset the influence of increasing physical inactivity on physical and physiological systems into older age. From a research perspective, comparison of masters athletes and performance-matched younger cohorts allows for further insight into the specific influence of the ageing process. Specifically, masters athletes have demonstrated a maintenance in HRR values following maximal exercise when compared to similarly trained younger cohorts [8] and improved resting HRV parameters when compared to sedentary age-matched cohorts [31]. These results suggest that maintaining systematic training may preserve cardiovascular autonomic modulation into older age. However, relatively little remains known about the autonomic modulation during recovery immediately following high-intensity interval training (HIT).
Regardless of age, it is common for competitive cyclists to compete several times over one day and utilise training practices such as HIT to simulate competition stress and promote desired physiological adaptations [25]. Indeed, the recovery of autonomic modulation to resting values following exercise has been shown to be dependent on exercise intensity [23], and parasympathetic reactivation has been shown to be suppressed in young cohorts immediately following sprint interval cycles when compared to baseline values [27] and sprint interval running exercise when compared to lower-intensity exercise protocols [6, 17]. Further, given that parasympathetic reactivation following exercise has also been shown to be influenced by age [33], an investigation into autonomic modulation following HIT in masters and young cyclists is warranted to extend current research examining the influence that physical training can have on preserving autonomic modulation following acute exercise stress into older age. Such knowledge may give insight into possible differences in recovery rates that could not only influence subsequent performance following HIT in masters athletes but also assess possible cardiovascular risks for masters athletes performing HIT.
Therefore, the aim of this study was to examine the autonomic cardiovascular modulation by comparing HR and HRV indices following an HIT protocol in well-trained masters and young cyclists.
Methods
Participants
Nine masters and eight young cyclists were recruited from local cycling and triathlon clubs to participate in the study. The participant’s demographic data are presented in Table 1. To be eligible for the study, all participants were required to be free from injury and medication that may have affected their ability to perform exercise or modify cardiovascular function and must have been involved in competitive cycling over the past 2 years. Prior to inclusion, all participants were informed about the study including potential risks and benefits and were required to give written consent. Participants were matched on training practices (hours per week) and performance [maximal oxygen consumption (VO2max) and peak power output (PPO)]. This study was given ethical clearance by the Central Queensland University Human Ethics Research Panel in accordance with the Helsinki Declaration.
Study overview
This study was completed over two sessions separated by at least 48 h. The first session included familiarisation and preliminary testing. The second session involved the collection of resting measures (10 min), the completion of an HIT protocol (detailed below), and the immediate recovery period (30 min). All exercise testing was conducted using an electromagnetically braked cycle ergometer (Velotron, Racermate; Seattle, WA, USA) and blood lactate was collected via standardised capillary protocols (Accutrend Plus, Roche Diagnostics; Mennheim, Germany). All resting and recovery HR and HRV measures were collected at a frequency of 1000 Hz with the participant laying supine in a quiet dark room in accordance with previous investigations [23, 27]. Measures where obtained using a Polar RS800cx (Polar Electro; Kempele, Finland) which has previously been reported to be a valid and reliable tool to measure HRV [32]. Participants were asked to not perform strenuous exercise over the 48 h preceding exercise testing and to follow their usual dietary intake.
Preliminary testing
Preliminary testing consisted of assessing anthropometric measures and a maximal graded exercise test (GXT). The GXT commenced after a standard warm up of 6 min at 100 watts (W). The GXT commenced at 150 W and workload increased 50 W every 3 min. VO2max was deemed as attained when the participants reached volitional exhaustion. Expired gas was continuously analysed throughout the GXT with an indirect calorimetry system (TrueOne 2400, Parvo Medics, Inc.; Sandy, UT, USA) which was calibrated according to the manufacturer’s instructions prior to each test. PPO (W) was calculated using the following formula [10]:
where PPO = maximal aerobic power; W (final) = workload (W) of final completed stage; t = duration of the final workload completed (s).
High-intensity interval exercise protocol
Once resting HR, HRV and lactate measures were collected, each participant performed a standardised warm-up before the commencement of the HIT protocol that consisted of 6 × 30-s exercise bouts at an intensity of 175% of the previously calculated PPO (Table 1). Each exercise bout was interspersed with 4.5 min of rest in which participants were allowed 2 min of active recovery at a self-selected cadence with a resistance of 50 W at a standardised time. The remainder of the between-bout recovery was performed passively seated on the cycle ergometer. Upon completion of the HIT, participants stepped off the cycle ergometer and immediately laid (<5 s) supine on a massage table adjacent to the ergometer where a lactate measure was immediately taken. Participants underwent 30 min of passive recovery in a quiet and darkened laboratory where HR and HRV were monitored. Respiratory rate was not controlled during the recovery period due to the high-intensity nature of the exercise protocol. The authors postulated that standardising the respiratory rate may have placed participants under greater stress and influenced the return of HR values to baseline during recovery [6].
Post-exercise heart rate recovery
The HR (bpm) and R–R interval data [time period between successive heart beats (ms)] related to the HRV measures were extracted to a personal computer using the Polar Pro Trainer 5 software (Polar Electro; Kempele, Finland). HRR was analysed in two ways, both of which are considered to be representative of parasympathetic reactivation following exercise [5]. Firstly, by calculating the absolute difference between the end of exercise HR (HRpeak) and HR 60 s into recovery (HRR60) [5]. Secondly, by applying a mono-exponential decay curve using the non-linear curve fitting function in Origin Pro 2015 software (Origin Lab; Northampton, MA, USA) to 30 min of HRR values [5, 17]. This method calculated the time constant of the HRR curve using an orthogonal distance regression algorithm using the following equation:
where Y(t) represents HR at any given time, Y (FR) is the final HRR value, A is the amplitude of the HRR curve, t is the time (s), TD is the time delay of the exponential component, and τ is the time constant of the HRR curve.
The goodness of the curve fit was assessed by the sum of the squares due to error (SSE) and the R-squared (R2) value. An SSE equalling zero represents no variation or random error between measured values and modelled values, and an R2 equalling one represents perfect curve modelling [16]. To reduce the SSE of the resultant time constant (τ) and time delay (TD), the initial parameters for amplitude (amplitude = final exercise HR–final recovery HR) and final recovery HR value of the curves were fixed using recorded data.
Post-exercise heart rate variability
Data taken from the Polar Pro Trainer 5 software were converted to a text file and analysed with HRV Analysis Software v2.2 (Biosignal Laboratory, University of Kuopio, Finland) [28]. Occasional ectopic beats were examined and erratic data were identified and replaced with interpolated adjacent R–R intervals using a medium artefact correction (±0.25 s of local mean). Due to methodological limitations of performing conventional HRV analysis with rapidly fluctuating autonomic conditions such as directly after exercise [29], during the first 10 min of recovery both the R–R interval and the root mean square of successive differences (RMSSD) of R–R intervals were obtained over subsequent 30-s non-overlapping segments (RMSSD30). RMSSD30 was calculated as a time-varying vagal parameter to monitor parasympathetic reactivation immediately following exercise [9].
Following the initial 5 min of recovery, subsequent 5-min R–R interval segments were analysed in both the time and frequency domains until cessation of the 30-min recovery period. The mean R–R interval and the natural logarithm of the RMSSD (LnRMSSD) for each 5-min segment were obtained from time domain analysis. Power frequency analysis of the 5-min segments was performed using fast Fourier transform algorithm after data were de-trended and resampled at 5 Hz to calculate the low-frequency (LF) band (0.04–0.15 Hz) and high-frequency (HF) band (0.15–0.4 Hz). The natural logarithm of the LF (LnLF) and HF (LnHF) power as well as the normalised LF power [LFnu = LF (ms2)/total power (ms2)–VLF (ms2)], normalised HF power [HFnu = HF (ms2)(total power (ms2)–VLF (ms2)] were derived for each 5-min segment in the frequency domain. LF measures have recently been shown to be a measure of baroreflex function [19] and HF measures are commonly accepted measures of parasympathetic activity [29].
Statistical analyses
All data are presented as mean ± standard deviations (SD) unless stated otherwise. The distribution of all data was tested with the Shapiro–Wilk normality test. When data were skewed, data were transformed using the natural logarithm. Natural logarithm data were taken for RMSSD, LF and HF parameters. Independent t tests were used to compare between-group differences for demographic data, HR-derived data, and lactate values. For RMSSD30 , a 2 × 21 repeated measures ANOVA, and for short-term HRV parameters, 2 × 6 repeated measures ANOVA were utilised to examine the effects of age and time. When statistical significance was identified, independent t tests were used to further delineate between-group differences. All data were assessed using Mauchly’s test for sphericity and whenever a test was violated, the Greenhouse–Geisser test was used. All statistical analyses were conducted using IBM SPSS Statistics (Version 22, IBM Corporation; Armonk, NY, USA) and statistical significance was accepted at a P < 0.05 level.
Results
No significant differences between groups were found for demographic data or HIT workload (P > 0.05), except for age (P < 0.001). All HRR and lactate data are presented in Table 2. The SSE was low for masters (2.94 ± 0.80 s) and young cyclists (3.19 ± 0.73 s) and the R-squared values were all >0.98 suggesting good curve fits. No significant between-group differences were observed for HRR or lactate values, except for HRpeak (P = 0.004) and HRRamp (P = 0.014).
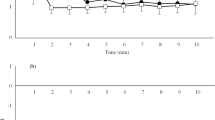
The time courses of the R–R intervals and RMSSD30 for the first 10 min of recovery are presented in Figs. 1 and 2. A significant interaction effect was found for the RMSSD30 (F20, 300 = 1.792, P = 0.021) time course with the masters group exhibiting higher RMSSD30 values compared to the young group. The between-group differences at specific time points for the time course of the RMSSD30 are represented in Fig. 2.
Mean ± standard deviation of the root mean square of successive differences of the R–R intervals measured on successive 30-s segments (RMSSD30s) during the first 10-min recovery period for masters (n = 9) and young (n = 8) cyclists following high-intensity interval training. Asterisk significant group difference between masters and young cyclists (P < 0.05)
Between-group HRV indices for the 30-min recovery time period are presented in Table 3. Significant interaction effects were found for LnRSMMD (F = 4.595, P = 0.008), LnHF (F = 4.429, P = 0.012) and LFnu (F = 3.980, P = 0.016). No significant interaction effects were found for R–R intervals (P = 0.693), LnLF (P = 0.472) and HFnu (P = 0.136); however, main effects for time were found (F = 151.10, P < 0.0001; F = 43.85, P < 0.0001, F = 9.915, P = 0.0001). Post hoc analyses found significant between-group differences for LnLF25–30 (0.031), LnHF5–10 (P = 0.037), LFnu5–10 (P = 0.015), LFnu10–15 (P = 0.033), LFnu15–20 (P = 0.043), HFnu5–10 (P = 0.039) and HFnu10–15 (P = 0.032) with the masters athletes exhibiting higher HF and lower LF values.
Discussion
The aim of this study was to examine and compare autonomic cardiovascular modulation following an HIT bout in well-trained masters and young cyclists. The present results suggest no significant differences in HR-based indices of parasympathetic reactivation (HRR60 and HRRτ) between masters and young cyclists following HIT. However, significant differences were observed within the HRV indices of autonomic modulation. Specifically, the masters cyclists showed significantly higher RMSSD30 values suggesting greater parasympathetic reactivation in the first 10 min of recovery. Furthermore, significant between-group differences in HFnu and LFnu over the 30-min recovery period also suggest a greater parasympathetic reactivation following HIT in the masters cyclists compared to the young cyclists. Therefore, the present findings suggest an altered autonomic modulation in the masters cyclists compared to young cyclists following HIT with the masters cyclists in the present study exhibiting greater parasympathetic reactivation following HIT.
As expected, the present study found significantly lower HRpeak and HRRamp in the masters cyclists which can be attributed to the age-related differences in maximal heart rate [14]. However, despite lower HRpeak in the masters cyclists, no significant between-group differences for HRR60 and HRRτ where observed following HIT. Similar dissociations between HRpeak and HRR parameters were reported by Nakamura et al. [17] who found no difference in HRR60 and HRRτ despite differences in HRpeak induced by different exercise protocols in national-level handball players (n = 13; 23.5 ± 4.1 years). Comparable HRR60 and HRRτ between young (24 ± 2 years) and older (51 ± 2 years) trained participants were also reported by Darr et al. [8] who performed linear regression analysis on the fast and slow phases of HRR curves. The authors reported no significant differences between groups in the HRR slopes [8]. Despite the different methodologies utilised, the results of the seminal Darr et al. [8] study support those of the present study suggesting that parasympathetic reactivation as measured via HRR after HIT appear to be maintained in athletes who continue systematic training into older age.
Although no significant between-group differences were found for HRR, significant differences were observed in a number of HRV indices. These results support previous research suggesting differences between HRR and HRV measures of parasympathetic activity in trained athletes following exercise [11]. The significantly lower recovery RSMMD30 values in the young cyclists from 90 s to 10 min during recovery (expect the 450 s time-point) suggests a delayed parasympathetic reactivation following the HIT protocol (Fig. 2). The lower parasympathetic activity in the young cyclists compared to the masters continued into the short-term HRV measures with the young cyclists showing significantly lower HFnu5-10 and HFnu10–15 during recovery. It should be noted that the masters athletes in the current study demonstrated high variability in their HRV responses to HIT which suggests that the recovery of autonomic modulation as measured by HRV following HIT may be highly individualised in master athletes and may be representative of this cohort only.
Unfortunately, to the authors’ knowledge, no previous studies have compared the autonomic modulation of masters and young athletes following exercise. Leti and Bricout [12] noted that the sympathetic modulation recorded via nocturnal HRV indices in the 10 senior runners (51 ± 5 years) they tested over 12 weeks of training were lower than other values reported in current literature, regardless of age or training status. Taken together with work by Ueno and Moritani [31] who reported greater parasympathetic activity and baroreflex function in aged endurance runners (66.9 ± 0.8 years) compared to their sedentary age-matched counterparts (65.4 ± 1.0 years), the present study supports current research suggesting that maintaining physical training into older age has a beneficial influence on the autonomic control of the cardiovascular system.
Although the ability of physical activity and structured exercise to improve HRV indices of parasympathetic activity in older adults is well-documented [1], the greater parasympathetic reactivation in the masters cyclists compared to the young cyclists in the current study was unexpected. A slowed parasympathetic reactivation has been suggested to be significantly related to the contribution of the anaerobic energy system due to the associated plasma metabolites [6]. However, the standardised relative workloads (175% PPO) administered in the tests used in the present study resulted in similar lactate concentrations (masters: 14.5 ± 5.3 mM; young: 13.9 ± 4.9 mM) between groups at the cessation of exercise. Therefore, it is unlikely that the delayed parasympathetic reactivation of the young cyclists was due to an increased anaerobic contribution at the end of exercise. The masters cyclists did, however, exhibit a significantly lower HRpeak at the end of exercise which is thought to be due to an age-related decrease in sensitivity to catecholamines [21]. Theoretically, a decreased sensitivity to catecholamines may have led to a decreased cardiovascular sympathetic drive during the HIT in the masters cyclists which could have could have led to a seemingly stronger post-exercise parasympathetic response. This suggests that perhaps a greater sympathetic drive during HIT in the young cyclists may have led to a blunted parasympathetic reactivation during recovery, potentially explaining the between-group differences.
A further possible explanation for the between-group differences in the HRV response could relate to the baroreflex response and blood pressure regulation following the HIT. The authors acknowledge that a limitation of the present study was that the blood pressure response was not measured during recovery as blood pressure changes can influence HR modulation [7] and has been suggested to be affected by age [24]. Additionally, previous studies have reported that blood pressure responses mainly occur in the first 15 min following HIT exercise [27], which is when the majority of the between-group differences in autonomic function in the present study were observed (Fig. 2; Table 3). Considering that the present study found significant differences in LFnu5–10, LFnu10–15 and LFnu15–20, which suggests differences in the baroreflex function between masters and young cyclists, blood pressure regulation immediately following exercise may have attributed to the different autonomic responses between groups and warrants further investigation. Additionally, another limitation of the current investigation is that respiration rate was not controlled or monitored during recovery. Therefore, between-group differences in respiration rate could have influenced the autonomic cardiovascular regulation during recovery following the HIT protocol [4]. Future research should look to standardise and monitor respiration rate as well as monitor blood pressure following exercise sessions to gain further insights into age-related differences in the autonomic response of masters and young athletes.
In summary, the present study is the first to examine autonomic cardiovascular modulation in well-trained masters and young cyclists. This study provides novel insight into the effect of age and continued sports participation on autonomic modulation following exercise, with masters cyclists demonstrating a greater parasympathetic response following HIT. Practically, these results suggest that the HIT protocol utilised in the current study did not induce negative influences on autonomic cardiovascular modulation in the masters cyclists during recovery. This finding suggests that HIT training is a safe training modality for masters athletes; however, without blood pressure responses being measured it remains difficult to draw comprehensive conclusions related to overall autonomic recovery in masters athletes. Additionally, the current findings related to autonomic cardiovascular modulation suggest that the duration for autonomic modulation to return to resting values in masters athletes may be similar to young athletes and that masters athletes can follow similar acute recovery procedures.
Conclusion
The current study is the first to compare a number of HR- and HRV-derived indices of autonomic cardiovascular modulation in training- and performance-matched masters and young cyclists. Although no between-group differences were found in HR-derived measures of parasympathetic reactivation, HRV indices demonstrated a stronger parasympathetic reactivation in masters cyclists compared to young cyclists during recovery from HIT. These findings suggest that continued physical training into older age has regulatory effects on autonomic modulation following exercise.
References
Albinet C, Boucard G, Bouquet C, Audiffren M (2010) Increased heart rate variability and executive performance after aerobic training in the elderly. Eur J Appl Physiol 109:617–624
Billman GE (2002) Aerobic exercise conditioning: a nonpharmacological antiarrhythmic intervention. J Appl Physiol 92:446–454
Borges N, Reaburn P, Driller M, Argus C (2015) Age-related changes in performance and recovery kinetics in masters athletes: a narrative review. J Aging Phys Act 24(1):149–157
Borresen J, Lambert MI (2008) Autonomic control of heart rate during and after exercise. Sports Med 38:633–646
Buchheit M, Gindre C (2006) Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol 291:H451–H458
Buchheit M, Laursen PB, Ahmaidi S (2007) Parasympathetic reactivation after repeated sprint exercise. Am J Physiol Heart Circ Physiol 293:H133–H141
Carter JB, Banister EW, Blaber AP (2003) Effect of endurance exercise on autonomic control of heart rate. Sports Med 33:33–46
Darr KC, Bassett DR, Morgan BJ, Thomas DP (1988) Effects of age and training status on heart rate recovery after peak exercise. Am J Physiol 254:H340–H343
Goldberger JJ, Le FK, Lahiri M, Kannankeril PJ, Ng J, Kadish AH (2006) Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol 290:H2446–H2452
Hawley J, Noakes T (1992) Peak power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur J Appl Physiol Occup Physiol 65:79–83
Lee CM, Mendoza A (2012) Dissociation of heart rate variability and heart rate recovery in well-trained athletes. Eur J Appl Physiol 112:2757–2766
Leti T, Bricout VA (2013) Interest of analyses of heart rate variability in the prevention of fatigue states in senior runners. Auton Neurosci 173:14–21
Lipinski MJ, Vetrovec GW, Froelicher VF (2004) Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am J Cardiol 93:445–449
Londeree BR, Moeschberger ML (1982) Effect of age and other factors on maximal heart rate. Res Q Exerc Sport 53:297–304
Messinger-Rapport B, Snader CEP, Blackstone EH, Yu D, Lauer MS (2003) Value of exercise capacity and heart rate recovery in older people. J Am Geriatr Soc 51:63–68
Motulsky H, Christopoulos A (2004) Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. GraphPad Software Inc, San Diego
Nakamura F, Soares-Caldeira L, Laursen P, Polito M, Leme L, Buchheit M (2009) Cardiac autonomic responses to repeated shuttle sprints. Int J Sports Med 30:808
Plews D, Laursen P, Kilding A, Buchheit M (2012) Heart rate variability in elite triathletes, is variation in variability the key to effective training? A case comparison. Eur J Appl Physiol 112:3729–3741
Rahman F, Pechnik S, Gross D, Sewell L, Goldstein DS (2011) Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin Auton Res 21:133–141
Reaburn P, Dascombe B (2009) Anaerobic performance in masters athletes. Eur Rev Aging Phys A 6:39–53
Seals DR, Taylor JA, Ng AV, Esler MD (1994) Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc 26:568–576
Seiler S, Haugen O, Kuffel E (2007) Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc 39:1366
Seiler S, Haugen O, Kuffel E (2007) Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc 39:1366–1373
Shantsila A, McIntyre DB, Lip GY, Fadel PJ, Paton JF, Pickering AE, Fisher JP (2015) Influence of age on respiratory modulation of muscle sympathetic nerve activity, blood pressure and baroreflex function in humans. Exp Physiol 100:1039–1051
Sloth M, Sloth D, Overgaard K, Dalgas U (2013) Effects of sprint interval training on VO2max and aerobic exercise performance: a systematic review and meta-analysis. Scand J Med Sci Sports 23:e341–e352
Soares-Miranda L, Sattelmair J, Chaves P, Duncan G, Siscovick DS, Stein PK, Mozaffarian D (2014) Physical activity and heart rate variability in older adults: the cardiovascular health study. Circ 113:005361
Stuckey M, Tordi N, Mourot L, Gurr L, Rakobowchuk M, Millar P, Toth R, MacDonald M, Kamath M (2012) Autonomic recovery following sprint interval exercise. Scand J Med Sci Sports 22:756–763
Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA (2014) Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed 113:210–220
Task Force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standard of measurement, physiological interpretation and clinical use. Circulation 93:1046–1065
Tzankoff SP, Norris AH (1979) Age-related differences in lactate distribution kinetics following maximal exercise. Eur J Appl Physiol Occup Physiol 42:35–40
Ueno LM, Moritani T (2003) Effects of long-term exercise training on cardiac autonomic nervous activities and baroreflex sensitivity. Eur J Appl Physiol 89:109–114
Williams DP, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF, Koenig J (2016) Two‐week test–retest reliability of the Polar® RS800CX™ to record heart rate variability. Clin Physiol Funct Imaging. Published ahead of print doi:10.1111/cpf.12321
Zhang J (2007) Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther 30:374–379
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Borges, N.R., Reaburn, P.R., Doering, T.M. et al. Autonomic cardiovascular modulation in masters and young cyclists following high-intensity interval training. Clin Auton Res 27, 83–90 (2017). https://doi.org/10.1007/s10286-017-0398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-017-0398-6