Abstract
Objective
The systemic illnesses associated with chronic lead exposure are partially explained by the interaction between lead and calcium metabolism. Lead exposure is posited to alter calcium levels either by altering calcium homeostasis markers or altering bone remodeling. The present study investigated the interaction between blood lead levels and calcium homeostasis markers and bone remodeling markers among lead-smelting plant workers.
Method
Adult male workers employed at the lead-smelting plant were clinically investigated as part of their regular occupational health assessment program. Additionally, control participants without occupational lead exposure, employed in administrative and white-collar jobs were invited to participate in the study. Sociodemographic and occupational details were collected by pre-standardized semi-structured questionnaires from all consenting participants, followed by clinical examination and blood collection. Blood lead levels were estimated using microwave-assisted acid digestion and the inductively coupled plasma mass spectrometry technique. Serum calcium and total protein and alkaline phosphatase levels were estimated as per standard biochemical techniques. 25-hydroxy vitamin-D3, calcitriol, and osteocalcin were estimated using the enzyme-linked immunosorbent assay. In addition to comparative analysis for comparing the two groups, independent linear regression models were explored to investigate the associations between serum calcium and blood lead and osteocalcin levels.
Result
A total of 189 lead-exposed men employed at the lead-smelting plant and 25 male control participants consented to participate. The two groups were similar in age, diet, and body mass index. Occupationally exposed individuals exhibited significantly lower serum calcium and higher bone remodeling markers (osteocalcin and alkaline phosphatase) as compared to controls. However, the serum 25-hydroxy vitamin-D3 and calcitriol levels were not significantly different between the two groups. Lastly, the serum lead and osteocalcin were weakly but significantly associated with serum calcium levels after controlling for variations in total protein, diet, 25-hydroxy vitamin-D3, calcitriol, and alkaline phosphatase in the study participants.
Conclusion
Current observations reinforce the adverse role of lead exposure on calcium metabolism. Although lead exposure is posited to affect calcium metabolism by multiple pathways, current study observations favor the bone remodeling pathway. The observations recommend periodic screening for calcium and bone health among lead-exposed adults.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb), a hazardous heavy metal, is an occupational and environmental pollutant with the potential to cause serious systemic illnesses. A common pathological effect of chronic Pb exposure is the perturbation of calcium (Ca) homeostasis. Maintaining Ca homeostasis is quintessential considering its paramount role in the musculoskeletal system and its cardinal role as a secondary messenger in various physiological activities at the molecular level (Yu & Sharma 2018). Therefore, it is compelling to investigate the changes in Ca homeostasis markers on chronic Pb exposure.
Intriguingly, Pb exhibits greater affinity than the divalent ions, thereby preferentially binding to osteocalcin (OC) over Ca (Ciosek et al. 2021; Kalahasthi et al. 2020). Existing literature suggests that Pb directly reduces bone mineral density (BMD) and cortical bone width, increasing the susceptibility to fractures and as well impair the reunion of the fractured bone. Additionally, Pb-induced imbalance in bone remodeling enhanced bone formation, and resorption results in the formation of poor-quality bones. Hence, Pb exposure, Ca metabolism, and bone density/health share a complex relation. The other mechanism posited in Pb-induced changes in Ca homeostasis includes the inhibition of vitamin D activation. Chronic Pb exposure is associated with impaired 1,25-dihydroxyvitamin-D3 (calcitriol) synthesis, by preventing renal 1α-hydroxylation of 25-hydroxyvitamin-D3 (25-HVD3) (Edelstein et al. 1984; Rosen 1985; Smith et al. 1981) and thereby lowering Ca absorption. Lastly, Pb may as well directly compete with and inhibit Ca absorption from the gastrointestinal tract (Fullmer 1992).
A recent systematic review observed a significant reduction in serum Ca among occupationally Pb exposed, with a trend of lower 25-HVD3 and inconsistent association with calcitriol levels (Upadhyay et al. 2022a, b). However, the systematic review identified a single study reporting bone remodeling markers among Pb-exposed adults (Ayla Akbal et al. 2014a, b). Hence, the current study investigated the changes in Ca homeostasis markers among adults with occupational Pb exposure and additionally explored the potential pathways via which Pb influences Ca levels.
Methods
The data for this cross-sectional observational study were obtained from a larger study that evaluated the clinical fitness of adult male workers employed at a Pb smelting plant as part of their regular occupational health assessment. The smelting plant authorities reported of otherwise healthy and medically fit workers being employed at their premises. Details of the recruitment and clinical assessment are previously described in detail (Upadhyay et al. 2022a, b; Upadhyay et al. 2023). Notably, the general clinical assessment of participants confirmed the physical fitness of the participants. In addition to the regular assessment, the workers were invited to participate in the current study after prior approval from the institutional ethical committee.
Consenting male controls without obvious occupational Pb exposure were recruited. Participants with a medical history of either pre-existing musculoskeletal, renal, hepatic, or endocrinological diseases (except diabetes) were excluded from the current study. All participants were interviewed by trained staff with a semi-structured, pre-validated questionnaire to collect socio-demographics and occupational details, followed by a detailed clinical examination by a physician (AV) including measurement of blood pressure (BP). Participants consuming vegetarian and animal products including meat were identified as “mixed diet”, while those restricted to vegetarian and milk (non-meat) were categorized as vegetarian. Lastly, under aseptic conditions, 5 ml of venous blood was drawn from each participant. Of this, 2 ml was transferred into sodium heparin-coated vials for analysis of blood Pb levels, while the remaining 3 ml was transferred into a plain tube. The serum was separated by centrifugation at 3000 g for 10 min to analyze biochemical parameters. The samples were transported to the laboratory under cold chain conditions for sample processing, storage, and analysis.
Blood pressure was recorded using a calibrated digital sphygmomanometer (Omron HealthCare, Kyoto, Japan) adhering to the American Heart Association recommendations. Briefly, each participant was evaluated thrice with a 3–5 min interval between each measurement, after an initial 5 min resting period. The average of the second and third measurements was considered for the study.
Bodyweight of the consenting participants was measured using a pre-validated weighing machine (Omron), ensuring the participant was comfortable, wore light clothes, and was without any footwear or other articles that may alter the weight. Height was measured using non-stretchable measuring tape mounted on a vertical wall, with participants standing erect, feet close to each other, without any footwear/head wearables standing close to the wall (posterior heel, gluteal region, and posterior of the head touching the wall) and ensuring Frankfort horizontal plane perpendicular to the vertical wall. Body mass index was calculated by the conventional method (i.e., a ratio of weight in kilograms to the square of the height in meters—(Weight in Kg)/(height in m2)).
Blood lead levels (BLL) of all participants were estimated using microwave-assisted acid digestion and the ICP-MS technique. Briefly, 1 ml of blood sample was digested with 1 ml of trace metal grade concentrated nitric acid and 4 ml of deionized distilled water using a microwave digestion system (multi-wave go, Anton parr) at 180 °C temp, 1000 W power for 45 min in two programmable steps. In the first step, the temperature gradually increased to 120 °C with a ramp of 10 min at 1000 W power and held for 10 min. In the second step, the temperature was raised to 180 °C with a ramp of 10 min and held for 15 min. The residues were cooled to room temperature and diluted with deionized double distilled water. The digested samples were analyzed with ICPMS (2030, shimadzu) in collision mode using helium as carrier gas at 1.2 L/min flow rate for removal of isobaric interferences and increased sensitivity. The calibration curve was prepared from a set of five different concentrations as per the expected sample concentrations and linear dynamic range. The linearity obtained for the calibration curve was 0.9998. Quality control check was performed at three different concentration levels with BIO-RAD Lyphochek whole blood metal control. The measured concentration for all three levels was in agreement with certified mean values and respective concentration ranges.
Serum Ca levels and total protein levels were estimated as per by Arsenazo III, endpoint (linearity up to 16 mg/dL and sensitivity was 0.04 mg/dL) and Biuret, endpoint assay (linearity up to 15 g/dL and sensitivity 0.05 g/dL), respectively, whereas alkaline phosphatase (ALP) activity were estimated by modified IFCC, kinetic assay (linearity up to 1200 U/l and sensitivity 1.8 U/l) using Meril auto-quant 100 biochemistry analyzer as per the prescribed protocols by the manufacturer. The validity of the test was confirmed with BioNorm (assayed normal) and BioPath (assayed abnormal) quality control checks.
The levels of 25-HVD3 and calcitriol were estimated using competitive ELISA as per the prescribed protocol by the manufacturer (Elabscience Biotechnology Inc., USA). Wherein, the antigen in the sample or standard was to compete with a fixed amount of antigen on the solid phase supporter for sites on the Biotinylated Detection Antibody specific to antigen. The optical density (OD) was measured spectrophotometrically at 450 nm wavelength. The concentration of antigen in tested samples was determined by comparing the OD of the samples to the standard curve. For 25-OHVitamin-D3 assay, the detection range was 3.13–200 ng/mL and sensitivity was 1.88 ng/mL, whereas for calcitriol assay, the detection range was 7.81–500 pg/mL and sensitivity was 4.69 pg/mL.
The levels of osteocalcin (OC) were estimated using sandwich ELISA as per the prescribed protocol by the manufacturer (Elabscience Biotechnology Inc., USA). Wherein, the analyte protein available with the standard or sample were combined to form a sandwich complex with the antibody specific to human OC precoated on microplates and a biotinylated detection antibody specific for Human OC. The optical density (OD) was measured spectrophotometrically at a 450 nm wavelength. The OD value was proportional to the concentration of Human OC. The concentration of Human OC in the samples was determined by comparing the OD of the samples to the standard curve. The detection range of the assay was 1.25–80 ng/mL and sensitivity was 0.75 ng/mL.
All data recorded were imported and analyzed using SPSS version 24. Data were checked for completeness and cleaned prior to its analysis. Independent t-tests (for continuous data) and chi-square tests (categorical data) were employed to test the group differences in demographics, clinical and laboratory variables between the two groups. Finally, the associations between serum Ca level (as a continuous measure) and BLL and OC levels (as continuous measures) were explored using a linear regression model, controlling for variations from potential confounders (diet, serum protein, 25-HVD3, calcitriol, and ALP). A correlational statistic was performed before exploring the linear regression, to determine the potential linearity between serum Ca and the independent variables.
Results
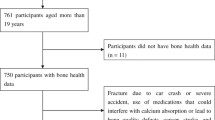
Only a fraction of the workers employed at the smelting plant consented to participate in the current study. The present study involved 189 Pb-exposed men employed at the Pb smelting plant and 25 male control participants. The flowchart of participants who approached, consented, and recruited is summarized in Fig. 1.
Salient socio-demographic and clinical details of the participants are included in Table 1. Briefly, the two groups were similar in age, diet, and BMI
The clinical parameters of the participants are described in Table 2. All clinical laboratory values, except serum 25-HVD3, were available for all the participants. Serum 25-HVD3 values of 12 Pb-exposed workers and one control participant could not be estimated because of technical issues involved in the sample transport and processing. Serum Ca levels of the recruited participants (i.e. range 8.07–14.77 mg/dL) ranged within the clinically normal range despite 32.3% of these participants having 25-HVD3 levels (< 30 ng/mL) suggestive of deficiency (Thacher & Clarke 2011).
Occupationally, Pb-exposed men exhibited significantly higher Pb levels as compared to the controls by virtue of their occupational Pb exposure. Interestingly, the Pb-exposed workers exhibited significantly lower serum Ca and higher bone remodeling markers (OC and ALP) as compared to controls. However, the serum 25-HVD3 and calcitriol levels were not significantly different between the two groups.
Serum Pb and OC were weakly but significantly associated with serum Ca levels after controlling for variations in total protein, diet, 25-HVD3, calcitriol, and ALP in the study participants.
Discussion
The current study investigated the influence of Pb exposure on Ca homeostasis and bone remodeling markers by involving occupationally Pb-exposed workers and controls without obvious occupational Pb exposure. Pb-exposed workers exhibited significantly lower serum Ca levels as compared to the controls. However, the 25-HVD3 and calcitriol levels were not different between the groups. Further, the Pb-exposed workers had significantly higher bone remodeling markers. Lastly, the serum Ca levels were significantly but weakly influenced by BLL and OC levels as revealed by the exploratory linear regression models.
The Pb-exposed workers by virtue of their occupational exposure had significantly higher BLL (median 37.3 μg/dL and IQR 26.95—46.5 μg/dL) as compared to the controls with no obvious occupational Pb exposure (median 4.94 μg/dL and IQR 3.39—5.87 μg/dL). The study controls resided > 150 kms from the smelting plant location and employed in a non-profit, public organization. Environmental contamination of Pb via the paint, ceramics, battery, plumbing and many more daily utilities are the potential non-occupational, environmental sources of Pb exposure among the controls. Although there exists no known physiological role for Pb in humans, BLL are reported among participants without obvious occupational Pb exposure due to environmental Pb exposure (Balachandar et al. 2023; Kalahasthi et al. 2022; Upadhyay et al. 2022a, b). Globally, the environmental and public health regulatory bodies enforce measures periodically to prevent environmental Pb exposure. Ban on Pb gasoline, Pb paints, Pb acid battery and Pb plumbing and use of alternatives without Pb are few regulations enforced toward cutting down the environmental Pb exposure (Kalra et al. 2013; Kessler 2014; Ansari et. al. 2020; Nichani et al. 2006; Singh & Singh 2006).
Current study observed significantly lower serum Ca levels among the Pb-exposed workers as compared to the controls. Although the serum Ca levels among the participants (Pb exposed and controls) were within the normal range. BLL is posited to affect the Ca metabolism and homeostasis at various levels (Upadhyay et al. 2022a, b). Pb exhibits higher affinity in the biological tissues replacing the divalent ions including Ca ions thereby potentially reducing the reserve as well the circulating levels (Dowd et al. 2001). Earlier preclinical studies demonstrated the potential inhibition of 1-α-hydroxylase enzyme in renal tubules leading to reduction in the active vitamin D metabolite (calcitriol) (Andrushaĭte & Bauman 1984; Andrushaĭte et al. 1982; Rahman et al. 2018). Lower serum Ca levels observed among the Pb-exposed individuals in clinical studies have been speculated to inhibitory action of Pb on the 1-α-hydroxylase enzyme (A. Akbal et al. 2014a, b; Batra et al. 2020; Dongre et al. 2013; Himani et al. 2020; Kristal-Boneh et al. 1998; Mazumdar et al. 2017). Notably, the serum 25-HVD3 and its active metabolite (calcitriol) were not significantly different between the two groups in the present study. Notably, the relative inverse pattern of 25-HVD3 (increased) and its active metabolite (reduced) among the Pb-exposed workers does not exclude the potential inhibitory role of Pb on the 1-α-hydroxylase enzyme.
Pb-exposed workers as compared to the controls, had significantly higher levels of the bone remodeling markers viz. OC and ALP. Earlier preclinical studies inferred the role of Pb in reducing bone mineral density by its preferential binding to OC (Ciosek et al. 2021). These studies demonstrated the active role of Pb in needless bone remodeling reflected by the increase in the bone remodeling markers rendering the bone potentially osteoporotic. Current observations support previous studies, indicating that Pb exposure affects Ca metabolism and homeostasis primarily by influencing bone remodeling (Ayla Akbal et al. 2014a, b; Kalahasthi et al. 2020).
Serum Ca levels were weakly but significantly associated with BLL and OC in the explorative linear regression analysis after controlling for the variations in the potential covariates. Interestingly, neither 25-HVD3 nor its metabolite (calcitriol) was significantly associated with serum Ca in the current study. Observations from existing literature regarding the role of 25-HVD3 or its metabolite in regulating the Ca metabolism among Pb exposed is inconclusive (Upadhyay et al. 2022a, b).
The present study reiterates the potential negative effects of Pb exposure on Ca homeostasis reported in the existing literature. Further, the observations suggest the probable pathways such as bone remodeling, by which the Pb exposure could potentially affect Ca metabolism. Although the current study estimated non-specific serum ALP, bone-specific ALP contributes to the majority of serum ALP in otherwise healthy adults (Sharma et al. 2014). Therefore, the variations in the ALP between the groups is likely to be Pb-associated bone remodeling, in view of the existing literature and raised serum OC in the Pb-exposed group. Additionally, in view of a single site and smaller sample size, the observed magnitude of the effect may be restricted to the current sample alone. However, the direction and the association between Pb exposure and Ca homeostasis cannot be undermined. Therefore, future studies with longitudinal multi-centered study design, investigating all the markers of Ca homeostasis (viz. Serum Ca, ionic Ca, 25-HVD3, Calcitriol, Parathyroid, Calcitonin), and bone remodeling (OC, bone-specific ALP, serum pyridinoline, deoxypyridinoline, tartrate-resistant acid phosphatase, and hydroxyproline) to unravel the precise levels of Pb affecting the Ca homeostasis, thereby to initiate corresponding interventions. Until confirmatory studies are available, Pb-exposed adults are recommended to periodically screen for the decrease in the Ca levels, symptomatic evaluation of hypocalcemia and osteoporosis of the long bones using DEXA (Dual energy X-Ray absorptiometry) are equivalent diagnostic techniques.
Conclusion
Current observations reinforce the adverse role of Pb exposure on Ca metabolism. Although Pb exposure is posited to affect Ca metabolism by multiple pathways, current study observations favor the bone remodeling pathway. Despite the Pb-exposed participants not exhibiting clinical hypocalcemia or symptoms of Ca deficiency, the observations recommend periodic screening for Ca and bone health among Pb-exposed adults. Future studies are recommended to estimate the dose–response relation between Pb exposure and Ca metabolism markers.
Data availability
Data will be made available on reasonable request.
References
Akbal A, Tutkun E, Yilmaz H (2014a) Lead exposure is a risk for worsening bone mineral density in middle-aged male workers. Aging Male 17(3):189–193. https://doi.org/10.3109/13685538.2013.836482
Akbal A, Tutkun E, Yılmaz H (2014b) Lead exposure is a risk for worsening bone mineral density in middle-aged male workers. Aging Male 17(3):189–193. https://doi.org/10.3109/13685538.2013.836482
Andrushaĭte RE, Bauman VK (1984) Effect of vitamin D on lead assimilation (Vliianie vitamina D na usvoenie svintsa). Vopr Pitan 6:46–49
Andrushaĭte RE, Bauman VK, Val’dman AR (1982) Relations between the supply of vitamin D and accumulation of Pb in chickens (Otlozhenie Pb v organizme tsypliat v zavisimosti ot obespechennosti vitaminom D). Biull Eksp Biol Med 93(2):30–32
Ansari JA, Mahdi AA, Malik PS, Jafar T (2020) Blood lead levels in children living near an informal lead battery recycling workshop in Patna, Bihar. J Health Pollut 10(25):200308. https://doi.org/10.5696/2156-9614-10.25.200308
Balachandar R, Viramgami A, Bagepally BS, Upadhyay K (2023) Association Between Blood Lead Levels and Thyroid Function: An Updated Systematic Review and Meta-Analysis. Indian J Clin Biochem. https://doi.org/10.1007/s12291-023-01113-8
Batra J, Thakur A, Meena SK, Singh L, Kumar J, Juyal D (2020) Blood lead levels among the occupationally exposed workers and its effect on calcium and vitamin D metabolism: A case-control study. J Family Med Prim Care 9(5):2388–2393. https://doi.org/10.4103/jfmpc.jfmpc_271_20
Ciosek Ż, Kot K, Kosik-Bogacka D, Łanocha-Arendarczyk N, Rotter I (2021) The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules. https://doi.org/10.3390/biom11040506
Dongre NN, Suryakar AN, Patil AJ, Hundekari IA, Devarnavadagi BB (2013) Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian J Clin Biochem 28(1):65–70. https://doi.org/10.1007/s12291-012-0241-8
Dowd TL, Rosen JF, Mints L, Gundberg CM (2001) The effect of Pb(2+) on the structure and hydroxyapatite binding properties of osteocalcin. Biochim Biophys Acta 1535(2):153–163. https://doi.org/10.1016/s0925-4439(00)00094-6
Edelstein S, Fullmer CS, Wasserman RH (1984) Gastrointestinal absorption of lead in chicks: involvement of the cholecalciferol endocrine system. J Nutr 114(4):692–700
Fullmer CS (1992) Intestinal interactions of lead and calcium. Neurotoxicology 13(4):799–807
Himani K, Kumar R, Ansari JA, Mahdi AA, Sharma D, Karunanand B, Datta SK (2020) Blood Lead Levels in Occupationally Exposed Workers Involved in Battery Factories of Delhi-NCR Region: Effect on Vitamin D and Calcium Metabolism. Indian J Clin Biochem 35(1):80–87. https://doi.org/10.1007/s12291-018-0797-z
Kalahasthi R, Barman T, Bagepally BS (2020) Assessment of Bone Turnover Biomarkers in Lead-Battery Workers with Long-Term Exposure to Lead. Int J Occup Environ Med 11(3):140–147
Kalahasthi R, Nagaraju R, Balachandar R, Bagepally BS (2022) Association between occupational lead exposure and immunotoxicity markers: A systematic review and meta-analysis. Toxicology 465:153047. https://doi.org/10.1016/j.tox.2021.153047
Kalra V, Sahu JK, Bedi P, Pandey RM (2013) Blood lead levels among school children after phasing-out of leaded petrol in Delhi. India Indian J Pediatr 80(8):636–640. https://doi.org/10.1007/s12098-013-0999-6
Kessler R (2014) Lead-based decorative paints: where are they still sold—and why? Nat Inst Environ Health Sci. https://doi.org/10.1289/ehp.122-A96
Kristal-Boneh E, Froom P, Yerushalmi N, Harari G, Ribak J (1998) Calcitropic hormones and occupational lead exposure. Am J Epidemiol 147(5):458–463. https://doi.org/10.1093/oxfordjournals.aje.a009471
Mazumdar I, Goswami K, Ali MS (2017) Status of Serum Calcium, Vitamin D and Parathyroid Hormone and Hematological Indices Among Lead Exposed Jewelry Workers in Dhaka. Bangladesh Indian J Clin Biochem 32(1):110–116. https://doi.org/10.1007/s12291-016-0582-9
Nichani V, Li WI, Smith MA, Noonan G, Kulkarni M, Kodavor M, Naeher LP (2006) Blood lead levels in children after phase-out of leaded gasoline in Bombay. India Sci Total Environ 363(1–3):95–106. https://doi.org/10.1016/j.scitotenv.2005.06.033
Rahman A, Al-Awadi AA, Khan KM (2018) Lead Affects Vitamin D Metabolism in Rats. Nutrients. https://doi.org/10.3390/nu10030264
Regulation on Lead contents in Household and Decorative Paints Rules, 2016, (2016). https://moef.gov.in/en/g-s-r-409e-08-04-2016-regulation-on-lead-contents-in-household-and-decorative-paints-rules-2016/
Rosen JF (1985) Metabolic and cellular effects of lead: a guide to low level lead toxicity in children. In: Mahaffey KR (ed) Dietary and Environmental Lead: Human Health Effects. Elsevier, New York, pp 157–185
Sharma U, Pal D, Prasad R (2014) Alkaline phosphatase: an overview. Indian J Clin Biochem 29(3):269–278. https://doi.org/10.1007/s12291-013-0408-y
Singh AK, Singh M (2006) Lead decline in the Indian environment resulting from the petrol-lead phase-out programme. Sci Total Environ 368(2–3):686–694
Smith CM, DeLuca HF, Tanaka Y, Mahaffey KR (1981) Effect of lead ingestion on functions of vitamin D and its metabolites. J Nutr 111(8):1321–1329
Thacher TD, Clarke BL (2011) Vitamin D insufficiency. Mayo Clin Proc 86(1):50–60. https://doi.org/10.4065/mcp.2010.0567
Upadhyay K, Viramgami A, Bagepally BS, Balachandar R (2022a) Association between blood lead levels and markers of calcium homeostasis: a systematic review and meta-analysis. Sci Rep 12(1):1850. https://doi.org/10.1038/s41598-022-05976-4
Upadhyay K, Viramgami A, Balachandar R, Pagdhune A, Sen S, Sarkar K (2022b) A Comparative Health Assessment of Occupationally Lead Exposed Individuals with Blood Lead Levels Range across Upper Acceptable Limit. Indian J Commun Med 47(3):343–346. https://doi.org/10.4103/ijcm.ijcm_756_21
Upadhyay K, Viramgami A, Balachandar R, Pagdhune A, Shaikh I, Sivaperumal P (2023) Development and validation of Graphite Furnace Atomic Absorption Spectrometry method and its application for clinical evaluation of blood lead levels among occupationally exposed lead smelting plant workers. Anal Sci 39(4):517–526. https://doi.org/10.1007/s44211-022-00260-x
Yu E, Sharma S (2018) Physiology, calcium. In: StatPearls. StatPearls Publishing, Treasure Island, FL. PMID: 29489276
Acknowledgements
The authors would like to express their sincere gratitude to the management of the lead-smelting plant unit for their assistance in facilitating access to subjects and collecting data for this study. They are deeply thankful to all the workers at the lead-smelting plant for their enthusiastic response and valuable contribution to the data collection process. The authors also acknowledge the tireless efforts of their technical staff in gathering data from the field and conducting instrumental analysis. They would like to acknowledge the support provided by the administration of the parent institute in successfully carrying out this project. Additionally, the authors extend their appreciation to all individuals who have directly or indirectly contributed to this study.
Funding
The present study was conducted with institutional support and no external funding was received.
Author information
Authors and Affiliations
Contributions
RB design, definition of intellectual content, literature search, data analysis, manuscript preparation, manuscript editing and review. AV concept, design, definition of intellectual content, literature search, data acquisition, data analysis. DS concept, definition of intellectual content, data acquisition, data analysis. PS concept, design, literature search, data acquisition. KU concept, design, definition of intellectual content, literature search, data acquisition, manuscript preparation, manuscript editing and review.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest to declare.
Ethical approval
The required ethical clearance was obtained from the Human Ethics Committee of the parent institute before initiating the study. The study followed all the methods and protocols for human experiments as recommended by the national ethical guidelines for biomedical and health research involving humans.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balachandar, R., Viramgami, A., Singh, D. et al. Unraveling the interaction between lead and calcium in occupationally exposed males: an exploratory observation study. Int Arch Occup Environ Health 96, 1393–1399 (2023). https://doi.org/10.1007/s00420-023-02018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-023-02018-y