Abstract
The microanatomical features of the oesophageal gastric tract in tetrapod representatives and their function, especially those related to the mucosal layer, have not yet been fully investigated. The mucosal layer cells and their function in the oesophageal gastric tract differ structurally and functionally in tetrapod representatives based on interspecies difference and the type of food and feeding habits. The present study was, therefore, postulated to compare the mucosal microanatomical structure and histochemical biodistribution of different mucin types in oesophageal gastric tract tissues of four tetrapod species. A representative of each tetrapod class was selected, as follows: the Egyptian toad Bufo regularis, the lizard Trachylepis quinquetaeniata, the domestic pigeon Columba livia domestica and the albino mouse Mus musculus for Amphibia, Reptilia, Aves and Mammalia, respectively. Microanatomically, in lower tetrapods (toad and lizard), the mucosal layer of the oesophagus was composed of simple ciliated columnar epithelium with goblet cells, whereas in higher tetrapods (pigeon and mouse) it was composed of stratified squamous epithelium, with non-keratinised epithelium in the pigeon but keratinised epithelium in the mouse. However, the gastric mucosal layer of the stomach in lower tetrapods consists of simple columnar epithelium and gastric glands. Similarly, the mucosa of the pigeon’s proventriculus consists of simple columnar epithelium with proventricular glands opened into the lumen, whereas mouse mucosa consists of simple columnar epithelium which folds and forms gastric glands with gastric pits having a variety of cell types. Histochemically, the neutral mucin profile biodistribution in the oesophagus mucosal layer was variable. It was strongly positive in the toad and lizard, but was weak in the pigeon and completely negative in the mouse. In contrast it was strongly positive in the gastric mucosa of the toad, lizard and pigeon, but was weak in the mouse's gastric mucosa. On the other hand, the signals of carboxylated and sulfated mucins were found to be different. They were strong in the mucosa of the lizard oesophagus. In contrast, the carboxylated mucins in the gastric mucosa were positive in all representatives except the mouse. The sulfated mucins were, however, seen localised in the mucosal layer cells of the lizard and pigeon only. The study revealed that the microanatomical structures and functions as well as mucin distribution profiles in the oesophageal gastric tract are in line with interspecies difference and the type of food and feeding habits. However, this may need further investigations including more tetrapod representatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetrapods belong to the vertebrates that have four limbs or leg-like appendages. In taxonomy, Tetrapoda includes four classes: Amphibia, Reptilia, Aves and Mammalia (Narkiewicz and Narkiewicz 2015). These four classes share many anatomical characteristics, especially those concerning the gastrointestinal tract (GIT). However, some microanatomical features of the GIT, especially those related to the cells of the mucosa layer, are completely different. The type and normal function of mucosal cells may differ from a class to another. Anatomically, the GIT of tetrapods is composed mainly of the oesophagus, stomach, small intestine and large intestine (Kardong 2006). Comparatively, most amphibians and reptilians have a short oesophagus followed by the stomach and small and large intestines in the GIT (Romer 1970; Divers and Stahl 2018; Divers and Stahl 2018). The stomach in amphibians is composed mainly of fundic and pyloric portions (Abo-Taira et al. 1988; Machado-Santos et al. 2014). The avian GIT is also composed of an oesophagus, stomach and small and large intestines (King et al. 1984; Al-Juboury 2016). Differently, the stomach of aves is divided anatomically into two parts, proventriculus (oesophageal side) and ventriculus (intestinal side) (Abumandour 2013). The GIT of mammals is mainly composed of the upper gastrointestinal tract, including the oesophagus and stomach, and the lower gastrointestinal tract including the small and large intestine. The stomach in mammalians is composed mainly of cardiac, fundic and pyloric portions, while the small intestine consists of the duodenum, jejunum and ileum (Kardong 2006).
Histologically, the oesophageal wall of tetrapods is mainly composed of four layers, namely the mucosa, submucosa, muscularis and serosa from inside to outside, respectively (Kardong 2006). Microanatomically, the oesophageal mucosal layer of some amphibians and reptilians is composed of ciliated columnar epithelium with a wide spread of goblet cells and tubular glands (Jacobson 2007; Romer 1970). On the contrary, the oesophageal mucosa of avian and mammalian species is, however, composed of non-keratinised and keratinised squamous epithelium (Malewitz 1965; Grossman 1985; Crole and Soley 2010). The stomach in amphibians and reptilians is composed mainly of two portions (fundic and pyloric) with four layers (Gans and Parsons 1977; Liquori et al. 2002, 2005; Kardong 2006). The mucosal layer in the amphibian stomach is composed mainly of simple columnar epithelium forming longitudinal folds (Bizjak Mali and Bulog 2004; Machado-Santos et al. 2014), whereas the gastric mucosa in reptilians is composed of branched tubular glands which consist of a gastric pit and glandular body with mucous neck cells (Jacobson 2007). Similarly, in aves and mammals, the stomach consists of four layers. The proventriculus mucosal layer of aves is lined by simple columnar epithelial cells. Additionally, the tubular mucous glands are located in the lamina propria and open into the lumen of the proventriculus via their ducts. According to Malewitz (1965), the stomach in mammals is divided into glandular and non-glandular portions. The glandular portion mucosa is composed of tall columnar epithelium which lines the gastric gland. Furthermore, the epithelium of the gastric glands has different types of cells, including the chief, mucous neck and parietal cells.
Comparatively, there are many microanatomical differences in the oesophagus and stomach of tetrapods, especially those concerned with mucosal cells. As a result, the mucous biodistribution and the function of mucosal layer cells may differ from species to species in these organs. For example, in amphibians, mucins are secreted by goblet cells in the oesophagus and by epithelial cells and mucous neck cells of the stomach (Liquori et al. 2007). It was proposed that sulfated mucins have a vital role in protecting the epithelia of the oesophagus from enzymatic degradation by bacterial glycosidase (Mikuni-Takagaki and Hotta 1979; Robertson and Wright 1997). Additionally, some representatives of amphibians such as Rinella icterica (Bufonidae), Duttaphrynus melanostictus and Bufo viridis showed a positive signal of goblet cells in the oesophagus and stomach for neutral and acidic mucins (Loo and Wong 1975; Kiernan 1999; Liquori et al. 2007). Previous studies showed differences regarding the qualitative expression of neutral, sulfated and carboxylated mucosubstances in the stomach of amphibians (Sheahan and Jervis 1976; Ferri et al. 1999). For example, in Rinella icterica, mucous neck cells of the stomach fundic region secrete neutral glycoprotein and galactose residues, while in Rana aurora aurora, epithelial cells of the stomach exhibit a large amount of neutral carbohydrates (Ferri et al. 2001). Meanwhile, in Varanus niloticus, as a reptilian model, the mucosa of the oesophagus showed a positive signal of neutral and acidic mucins. Functionally, the localisation of acidic mucins in the mucosa lubricates and allows large food particles (Ahmed et al. 2009). Additionally, Uromastyx aegyptiaca and Laudakia stellio showed a heavy content of carbohydrates and acidic mucins in most oesophageal goblet cells (Koca and Gürcü 2011; Hamdi 2012). However, the stomach of Varanus niloticus and Uromastyx aegyptiaca showed a positive signal for neutral carbohydrate content but a negative acidic one (Ahmed et al. 2009; Hamdi 2012).
For birds, the cervical oesophagus of Columba palumbus showed a weak signal for mucins in the lamina propria, while oesophageal glands showed a strong signal for acidic and neutral mucins (Al-Juboury 2016). It is expected that the type of birds’ food can affect the localisation and production of some carbohydrates in the oesophagus and stomach mucosa. For example, Columba palumbus mucosa was heavily loaded with acid mucopolysaccharides after feeding with grains, while Tyto alba fed with soft flesh showed abundant mucopolysaccharides (Al-Juboury 2016). Additionally, proventriculus mucosal folds of Coturnix coturnix and Columba livia showed a heavy accumulation of neutral and acidic mucins, especially within the cells of apical parts of their ducts (Zaher et al. 2012). In mammalians the oesophageal wall of Myotis myotis showed predominant localisation of glycoproteins in all layers (Paksuz and Paksuz 2021). Furthermore, neutral mucins were observed in the stomach of Acomys Spinosissimus, Crocidura cyanea and Amblysomus hottentotus, while the acid mucins showed variable reactions between these three species (Boonzaier et al. 2013).
Therefore, in this study we will compare the microanatomical features of the mucosal layer of the oesophageal gastric tract in some representatives of tetrapods using histological and histochemical analysis. Additionally, it is expected that digestion of different types of food by different species of tetrapods is in line with the biodistribution of some mucins in the oesophageal gastric tract. The data of this study provides some information about the relationship between the microanatomical structure and different types of mucin biodistribution with the function of different regions of the oesophageal gastric tract.
Materials and methods
Materials
The chemicals used in the present study including nuclear fast red, Alcian blue, basic fuchsin and periodic acid were purchased from Sigma-Aldrich (Ontario, Canada). The rodent chow was purchased from Feedmix Egypt Co., El Obour City, Elgharbia, Egypt.
Experimental design
Four groups of adult male tetrapod representatives (five animals in each group) were used in this study. The experimental groups were named as first, second, third and fourth for toads (Bufo regularis), lizards (Trachylepis quinquetaeniata), pigeons (Columba livia domestica) and mice (Mus musculus), respectively. The details of each animal species are described in Table 1. The experimental procedures in this study were approved by the Local Animal Experimentation Committee, Department of Zoology, Faculty of Science, Sohag University. Each animal group was maintained and housed in a special habitat with its own food at the Animal Facility, Department of Zoology, Faculty of Science, Sohag University.
Animal habitat and type of food
The male toads were collected from water canals near agricultural fields in Sohag Governorate during July and August 2020. They were raised in an artificial habitat designed to imitate the real toad’s habitat. The toad’s habitat was a glass cage with dimensions of 50 cm × 30 cm × 30 cm. The cage was settled with some stones, water and grass comparable to the natural habitat of the toads. The toads had free access to water, house flies (Musca domestica) and earthworms (Allolobophora caliginosa) as a standard food which were provided by Department of Zoology, Sohag University. The male lizards were collected from some agricultural fields in Sohag Governorate from June to August 2020. The artificial habitat of lizards was composed mainly of a glass cage similar to the toad cages in their dimensions, but the water was replaced with sand and medium to large rocks to imitate the hard natural habitats of lizards. The daily food introduced to the lizards was similar to that introduced to toads. The adult males of pigeons were raised in artificial cages designed specifically for pigeon raising. Due to the large size of pigeons, each pigeon was maintained in stainless-steel cages with dimensions of 50 cm × 40 cm × 40 cm) bedded with wooden sawdust and equipped with a water source and food container. Additionally, these cages were settled with mesh cover to allow entry of light. The daily foods introduced to the pigeons were wheat seeds (Triticum aestivum) and sorghum seeds (Sorghum bicolor). The adult males of mice were bred and raised in artificial cages similar to those of pigeons. The mice had access to rodent chow containing 23% protein, 5.5% fats and 60% polysaccharides. Additionally, the rodent chow constitutes have 2950 cal/kg.
Animal dissection and organ collection
All animals were housed and maintained in the animal facility under standard conditions, i.e, room temperature (25 °C) and 12 h light/12 h dark, for 2 weeks. Then animals were sacrificed using a diethyl ether overdose and dissected carefully in a sterilised area. The oesophagus of all animals, stomach of toads, lizards and mice, and proventriculus of pigeons were collected and fixed in Carnoy’s fixative for 1 h. The organs were then dehydrated in a graded series of ethyl alcohols, cleared by methyl benzoate and toluene, and then embedded in paraffin wax. Sections were cut at 7 µm by the rotary microtome (RM 2125RTS; Leica Biosystems, China). Sections were mounted on clean glass slides and incubated in an incubator (Venticell 55 Comfort, MMM Medcenter Einrichtungen, Germany) for 24 h at 35 °C.
Histological investigations
Firstly, sections were deparaffinized by xylene (two changes, 15 min each), rehydrated by a graded series (100% two changes, 90%, 70% and 50%) (5 min each) of alcohol to distilled water. Sections were then stained with haematoxylin for 1 min, washed with tap water (10 min) and rinsed in distilled water. Thereafter, sections were stained by eosin for 3–5 min and dehydrated through a graded series (50%, 70%, 90% and 100% two changes) (5 min each) of ethyl alcohol and cleared in xylene (two changes, 15 min each). Stained sections were mounted by DPX, covered by glass cover slides and examined under a light microscope (Axio Scope.A1, Carl ZEISS, Germany) (Carleton et al. 1967).
Histochemical investigations
Preparation of periodic acid–Schiff (PAS) reagent
Based on McManus (1946), PAS reagent included periodic acid solution (0.5%) for the oxidation of most of the neutral mucins into aldehydes, while Schiff reagent for the detection of aldehydes. Schiff reagent was prepared mainly of 1% basic fuchsin and 1.9% sodium metabisulfite dissolved in hydrochloric acid (0.15 M).
Preparation of Alcian blue solution
Based on Scott and Mowry (1970), Alcian blue staining solution was prepared at two different pH values, pH 2.5 and pH 1, for the detection of carboxylated and sulfated mucins, respectively. Alcian blue stain solution (1%) with pH 2.5 was prepared by adding 1 g Alcian blue to 100 ml of 3% acetic acid solution, while Alcian blue stain (1%) solution with pH 1 was prepared by adding 1 g Alcian blue stain to 100 ml of 0.1 M hydrochloric acid.
Neutral mucin detection
The PAS reagent was used to demonstrate neutral mucins in the tissues. In this study, dewaxed sections were rehydrated using a descending series of ethyl alcohols till distilled water. Then, sections were incubated in periodic acid as a weak oxidising agent of neutral mucins for 5 min to oxidise. Then, sections were rinsed in distilled water for several changes. Thereafter, sections were incubated in Schiff solution at room temperature for 15 min. Counterstaining was done by haematoxylin for 10 s and washing in tap water for 5 min, then by distilled water. Finally, sections were dehydrated by a graded series of ethyl alcohols, cleared by xylene and mounted by DPX (Bancroft and Gamble 2008).
Carboxylated mucin detection
Alcian blue staining protocol at pH 2.5 is usually used to detect carboxylated (non-sulfated) mucins in different tissues (Machado-Santos et al. 2014). In this protocol, sections were deparaffinised in xylene and rehydrated with distilled water. Then, hydrated sections were incubated for 30 min in Alcian blue stain solution at pH 2.5 at room temperature. Sections then were washed by tap water for 5 min followed by rinsing in distilled water and counterstained by nuclear fast red. Finally, sections were dehydrated, cleared by clearing reagent and mounted by DPX (Bancroft and Gamble 2008).
Sulfated mucin detection
To detect sulfated mucins in tissues, Alcian blue stain (pH 1) was used (Machado-Santos et al. 2014). Deparaffinised tissue sections were rehydrated in distilled water and incubated for 30 min in Alcian blue solution (pH 1) at room temperature. Sections were then washed by tap water for 5 min followed by distilled water and stained with nuclear fast red as a counterstain. Thereafter, stained sections were dehydrated by ethyl alcohols, cleared by xylene and mounted by DPX (Bancroft and Gamble 2008).
Semi-quantitative analysis
The staining intensities of sections stained by PAS, Alcian blue (pH 2.5) and Alcian blue (pH 1) to demonstrate the amount of neutral, carboxylated and sulfated mucins, respectively, were indicated as (+++) for highly positive, (++) for moderately positive, (+) for weak positive and (−) for a negative signal.
Results
Histological structure of the oesophagus in tetrapods
The oesophagus of all studied tetrapod representatives in this study consists of the four ordinary layers organised from outside inwards as follows: serosa/adventitia, muscularis, submucosa and mucosa. Comparatively, the structure of each layer, especially the mucosa, showed several varieties regarding the encountered cell types.
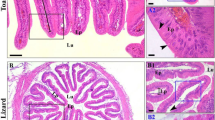
As shown in Fig. 1a, the mucosa of the toad’s oesophagus appeared as microfolds facing the inner lumen. Each microfold was composed mainly of lamina propria rich in blood vessels and small structures known as simple tubular glands which are mainly formed of simple cuboidal epithelium (Fig. 1a1). Furthermore, the apical regions of the mucosal lining epithelium facing the inner lumen are composed of partially ciliated columnar epithelium and scattered goblet cells (Fig. 1a2).
Photomicrographs of transverse sections of the oesophagus of the toad (a) and lizard (b) showing the general histological structure. The mucosa of the toad’s oesophagus is composed of tubular glands (Tg) surrounded by lamina propria (Lp). The lining epithelium (Ep) in the glands faces the inner lumen (Lu) and comprises partially ciliated columnar epithelial cells (bold arrow) and goblet cells (arrow heads). The mucosa of the lizard’s oesophagus is mainly composed of lamina propria (Lp) and lining epithelium (Ep) facing the inner lumen (Lu). The lining epithelium of a lizard’s oesophagus is composed of full ciliated columnar epithelial cells (bold arrows) and goblet cells (arrow heads). Stain: H&E. Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
As for the lizard’s oesophagus (Fig. 1b), morphologically, the mucosa layer was similar to that of the toad’s mucosa, being formed of microfolds that consist mainly of a lamina propria, a muscularis mucosa and a lining epithelium (Fig. 1b1). The lining epithelium faced the lumen and comprised completely ciliated columnar epithelium and scattered goblet cells (Fig. 1b2). Comparatively, there were no tubular glands in the lamina propria of the lizard’s oesophagus compared to that of toad’s oesophagus.
The mucosa of the pigeon’s oesophagus consists of more microfolds compared with the toad and lizard (Fig. 2a). The microfolds of the pigeon’s oesophagus contained muscularis mucosa located under the epithelium layer to support the mucosal folds. In addition to muscularis mucosa, lamina propria contained connective tissues and blood vessels (Fig. 2a1). In contrast to the toad and lizard, the oesophageal mucosal epithelium of the pigeon was composed of non-keratinised stratified squamous epithelium and faced the inner lumen of the oesophagus (Fig. 2a2).
Photomicrographs of transverse sections of the oesophagus of the pigeon (a) and mouse (b) showing the general histological structure. The mucosa of the pigeon’s oesophagus is composed of lamina propria (Lp) and non-keratinised stratified squamous lining epithelium (Ep) facing the inner lumen (Lu). The mucosa of the mouse oesophagus consists mainly of lamina propria (Lp) and keratinised stratified squamous lining epithelium (Ep) facing the inner lumen (Lu). Stain: H&E. Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Like the pigeon, the mouse’s oesophagus (Fig. 2b) was composed mainly of microfolds with lamina propria, muscularis mucosa and mucosal epithelium (Fig. 2b1). Lamina propria of the mouse’s oesophagus was composed of connective tissues rich in blood sinusoids in the vicinity of the muscularis mucosa. As in the case of the pigeon, the mucosal epithelium of the mouse oesophagus was composed of stratified squamous, but with keratinised epithelium facing the inner lumen (Fig. 2b2).
Histological structure of the stomach in tetrapods
Similar to the oesophagus, the stomach in all tetrapod representatives consists mainly of the four main layers; serosa, muscularis, submucosa and mucosa. Comparatively, the main histological difference between the studied tetrapod representatives was observed in the mucosal layer. The mucosal layer of the toad’s stomach was composed of a small lamina propria layer followed by muscularis mucosa, gastric glands and lining epithelium (Fig. 3a). The gastric glands were lined with simple cuboidal epithelium and were located just under the lining epithelium (Fig. 3a1). The lining epithelium faced the inner lumen and comprised simple cuboidal to columnar epithelium without goblet cells (Fig. 3a2).
Photomicrographs of transverse sections of the stomach of the toad (a) and lizard (b). The mucosa of the toad’s stomach consists of lamina propria (Lp) followed by muscularis mucosa (Mm), gastric glands (Gg) and lining epithelium (Ep). The lining epithelium of the toad’s stomach faces the inner lumen (Lu) and comprises cuboidal (bold arrows) to columnar simple epithelial cells (arrow heads). The mucosa of the lizard’s stomach consists also of lining epithelium (Ep) and gastric glands (Gg), surrounded with lamina propria (Lp) followed by muscularis mucosa (Mm). The lining epithelium consists of cuboidal (bold arrow) to columnar simple epithelial cells (arrow heads) facing the inner lumen (Lu). Stained with H&E. Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Similar to the toad’s mucosa, the mucosal layer of the lizard was composed mainly of lamina propria connected to muscularis mucosa, gastric glands and lining epithelium (Fig. 3b). The gastric gland in the lizard’s mucosa was also composed of a simple cuboidal epithelium (Fig. 3b1). Similar to the toad’s mucosa, the lining epithelium of the lizard’s stomach mucosa was composed of simple cuboidal to columnar epithelium (Fig. 3b2).
The mucosa of the pigeon’s proventriculus stomach (glandular stomach in birds) was composed mainly of many proventricular glands opening into the inner lumen (Fig. 4a). The mucosal epithelium of the proventricular glands consists of polygonal to columnar simple epithelial cells (Fig. 4a1). The lamina propria was located just under the proventricular glands (Fig. 4a2).
Photomicrographs of transverse sections of the stomach of pigeon (a) and mouse (b). The mucosa of the pigeon’s stomach consists of proventricular glands (Pg), which open into the inner lumen (Lu) of the stomach, and are lined with polygonal (zigzag arrow) to columnar simple epithelial cells (double-headed arrow). The proventricular glands are surrounded by a narrow lamina propria. The mucosa of the mouse’s stomach is mainly composed of gastric glands (Gg) intervened with lamina propria (Lp) and lining epithelium facing the inner lumen (Lu). The lining epithelium of the mouse’s gastric glands incorporates different types of gastric cells such as gastric pit cells (bold arrows), chief cells (arrows) and parietal cells (arrow heads). Stained with H&E. Scale bar: a and b = 50 µm; a1 = 10 µm; and a2, b1, b2 and b3 = 5 µm
The histological architecture of the mouse’s stomach mucosa was similar to that of the pigeon, being formed as a lining epithelium and many gastric glands surrounded by a narrow lamina propria and supported by muscularis mucosa (Fig. 4b). The gastric glands were lined by simple columnar epithelium (Fig. 4b1) comprising different cell types, including chief cells, gastric stem cells, parietal cells, mucous neck cells and gastric pit cells (Fig. 4b1, b2, b3).
Figure 5 summarises comparatively the differences between the histological structures of the mucosal layer in the oesophagus (Fig. 5a, b, c, d) and stomach (Fig. 5e, f, g, h) in the four tetrapod representatives. The mucosal layer of the oesophagus of the toad and lizard consisted of a simple columnar epithelium (Fig. 5a, b). In the pigeon's and mouse's oesophagus, it was composed of a stratified squamous epithelium (Fig. 5c, d). As for the stomach, the mucosal layer architectures of the toad, lizard and pigeon were similar, being formed mainly of a lining with simple columnar epithelium (Fig. 5e, f, g, h). The mucosal layer of the mouse stomach, however, was composed of simple columnar epithelium and a gastric gland with cuboidal to columnar cells (Fig. 5h).
Cartoon drawings showing the toad’s oesophageal mucosa (a) consisting of partially ciliated simple columnar epithelium and goblet cells. The lizard’s oesophageal mucosa (b) which consists of ciliated simple columnar epithelium and goblet cells. The pigeon’s oesophageal mucosa (c) with non-keratinised stratified epithelium. The mouse’s oesophageal mucosa (d) which is mainly composed of keratinised stratified epithelium as well as the gastric mucosa in the four tetrapod representatives (e–h), the toad, lizard, pigeon and mouse, respectively. Ep epithelium, Lp lamina propria, Tg tubular gland, Gc goblet cells, Hl horny layer, Mm muscularis mucosa, Ct connective tissue, Pg proventriculus gland, Gpc gastric pit cells, Prc progenitor cells, Pc parietal cells, Stc stem cells, Chc chief cells, Mnc mucous neck cells
Mucin biodistribution profiles in the oesophageal gastric tract of tetrapod representatives
Neutral mucins in the oesophagus
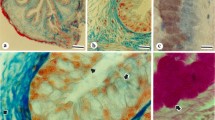
As shown in Fig. 6a, the neutral mucins had a negative signal in the muscularis and lamina propria layers. In contrast, the tubular glands and lining epithelium including columnar epithelial cells and goblet cells showed a moderate to strong positive signal (Fig. 6a1, a2). Likewise, in the lizard’s oesophagus (Fig. 6b), neutral mucins had weak to negative signals in the muscularis layer, and a weakly positive signal in lamina propria and submucosa, but a highly positive signal in the lining epithelium (columnar epithelial cells and goblet cells) of both the mucosal layer and tubular glands (Fig. 6b2).
Photomicrographs of transverse sections of the oesophagus stained with PAS and counterstained with haematoxylin showing localisation and biodistribution of neutral mucins (purple colour) in the mucosa of the toad (a) and lizard (b). The localisation of neutral mucins in the mucosa of the toad’s oesophagus have a moderate positive signal in the lining epithelium (Ep) (bold arrows) and tubular glands (Tg), but a negative signal in both lamina propria (Lp) and muscularis layer (Mu). The mucosa of the lizard’s oesophagus shows a highly positive signal of neutral mucins in the lining epithelium (Ep) (bold arrows), moderately positive in lamina propria (Lp) and submucosa (SM), moderately positive in tubular glands (Tg) and negative in the muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
In the pigeon, neutral mucins showed weak to moderate profiles in all oesophageal compartments except muscularis (Fig. 7a). In the muscularis layer, strong positive signals of neutral mucins were observed. Neutral mucins were moderately localised in the muscularis mucosa. Compared to toad’s and lizard’s mucosae and lamina propria (Fig. 6a, a1, a2), the lining epithelium of the pigeon's mucosa showed weaker neutral mucin profiles (Fig. 7a, a1, a2).
Photomicrographs of transverse sections of the oesophagus stained with PAS and counterstained with haematoxylin showing localisation and biodistribution of neutral mucins (purple colour) in the mucosa of the pigeon (a) and mouse (b). The neutral mucins in the mucosa of the pigeon’s oesophagus shows a weak to moderate signal in the lining epithelium (Ep) and lamina propria (Lp) and moderate to strong positive in the muscularis layer (Mu). The mucosa of the mouse’s oesophagus shows a negative signal of neutral mucins in the lining epithelium (Ep), weakly positive in lamina propria (Lp) and highly positive in the muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Like the pigeon, the neutral mucins of the mouse’s oesophagus (Fig. 7b) showed a highly positive signal in the muscularis and muscularis mucosae compared to other layers. The lamina propria of the mouse oesophagus showed a weakly positive signal (Fig. 7b1), and the lining epithelium showed a negative signal (Fig. 7b2).
Carboxylated mucins in the oesophagus
As shown in Fig. 8a, the carboxylated mucins in the mucosa of the toad’s oesophagus (stained with Alcian blue stain at pH 2.5) had a moderately positive signal in lamina propria. In contrast, they were negative neither in the tubular glands (Fig. 8a1) nor lining epithelium including columnar epithelial or goblet cells (Fig. 8a2). On contrary to the toad, carboxylated mucins showed negative signals in the muscularis and lamina propria of the lizard’s oesophagus (Fig. 8b, b1) and highly positive signals in the mucosal layer, especially the goblet cells of the lining epithelium (Fig. 8b2).
Photomicrographs of transverse sections of the oesophagus showing the biodistribution of carboxylated mucins (blue colour) in the mucosa of the toad (a) and the lizard (b). The localisation of carboxylated mucins in the mucosa of the toad’s oesophagus shows a negative signal in the lining epithelium (Ep) and tubular glands (Tg), but shows a moderately positive signal in lamina propria (Lp). The mucosa of the lizard’s oesophagus shows a highly positive signal of carboxylated mucins in the goblet cells of lining epithelium (arrow head), a negative signal in the lamina propria (Lp), weakly positive in the submucosa (SM) and negative in the muscularis layer (Mu). Stain: Alcian blue at pH 2.5; counterstained with nuclear fast red (red colour). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
In the pigeon, most oesophageal compartments showed a negative signal of carboxylated mucins, especially muscularis mucosa and lining epithelium (Fig. 9a). Interestingly, the lamina propria of the pigeon’s oesophagus had a weakly positive signal (Fig. 9a1, a2). Similar to the pigeon’s oesophagus, the carboxylated mucins showed a negative signal in all tissue compartments of the mouse’s oesophagus (Fig. 9b1), including the lamina propria and lining epithelium (Fig. 9b, b2).
Photomicrographs of transverse sections of the oesophagus mucosa of the pigeon (a) and mouse (b). The localisation of carboxylated mucins in the mucosa of the pigeon’s oesophagus shows a negative signal in the lining epithelium (Ep) and a weakly positive signal in lamina propria (Lp). The mucosa of the mouse’s oesophagus shows a negative signal of carboxylated mucins in all compartments, including the lining epithelium (Ep), lamina propria (Lp) and muscularis layer (Mu). Stain: Alcian blue at pH 2.5; counterstained with nuclear fast red (red colour). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Sulfated mucins in the oesophagus
The biodistribution profiles of sulfated mucins in all tissues in the oesophagus of tetrapod representatives were similar to those of the carboxylated mucins. The sulfated mucins showed a negative signal in all tissues of the toad’s oesophagus (Fig. 10a). Similar to carboxylated mucin profiles, the lizard’s oesophagus showed a highly positive signal of sulfated mucins in the epithelial goblet cells, while other tissues showed a negative signal (Fig. 10b). As shown in Fig. 11, the sulfated mucins showed a negative signal in all tissues of the pigeon’s (Fig. 11a) and mouse’s oesophagus (Fig. 11b).
Photomicrographs of transverse sections stained with Alcian blue stain at pH 1 and counterstained with nuclear fast red to illustrate the sulfated mucins biodistribution in the oesophagus mucosa of the toad (a) and lizard (b). The localisation of sulfated mucins in the mucosa of the toad’s oesophagus shows a negative signal in the lining epithelium (Ep), goblet cells (arrow head) and tubular glands (Tg), but shows a weak signal in lamina propria (Lp). The mucosa of the lizard’s oesophagus displayed a highly positive signal of sulfated mucins in the goblet cells of lining epithelium (arrow head), and a negative signal in the lamina propria (Lp), weakly positive in the submucosa (SM) and negative in the muscularis layer (Mu). Stained with Alcian blue stain at pH 1 and counterstained with nuclear fast red. Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Photomicrographs of transverse sections of the oesophagus stained with Alcian blue stain at pH 1 and counterstained with nuclear fast red showing the sulfated mucin biodistribution in the mucosa of the pigeon (a) and the mouse (b). The sulfated mucins in the mucosa of both the pigeon’s and mouse’s oesophagus shows a negative signal in almost all compartments, including the lining epithelium (Ep), lamina propria (LP) and muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm. Mu muscularis
Neutral mucins in the stomach
In the toad’s stomach, the neutral mucin localisation showed a highly positive signal in the mucosal layer but a negative signal in the muscularis layer (Fig. 12a). Obviously, the mucosal layer, the tubular glands (Fig. 12a1) and the lining epithelium (Fig. 12a2) showed a high density of neutral mucin profiles in the epithelial cells. In the lizard’s stomach, all tissue compartments showed a positive signal of neutral mucins (Fig. 12b). The muscularis layer of the lizard’s stomach displayed a weakly positive signal. In contrast, submucosa, lamina propria and gastric gland tissues (Fig. 8b1) exhibited a moderately positive signal of neutral mucins. In addition, the lining epithelium of the mucosal folds showed a strong positive signal, indicating highly accumulated neutral mucins (Fig. 8b2).
Photomicrographs of transverse sections of the stomach stained with PAS and counterstained with haematoxylin to localise the neutral mucins in the stomach mucosa in (a) the toad and (b) the lizard. The localisation of neutral mucins in the mucosa of the toad’s stomach shows a highly positive signal both in the lining epithelium (Ep) and tubular glands (Tg), and a weakly positive signal in the muscularis (Mu). The mucosa of the lizard’s stomach shows a highly positive signal of neutral mucins in the lining epithelium (Ep), moderately positive in the lamina propria (LP), submucosa (SM) and tubular glands (Tg), but a weakly positive signal in the muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
As shown in Fig. 13a, the neutral mucins in the pigeon’s proventriculus showed a weakly positive signal in lamina propria, submucosa and epithelium of the proventriculus glands. Neutral mucins showed a highly positive signal in the lining epithelium (Fig. 13a1, a2). In the mouse’s stomach, however, most tissue compartments showed a negative signal of the neutral mucins (Fig. 13b). Obviously, most glandular epithelial cells had a negative neutral mucins signal (Fig. 13b), except the gastric pit cells which revealed a moderate positive signal of neutral mucins (Fig. 13b1, b2).
Photomicrographs of transverse sections of the stomach stained with PAS and counterstained with haematoxylin to localise the neutral mucins of the stomach mucosa in the pigeon (a) and the mouse (b). The localisation of neutral mucins in the mucosa of the pigeon’s stomach shows a highly positive signal in the lining epithelium (Ep) and weakly positive signals in the lamina propria (LP) and the submucosa (SM). The mucosa of the mouse’s stomach shows a moderately positive signal of neutral mucins in the lining epithelium (Ep), but a negative signal in the gastric glands (Gg). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Carboxylated mucins in the stomach
The localisation of carboxylated mucins in the toad’s stomach showed a negative signal in the muscularis, submucosa, muscularis mucosa (Fig. 14a). The columnar epithelial cells of the lining epithelium (Fig. 14a1) and the cuboidal cells of the tubular glands (Fig. 12a2) displayed a weakly positive signal. Furthermore, the signal indicating the carboxylated mucins in the lizard’s stomach was negative in the muscularis and muscularis mucosa layers, but a weak positive signal in the lamina propria and submucosa (Fig. 14b). In addition, the lining epithelium of lizard mucosa showed a moderately positive signal (Fig. 14b1), while the brush border showed a highly positive one (Fig. 14b2).
Photomicrographs of transverse sections of stomach stained with Alcian blue stain at pH 2.5 and counterstained with nuclear fast red to illustrate the localisation of carboxylated mucins (blue) in the mucosa of the toad (a) and the lizard (b). The carboxylated mucins in the mucosa of the toad’s stomach shows a weakly positive signal in the lining epithelium (Ep) of the tubular glands (Tg). The mucosa of the lizard’s stomach shows a moderately positive signal of carboxylated mucins in the lining epithelium (Ep), a highly positive signal in the brush border (arrow heads), a weakly positive signal in the lamina propria (Lp), submucosa (SM), and negative in the muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
In the pigeon’s proventriculus, the carboxylated mucins showed a weakly positive signal in the lamina propria and submucosa (Fig. 15a). The polygonal cells of the proventricular glands showed a negative signal, whereas the columnar epithelial cells of the lining epithelium of mucosal folds showed a moderately positive signal (Fig. 15a1, a2). As shown in Fig. 15b, the carboxylated mucin profiles in the mouse’s stomach were weakly positive in the muscularis layer, gastric glands and the lining epithelium (Fig. 15b1, b2).
Photomicrographs of transverse sections of stomach stained with Alcian blue stain at pH 2.5 and counterstained with nuclear fast red to show the biodistribution of carboxylated mucins (blue) of the mucosa of (a) the pigeon and (b) the mouse. The presence of carboxylated mucins in the mucosa of the pigeon’s stomach shows a moderately positive signal in the lining epithelium (Ep) and a weakly positive signal in both lamina propria (Lp) and submucosa (SM). The mucosa of the mouse’s stomach shows a negative signal of carboxylated mucins in the lining epithelium (Ep), but a weakly positive in the gastric glands (Gg), and the muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Sulfated mucins in the stomach
The sulfated mucin profiles in all gastric layers of tetrapod representatives were slightly weaker than those of carboxylated mucins. The localisation of sulfated mucins in the toad’s stomach showed a negative signal in all stomach layers, including the lining epithelium, tubular glands and lamina propria (Fig. 16a, a1, a2). As shown in Fig. 16b, the localisation of sulfated mucins in the lizard’s stomach showed a negative signal in the muscularis layer, lamina propria and submucosa. In contrast, the lining epithelium and brush border of stomach mucosa showed a moderately positive signal (Fig. 16b1, b2). The biodistribution profiles of sulfated mucins in the pigeon’s proventriculus were similar to that of the lizard (Fig. 17a). The sulfated mucins in the pigeon’s proventriculus were not localised in proventriculus glands, submucosa and lamina propria, while the lining epithelium of the mucosal layer showed a moderately positive signal (Fig. 17a1, a2). In the mouse’s stomach, sulfated mucins were not found in any of the layers, as shown in Fig. 17b.
Photomicrographs of transverse sections of the stomach stained with Alcian blue stain at pH 1 and counterstained with nuclear fast red showing the localisation of the sulfated mucins (blue) in the mucosa of (a) the toad and (b) the lizard. The localisation of sulfated mucins in mucosa of the toad’s stomach shows a negative signal in all gastric compartments, including the lining epithelium (Ep), tubular glands (Tg) and lamina propria (Lp). The mucosa of the lizard’s stomach shows a weakly positive signal of sulfated mucins in the lining epithelium (Ep), moderately positive signal in the brush border, weakly positive signal in the lamina propria (Lp) and submucosa (SM), and negative signal in the muscularis layer (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Photomicrographs of transverse sections of the stomach stained with Alcian blue stain at pH 1 and counterstained with nuclear fast red showing the localisation of the sulfated mucins in the mucosa of (a) the pigeon and (b) the mouse. The signal of sulfated mucins in the mucosa of the pigeon’s stomach have a moderate positive signal in the lining epithelium (Ep) and a negative signal in the lamina propria (LP) and the submucosa (SM). The mucosa of the mouse’s stomach shows a negative signal of sulfated mucins in the lining epithelium (Ep), gastric glands (Gg), lamina propria, submucosa (SM) and muscularis (Mu). Scale bar: a and b = 50 µm; a1 and b1 = 10 µm; and a2 and b2 = 5 µm
Figure 18 and Table 2 present a summary of the localization and biodistribution profiles of the neutral, carboxylated and sulfated mucins in both oesophagus and stomach tissues of tetrapod representatives. The neutral mucins showed a highly positive signal in the epithelial and goblet cells in the epithelial layer of the toad’s and lizard’s oesophagus. However, the apical cells in the epithelial layer of the pigeon’s oesophagus showed a weak positive signal, and the epithelial layer of the mouse oesophagus showed a completely negative signal of the neutral mucins. The carboxylated and sulfated mucins were highly expressed only in the goblet cells of the epithelium of the lizard’s oesophagus. Most tissues of the oesophagus in other tetrapod representatives (toad, pigeon and mouse) showed moderate to negative signals of carboxylated as well as sulfated mucins.
Cartoon drawings of the epithelial layer of (a–c) oesophagus and (d–f) stomach of tetrapod representatives. The biodistribution of neutral mucins (a and d), carboxylated (b and e) and sulfated mucins (c and f) in oesophagus and stomach epithelia of (I) toad, (II) lizard, (III) pigeon and (IV) mouse. The neutral mucins are localised in the epithelium of the oesophagus and stomach in all tetrapods. However, the carboxylated and sulfated mucin biodistribution in the oesophageal gastric epithelium differs in the different tetrapod representatives
Discussion
The microanatomical features in the GIT of most tetrapod representatives displayed dramatic differences which reflect a variation in functions of different GIT regions of different species under investigation. Generally, there was a difference in the morphology and gross anatomy of the four studied species. Interestingly, the main microanatomical differences between tetrapod representatives were mainly encountered in the mucosal layer. In most amphibian representatives (for example, Bufo regularis in the present study and Bufo viridis in previous studies), the mucosal layer was composed of ciliated columnar epithelium with intervening tubular glands having a large number of scattered goblet cells (Liquori et al. 2002; Pelli-Martins et al. 2012). In contrast to the toad, the oesophageal mucosa of the lizard (Trachylepis quinquetaeniata) was composed of folds lined with simple ciliated columnar epithelium with scattered goblet cells, but lacked the oesophageal glands. However, such tubular glands have been found in some reptilians species such as snakes, which is due to their feeding style and habits (Jacobson 2007). In accordance with these findings, many other reptilian species such as Uromastyx aegyptiaca exhibited oesophageal glands (Hamdi 2012). The present study revealed that, in contrast to amphibian and reptilian forms, the mucosal layer in the cervical oesophagus of the pigeon and mouse was composed of longitudinal folds with non-keratinised and keratinised stratified squamous epithelium. These findings are in agreement with those of Malewitz (1965) and Crole and Soley (2010).
The above-mentioned differences in the microanatomical features in the mucosal layer between the lower tetrapod representatives (toad and lizard) and the higher tetrapod representatives (pigeon and mouse) are in keeping with the difference in the type of food and feeding habits of the four species. Additionally, it is assumed that these microanatomical differences may necessitate a slight modification in oesophageal gastric tract function. For example, in lower tetrapod representatives, the mucosal epithelial cilia and goblet cell secretion is essential for the distribution of mucous in the oesophagus in order to lubricate the passage of food and protect the mucosal layer from abrasive food (Pelli-Martins et al. 2012). On the other hand, the microanatomical and histological structure of the oesophagus in higher tetrapod representatives, the pigeon and mouse, as revealed by the present study, may fit its function, which may vary in the different species. The pigeon and mouse oesophageal mucosae have a thick keratinised and/or non-keratinised stratified squamous epithelium that may protect the mucosa from the harmful effect of food transport into the stomach (Gelis 2013). The function of the oesophagus is to aid in food transport and passage rather than in chemical digestion activity (Paksuz and Paksuz 2021). The lack of oesophageal glands and epithelial goblet cells in higher tetrapod representatives as revealed by the present study is in keeping with the main function of the oesophagus. In this context, it can be believed that the oesophagus of lower tetrapod representatives may play an essential role in food digestion due to the presence of oesophageal gland and/or numerous mucous and goblet cells and due to goblet cell secretion.
The stomach mucosal layer in the toad (the amphibian model in the present study) was shown to be formed of simple cuboidal to simple columnar epithelium without goblet cells, while the gastric glands were composed of simple cuboidal epithelium and open into the gastric lumen. These results agree with those of Machado-Santos et al. (2014), who studied the microanatomical structure of the GIT in Rinella icterica as a model of amphibians and published similar findings. However, some species of amphibians such as Proteus anguinus have goblet cells in stomach mucosa (Bizjak Mali and Bulog 2004). Similar to the toad’s stomach, in the lizard (the reptilian model in this study) the mucosa of the stomach is composed of gastric glands with gastric pits. The gastric pits of the lizard’s stomach were lined with simple columnar epithelium and open into the lumen. Likewise, the epithelium of the lizard’s gastric glands is composed of simple cuboidal to columnar epithelium, but lacking the goblet cells (Jacobson 2007). These findings agree with those of Hamdi (2012) and Bizjak Mali and Bulog (2004). However, they contradict those of Chou (1977), who stated that the mucosal epithelium of the lizard’s stomach contains goblet cells.
In the pigeon (the avian model in this study), the proventriculus mucosa was composed of proventricular glands, which are formed of polygonal secretory cells and apical columnar epithelium. These findings are in agreement with those of Al-Saffar (2016), who reported that avian proventricular glands have a variety of cell types, including apical columnar cells and polygonal secretory cells. Likewise, the present study revealed that the mouse mucosa is also composed of gastric glands which have different types of cells such as chief cells, parietal cells, mucous neck cells and gastric pit cells. This is in accordance with Malewitz (1965), who reported similar findings. The mucous cells encountered in the stomach of higher tetrapod representatives (pigeon and mouse) perform various functions. They may protect stomach mucosa from mechanical injury, pathogens and aggressive pepsin. Additionally, gastric pit cells may support surface neutralisation of acids by mucosal bicarbonate (Ferri et al. 2001).
The localisation and distribution of different types of mucins (neutral and acidic) in the oesophageal gastric tract was evaluated in the main tissues and cells of each class representative. In general, mucins are composed of polysaccharide chains covalently linked to a protein core (O-glycosidic linkage) (Gendler and Spicer 1995). The polysaccharide chains of mucins vary from neutral mucins to weakly acidic (carboxylated) mucins to strongly acidic (sulfomucins) (Bancroft and Gamble 2008). The findings of the current investigation of lower tetrapod representatives are in keeping with mucus being secreted into the oesophagus by goblet cells to facilitate food passage and protect the mucosal epithelium against abrasive food, as well as participation in the digestion process. Furthermore, it was proposed that sulfated mucins are involved in the chemical defence of epithelia against enzymatic degradation of the mucus barrier by bacterial glycosidase (Eggert-Kruse et al. 2000). In contrast to lower tetrapod models, in higher tetrapod models, the pigeon cervical oesophagus and mouse oesophagus were characterised by the absence of glands. This suggests that the main function of the oesophagus in these two groups is in keeping with food transfer from the mouth to the stomach.
In the current study, the neutral mucins were seen localised in tubular glands, lining epithelium and goblet cells of the mucosal layer in the toad’s oesophagus. Conversely, the sulfated and carboxylated mucins were not seen in most oesophageal compartments. Our findings are corroborated by those of Liquori et al. (2002), who reported the presence of neutral mucins in the oesophageal mucosa of Bufo virides and Rinella icterica. In contrast, another study on the oesophagus of Bufo virides and Rinella icterica indicated a positive signal of acidic mucins in the mucosal layer, especially in their tubular glands and goblet cells (Pelli-Martins et al. 2012). As for the lizard’s oesophagus in this study, neutral and acidic mucins were seen in goblet cells. This finding is supported by those of Hamdi (2012) and Hamdi et al. (2014), who reported that goblet cells in the oesophagus of Uromastyx aegyptiaca and Chamaeleon africanusas exhibited an apparent localization of both neutral and acidic mucins.
The pigeon’s cervical oesophagus showed a weakly positive signal for neutral mucins and a negative signal for acidic mucins. However, previous studies on the pigeon’s thoracic oesophagus showed a high positive signal of neutral and acidic mucins in mucosal folds and oesophageal glands (Rajabi and Nabipour 2009; Hadi and Mohamed 2015). Furthermore, the present study showed a weak profile of neutral mucins in lamina propria and a negative one in the lining epithelium of the mouse’s oesophagus. In contrast, the oesophagus of the mammalian forms, Myotis myotis and Pipistrellus, showed positive localisation of neutral and acidic mucins in the lining epithelium layer. It is possible that mucin localisation in the oesophagus of the mouse is due to the presence of salivary secretions (Strobel et al. 2015; Paksuz and Paksuz 2021).
The stomach of the toad as shown by the present study had a strong signal of neutral mucins in the lining epithelium and gastric glands, whereas it had a negative signal for acidic mucins in all compartments except for lamina propria, which showed a positive signal for carboxylated mucins. The biodistribution profiles of mucins in the toad's stomach revealed by our study is in agreement with that of Bufo viridis and Bufo melanostictus, the gastric lining epithelial cells of which produce only neutral mucins (Loo and Wong 1975; Liquori et al. 2002). Furthermore, these findings are corroborated by those of Ferri et al. (2001), who reported a strong localization of neutral mucins in the epithelial cells of the stomach of Rana aurora aurora.
Furthermore, the mucosal layer of the lizard's stomach showed a moderately to a highly positive signal of neutral mucins in gastric glands and lining epithelium, whereas it showed a highly to a moderately positive signal of acidic mucins in the brush border, lining epithelium and lamina propria. Interestingly, the biodistribution profiles in the lizard's stomach are similar to those of Uromastyx aegyptiaca as a model for reptilians (Hamdi 2012). However, the stomach of Laudakia stellio showed higher localisation of neutral and acidic mucins in the epithelial secretory cells of the mucosal layer (Koca and Gürcü 2011).
The pigeon proventriculus showed high contents of both neutral and acidic mucins in the lining epithelium. This finding is corroborated by that of Al-Saffar (2016), who revealed a strong localization of mucins in the pigeon proventriculus. This is further supported by Zaher et al. (2012) and Hamdi (2013), who reported similar observations on the avian forms Coturnix coturnix and Elanus caeruleus.
The mucosal layer of the mouse's stomach showed a moderate localisation of neutral mucins in the gastric pits only, but a low content of carboxylated mucins in lamina propria and gastric glands. However, the sulfated mucins were absent in all layers of the mouse's stomach. Similar findings were shown in some layers in the stomach of Acomys Spinosissimus such as neutral mucins in the gastric pits. In comparison, acidic mucins were seen localised with a weak signal in some mucous neck cells in Acomys Spinosissimus as a mammalian model. Additionally, Crocidura cyanea a showed strong positive signal of neutral mucins in mucous cells, but a negative signal for acidic mucins (Boonzaier et al. 2013). The mucins in the stomach and gastric lumen play a major role in protecting the mucosal epithelium from mechanical injury and lubricating food passage to the intestine, and in protecting the mucosa from enzymatic activity (Ferri et al. 2001).
In conclusion, the present study showed histological, histochemical and microanatomical differences in the mucosal layer of the oesophageal gastric tract among the four tetrapod representatives. It is expected that the variations observed in the histological structure and the biodistribution of the different types of mucins is in line with the function of different regions in the oesophageal gastric tract. Additionally, it is expected that the type of the food may affect the mucin production for food digestion and foreign substance fighting. Further investigations are needed for our hypothesis regarding the relationship between the oesophageal gastric tract tissues and their function against different types of food or other substances. Furthermore, the data of this study are essential to understanding the relationship between microanatomical features and their functions in the oesophageal gastric tract against food digestion and foreign substance invasion.
References
Abo-Taira AM, Mansour AB, Amer MA, Zaher MM (1988) Anatomical, morphometrical and histological studies on the alimentary tract of the lacertid lizard, Acanthodactylus boskianus (Family Lacertidae). Proc Egypt Acad Sci 38:87–101
Abumandour MM (2013) Morphological studies of the stomach of falcon. Sci J Veter Adv 2:30–40
Ahmed Y, Ea E, Ae Z (2009) Histological and histochemical studies on the esophagus, stomach and small intestines of vara-nus niloticus. J Vet Anat 2:35–48. https://doi.org/10.21608/jva.2009.45136
Al-Juboury R (2016) Comparative anatomical, histological and histochemical studies of the oesophagus in two different Iraqi birds (Columba palumbus and Tyto alba). Int J Adv Res Biol Sci 12:188–199. https://doi.org/10.13140/RG.2.1.2961.2403
Al-Saffar FJ (2016) Histomorphological and histochemical study of stomach of domestic pigeon (Columba livia domestica). Iraqi J Vet Med 40:89–96. https://doi.org/10.30539/iraqijvm.v40i1.144
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Elsevier, Amsterdam, pp 183–186
Bizjak Mali L, Bulog B (2004) Histology and ultrastructure of the gut epithelium of the neotenic cave salamander, proteus anguinus (Amphibia, Caudata). J Morphol 259:82–89. https://doi.org/10.1002/jmor.10171
Boonzaier J, Van der Merwe EL, Bennett NC, Kotzé SH (2013) A comparative histochemical study of the distribution of mucins in the gastrointestinal tracts of three insectivorous mammals. Acta Histochem 115:549–556. https://doi.org/10.1016/j.acthis.2012.12.003
Carleton HM, Drury RAB, Wallington EA (1967) Carleton’s histological technique. Oxford University Press, New York, pp 127–130
Chou LM (1977) Anatomy, histology and histochemistry of the alimentary canal of gehyra mutilata (reptilia, lacertilia, gekkonidae). J Herpetol 11:349–357. https://doi.org/10.2307/1563248
Crole MR, Soley JT (2010) Gross morphology of the intra-oral rhamphotheca, oropharynx and proximal oesophagus of the emu (dromaius novaehollandiae). Anat Histol Embryol 39:207–218. https://doi.org/10.1111/j.1439-0264.2010.00998.x
Divers SJ, Stahl SJ (2018) Mader’s reptile and amphibian medicine and surgery-e-book. Elsevier, Amsterdam
Eggert-Kruse W, Botz I, Pohl S et al (2000) Antimicrobial activity of human cervical mucus. Hum Reprod 15:778–784. https://doi.org/10.1093/humrep/15.4.778
Ferri D, Liquori GE, Scillitani G (1999) Morphological and histochemical variations of mucous and oxynticopeptic cells in the stomach of the seps, Chalcides chalcides. J Anat 194:71–77. https://doi.org/10.1046/j.1469-7580.1999.19410071.x
Ferri D, Liquori GE, Natale L et al (2001) Mucin histochemistry of the digestive tract of the red-legged frog Rana aurora aurora. Acta Histochem 103:225–237. https://doi.org/10.1078/0065-1281-00582
Gans C, Parsons TS (1977) Biology of the reptilia. Morphology. Academic Press, New York
Gelis S (2013) Evaluating and treating the gastrointestinal system. Clin Avian Med 1:412–416
Gendler SJ, Spicer AP (1995) Epithelial mucin genes. Annu Rev Physiol 57:607–634. https://doi.org/10.1146/annurev.ph.57.030195.003135
Grossman JD (1985) Anatomia dos animais domestic, 5th edn. Guanabara Koogan, Rio de Janeiro
Hadi K, Mohamed A (2015) Comparative anatomical and histological study of the esophagus of local adult male and female homing pigeon (Columba livia domestica). AL-Qadisiya J Vet Med Sci 14:80–87. https://doi.org/10.29079/vol14iss1art333
Hamdi H (2012) Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I. Uromastyx aegyptiaca. Life Sci J 9:84–104
Hamdi H (2013) Anatomical, histological and histochemical adaptations of the avian alimentary canal to their food habits: ii- elanus caeruleus. Int J Sci Eng Res 4:1355–1364
Hamdi H, El Ghareeb A, el wahab, Zaher M, et al (2014) Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-chamaeleon africanus. World Appl Sci J 30:1306–1316
Jacobson E (2007) Overview of reptile biology, anatomy, and histology. CRC Press, New York, pp 1–130
Kardong KV (2006) Vertebrates: comparative, anatomy, function. Washington State University, Evolution
Kiernan JA (1999) Histological and histochemical methods:theory and practice. Shock 12:479
King AS, McLelland J, King AS (1984) Birds: their structure and function. Baillière Tindall, London
Koca YB, Gürcü B (2011) Morphological and histochemical investigations of esophagogastric tract of a lizard, laudakia stellio (Agamidae, Linnaeus 1758). Acta Biol Hung 62:376–387. https://doi.org/10.1556/ABiol.62.2011.4.4
Liquori GE, Scillitani G, Mastrodonato M, Ferri D (2002) Histochemical investigations on the secretory cells in the oesophagogastric tract of the Eurasian green toad, Bufo viridis. Histochem J 34:517–524. https://doi.org/10.1023/a:1024766124211
Liquori GE, Zizza S, Mastrodonato M et al (2005) Pepsinogen and H, K-ATPase mediate acid secretion in gastric glands of Triturus carnifex (Amphibia, Caudata). Acta Histochem 107:133–141. https://doi.org/10.1016/j.acthis.2005.03.002
Liquori GE, Mastrodonato M, Zizza S, Ferri D (2007) Glycoconjugate histochemistry of the digestive tract of triturus carnifex (Amphibia, Caudata). J Mol Histol 38:191–199. https://doi.org/10.1007/s10735-007-9087-4
Loo SK, Wong WC (1975) Histochemical observations on the mucins of the gastrointestinal tract in the toad (Bufo melanostictus). Acta Anat 91:97–103. https://doi.org/10.1159/000144374
Machado-Santos C, Pelli-Martins AA, Abidu-Figueiredo M, de Brito-Gitirana L (2014) Histochemical and Immunohistochemical Analysis of the Stomach of Rhinella icterica (Anura, Bufonidae). J Histol 2014:872795. https://doi.org/10.1155/2014/872795
Malewitz TD (1965) Normal histology of the digestive tract of the mouse. Okajimas Folia Anat Jpn 41:21–47. https://doi.org/10.2535/ofaj1936.41.1_21
McManus JFA (1946) Histological demonstration of mucin after periodic acid. Nat Nat 158:202
Mikuni-Takagaki Y, Hotta K (1979) Characterization of peptic inhibitory activity associated with sulfated glycoprotein isolated from gastric mucosa. Biochim Biophys Acta 584:288–297. https://doi.org/10.1016/0304-4165(79)90274-5
Narkiewicz K, Narkiewicz M (2015) The age of the oldest tetrapod tracks from Zachełmie, Poland. Lethaia 48:10
Paksuz EP, Paksuz S (2021) Histomorphometric and histochemical characteristics of the oesophagus of the greater mouse-eared Bat, Myotis myotis (Borkhausen, 1797). Anat Histol Embryol 50:701–706. https://doi.org/10.1111/ahe.12678
Pelli-Martins A, Santos C, Sales A, de Brito GL (2012) Histochemical, immunohistochemical, and ultrastructural observations of the esophagus morphology of Rinella icterica (Anuran, Bufonidae). Acta Zool. https://doi.org/10.1111/j.1463-6395.2011.00510.x
Rajabi E, Nabipour A (2009) Histological study on the oesophagus and crop in various species of wild bird. Avian Biol Res 2:161–164. https://doi.org/10.3184/175815509X12474789336122
Robertson AM, Wright DP (1997) Bacterial glycosulphatases and sulphomucin degradation. Can J Gastroenterol 11:361–366. https://doi.org/10.1155/1997/642360
Romer AS (1970) Vertebrate body. Wiley, New York, p 486. https://doi.org/10.1002/sce.37304103194
Scott JE, Mowry RW (1970) Alcian blue–a consumers’ guide. J Histochem Cytochem 18:842. https://doi.org/10.1177/18.11.842
Sheahan DG, Jervis HR (1976) Comparative histochemistry of gastrointestinal mucosubstances. Am J Anat 146:103–131. https://doi.org/10.1002/aja.1001460202
Strobel S, Encarnação JA, Becker NI, Trenczek TE (2015) Histological and histochemical analysis of the gastrointestinal tract of the common pipistrelle bat (Pipistrellus pipistrellus). Eur J Histochem 59:2477. https://doi.org/10.4081/ejh.2015.2477
Zaher M, El-Ghareeb AW, Hamdi H, AbuAmod F (2012) Anatomical, histological and histochemical adaptations of the avian alimentary canal to their food habits: I-coturnix coturnix. Life Sci J 9(3):253–275
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Awaad, A., Rushdy, A. & Adly, M.A. Comparative microanatomical and histochemical biodistribution profiles of different types of mucins in oesophageal gastric tract mucosa of some tetrapod representatives. Histochem Cell Biol 157, 217–238 (2022). https://doi.org/10.1007/s00418-021-02049-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-021-02049-x