Abstract
Purpose
Common methods of measuring severity of Fuchs endothelial corneal dystrophy (FECD) are limited in objectivity, reliability, or start with a variable baseline that prevents distinguishing healthy from affected eyes. The aim of this study was to describe a method of grading FECD that overcomes these limitations.
Methods
Fifteen patients with Fuchs endothelial corneal dystrophy were included in the study. Guttae were imaged with a slit lamp beam 8 mm tall; the bottom 4 mm half of each image was divided into two equally-sized sections. Guttae were counted by four independent graders blinded to disease severity scores. The peripheral:central guttae ratio was compared to modified Krachmer clinical severity scores. The peripheral:central guttae ratio was compared between mild (severity 0.5–3) versus moderate-to-severe (severity 4–5) disease. Receiver operating characteristics defined optimal ratio cutoffs for mild versus moderate-to-severe disease.
Results
Increased peripheral guttae and peripheral:central guttae ratio correlated with Krachmer severity (p = 0.021 and p = 0.009, respectively). The difference between mild and moderate-to-severe cases for the peripheral:central guttae ratio was significant (p < 0.001). Inter-rater reliability of total guttae count was high (coefficient = 0.82, p < 0.001). A peripheral:central guttae ratio of 0.16 was the ideal cut-off point (area under the curve = 0.79, sensitivity = 0.78, and specificity = 0.80).
Conclusion
In this pilot study, the peripheral:central ratio of guttae correlates with subjective clinical severity of Fuchs dystrophy. It starts at a common baseline, has good inter-rater reliability, does not require dilation, and can be conducted with a smartphone and slit-lamp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Fuchs endothelial corneal dystrophy (FECD) is a progressive, hereditary condition that affects approximately one in 30 Americans [1] and is a leading indication for corneal transplantation [2]. It results in the formation of guttae, which are excrescences of Descemet membrane [3] that are associated with endothelial cell loss [4], corneal edema, and decreased vision [5].
For clinicians and researchers seeking to determine clinical severity of disease, numerous methods of identifying severity exist, each with their own limitations. Pachymetry is frequently used to track disease progression [6], but baseline corneal thickness varies by person and population [7]. The peripheral-to-central ratio of corneal thickness [8], another objective method of severity, also may vary by populations with different baseline average corneal thicknesses. Specular microscopy and confocal microscopy are helpful to determine endothelial cell density (ECD) which decreases over time in FECD and has been proposed as an objective measure of disease progression in previous studies using confocal microscopy [9]. However, the field of view is relatively small compared to the rest of the cornea, and is often filled with guttae once disease reaches a moderate or severe stage [10].
In the assessment of patients with FECD, clinicians frequently use the Krachmer scale, which grades disease severity on a scale of 1 (least severe) to 5 (most severe) and is based on the number of guttae seen and presence of corneal and/or stromal edema [11]. Since its introduction in the 1970’s, researchers have modified this approach [12], but interrater reliability remains a significant limitation.8 Retroillumination photography analysis, in which the total number of guttae is summated, offers an objective measurement of disease severity that starts with a common baseline of zero guttae in unaffected individuals [13] and can effectively distinguish between various levels of severity [14]. However, this requires dilation and counts may be affected by cataract, posterior capsular opacification, or poor mydriasis.
An ideal approach would be objective, reliable, low-cost, non-contact, non-mydriatic, have a common baseline for all individuals, and be able to discern various levels of disease severity. In this pilot study, we describe an objective grading system for FECD based on shining a single slit of light through the cornea and analyzing the distribution of individual guttae in the periphery and center among multiple graders, an approach that combines strengths of currently available imaging modalities.
Materials and methods
Image acquisition from human subjects
Participants in the study presented to the subspecialty cornea clinic at the Wilmer Eye Institute at the Johns Hopkins Hospital between 1 July 2013 and 30 June 2015; patients either received a new diagnosis or had a pre-existing diagnosis of FECD. This study is a retrospective cross-sectional analysis and was approved by the Johns Hopkins University School of Medicine Institutional Review Board (IRB).
At the time of examination, subjects were seated in front of a Haag-Streit BM900 slit-lamp biomicroscope (Haag-Streit AG, Bern, Switzerland) that was operated by a licensed ophthalmologist with subspecialty training in cornea and external disease (AOE). During the slit lamp examination, each patient had their FECD graded using a modified Krachmer clinical disease severity scale (Table 1).
Using direct illumination at 16x magnification, the length of the beam was increased to 8 mm, with maximization of the light intensity and minimization the thickness of the beam. An iPhone 4S (Apple Inc., Cupertino, CA, USA) was freely held to the ocular by the operator to capture images of the slit lamp field of view. This phone is capable of capturing images with a maximum resolution of 3264 by 2448 pixels (8 megapixels) and has been described previously in both corneal photography [15] and external eye imaging [16]. The images that were collected were aimed as close as possible to the center of the cornea.
Image analysis protocol
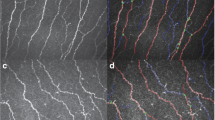
Images were downloaded to a secure, password-protected online repository hosted on the institutional server. Using ImageJ (Wayne Rasbad, National Institutes of Health, Bethesda, MD), images were divided as demonstrated in Fig. 1. Beginning at the center of the cornea and proceeding inferiorly, two equally-sized sections were created for the purpose of counting and statistical analysis: “central” cornea (containing the superior 2 mm of the divided slit beam) and “peripheral” cornea (containing the inferior 2 mm of the divided slit beam). Because the entire length of the slit beam was 8 mm, and two subdivisions were created after dividing the entire length of the beam in half, each of the two sections is approximately 2 mm in length.
Three medical student graders (RPB, MJF, and TP) and one cornea specialist grader (AOE) counted individual guttae in each image, and then recorded the number of guttae on individual spreadsheets, blinded to one another’s guttae counts. Each grader was also blinded to clinical disease severity scores when analyzing images. Additionally, graders rated each image for subjective quality on a scale of 1 being lowest and 5 being highest. An example of counted guttae in a sample image is observed in Fig. 2.
Statistical analysis protocol
Statistical analysis was carried out using RStudio Desktop 1.2.5033 (RStudio Inc., Boston, MA, USA). The ratio of guttae in the peripheral section to those in the center section was correlated to the clinical severity using Spearman’s rank correlation coefficient. Wilcoxon rank sum testing was used to compare cases of mild disease (modified Krachmer clinical severity of 0.5–2) to moderate-to-severe disease (clinical severity of 3–5). Intraclass correlation coefficients (ICC’s) were calculated to compare inter-rater reliability for guttae counts obtained from the four independent image graders using a two-way mixed model for absolute agreement. Inter-rater reliability was assessed using a framework described in a previous text, where moderate agreement is defined as ICC 0.5 to 0.75, good agreement is 0.75 to 0.9, and excellent agreement is 0.9 and above [17]. Receiver operating characteristic curves were generated to determine area under the curve (AUC) with varied levels of discrimination; cut off points were determined using Youden’s index analysis.
Results
Images were obtained from 15 patients, of whom 9 (60%) were female; a total of 15 images were included for analysis. The cohort was predominantly white (87%), with mean age of 67.13 years (range 48 to 93 years). Median FECD clinical severity was 4+ on the Krachmer Scale (range 0.5 to 5). The total number of guttae counted in the four sub-divisions of each image ranged from 0 to 66 (Table 2). Average subjective image quality as reported by the independent graders for the 15 images was rated as 3.01 (S.D. 1.47) on scale of 1 being the lowest quality and 5 being the highest quality (Table 3).
An increase in the number of peripheral guttae correlated with an increased Krachmer clinical severity (correlation coefficient = 0.297, p = 0.021), as did the peripheral:central guttae ratio (correlation coefficient = 0.333, p = 0.009). The peripheral:central guttae ratio of mild (clinical severity 0.5–2) and moderate-to-severe (clinical severity 3–5) cases were significantly different (Wilcoxon rank sum test, W = 165, p < 0.001).
Inter-rater reliability between graders was good when accounting for total number of guttae counted (ICC = 0.82, 95% CI 0.775–0.859, p < 0.001), number of central guttae (ICC = 0.790, 95% CI 0.551–0.920, p < 0.001), and number of peripheral guttae (ICC = 0.924, 95% CI 0.832–0.971, p < 0.001).
ROC characteristics were determined based on the above classification of Krachmer stage 0.5–3 as “Mild” disease and stage 4–5 as “Moderate-to-Severe.” A peripheral:central guttae ratio of 0.16 was determined to be the ideal cut-off point, yielding an area under the curve (AUC) of 0.79, a sensitivity of 0.78, and a specificity of 0.80 (Supplemental Fig. 1). Reclassification of “Mild” disease as Krachmer 0.5–2 and “Moderate-to-Severe” as Krachmer 3–5 yielded a less optimal cutoff point of 0.18 with decreased ROC characteristics and sensitivity (AUC = 0.57, sensitivity = 0.60, and specificity = 0.75).
Discussion
In this pilot study, we demonstrate a safe, repeatable, affordable, and objective method to determine severity of FECD using images captured on a slit-lamp using a smartphone camera. Currently, alternative forms of corneal imaging including specular microscopy, confocal microscopy and Scheimpflug imaging require equipment at significant cost; we sought to identify a method of imaging that could be conducted at a lower cost using equipment that is broadly available in eye care clinics. This study adds to the literature in that it describes a method for assessment of FECD severity that shares a common baseline of zero in unaffected individuals, provides insight into the peripheral status of the cornea, has excellent inter-rater reliability, does not require dilation, and correlates with the clinician’s clinical grading of severity.
With the advent of Descemet stripping only (DSO) surgery [18], recent attention has been directed to the role of guttae in contributing to the disease state in FECD. Ideal candidates for DSO surgery have central guttae but maintain a healthy peripheral cornea that can provide migrating cells to clear the central cornea. In this study, we defined “mild” disease as up to 3+ severity on the modified Krachmer scale, which implies that guttae are central, confluent, and extend to a maximum diameter of 5 mm, while leaving the peripheral cornea relatively spared; these are also patients who respond well to DSO. The approach that is described in this study could be helpful to identify patients with a low periphery-to-center ratio of guttae and, as a result, may assist clinicians in identifying candidates in whom DSO surgery should be further explored.
There are several important limitations to our study that inform important future areas of research and refinement. A question that arises is whether data from a single slit of light is able to be extrapolated across the cornea. In our previous work, we summated all the guttae in an image using retroillumination photography analysis, a method in which we counted at times over 10,000 guttae in a single picture [14]. In a post-hoc analysis, we found that total counts from two clock hours in a single axis correlated with the total corneal counts (unpublished data), suggesting that a single slit may be able to offer information that can potentially be extrapolated to the remaining uncounted cornea. Future investigations can examine whether a horizontal or vertical slit of light would be more representative of the whole cornea, and whether the image analysis method we have described can be automated to count an even greater number of guttae at varying angles. Additionally, in the present study we utilized a basic smartphone and placed the camera up to the ocular of the slit-lamp microscope in order to acquire images. To address the issue of holding the smartphone to the ocular, an adapter can be applied to stabilize the phone to the eyepiece, as has been described previously [19].
We were limited by the number and quality of images available for inclusion in the study. As camera quality on smartphones improves, the quality of the images obtained using our method will see a similar progression in the quality and countability of discrete guttae. Future studies could assess ease of image acquisition, reproducibility of images across platforms and different cameras, and reproduce these findings in a larger cohort of patients. Even with these limitations in mind, we consider this work to be a pilot study that demonstrates proof of concept and promising directions for future research.
In summary, the periphery-to-center ratio of individual guttae offers an objective, safe, reliable and affordable method for the assessment of patients with FECD.
Data availability
Study data is maintained by the investigators and is available upon request.
References
Eghrari AO, Riazuddin SA, Gottsch JD (2015) Fuchs corneal dystrophy. Prog Mol Biol Transl Sci 134:79–97
Eye Bank Association of America (2019) Analysis of surgical Use and indications for corneal transplant. 2019 Eye banking Statistical Report. https://restoresight.org/wp-content/uploads/2020/04/2019-EBAA-Stat-Report-FINAL.pdf. Accessed 4 May 2020
Eghrari AO, Gottsch JD (2010) Fuchs’ corneal dystrophy. Expert Rev Ophthalmol 5(2):147–159
Zhang J, Patel DV (2015) The pathophysiology of Fuchs' endothelial dystrophy – A review of molecular and cellular insights. Exp Eye Res 130:97–105
Wilson SE, Bourne WM (1988) Fuchs' dystrophy. Cornea 7(1):2–18
Kopplin LJ, Przepyszny K, Schmotzer B et al (2012) Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol 130(4):433–439
Feng MT, Kim JT, Ambrósio RJ et al (2012) International values of central Pachymetry in Normal subjects by rotating Scheimpflug camera. Asia Pac J Ophthalmol (Phila) 1:13–18
Repp DJ, Hodge DO, Baratz KH et al (2013) Fuchs' endothelial corneal dystrophy: Subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 120(4):687–694
McLaren JW, Bachman LA, Kane KM, Patel SV (2014) Objective assessment of the corneal endothelium in Fuchs’ endothelial dystrophy. Invest Ophthalmol Vis Sci 55:1184–1190
Adamis AP, Filatov V, Tripathi BJ et al (1993) Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol 38(2):149–168
Krachmer JH, Purcell JJ Jr, Young CW, Bucher KD (1978) Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol 96(11):2036–2039
Afshari N, Igo R, Morris N et al (2017) Genome-wide association study identifies three novel loci in Fuchs’ endothelial corneal dystrophy. Nat Commun 8:148–198
Eghrari AO, Mumtaz AA, Garrett B, Rezaei M, Akhavan MS, Riazuddin SA, Gottsch JD (2017) Automated Retroillumination photography analysis for objective assessment of Fuchs’ corneal dystrophy. Cornea 36(1):44–47
Eghrari AO, Garrett BS, Mumtaz AA, Edalati AE, Meadows DN, McGlumphy EJ, Iliff BW, Gottsch JD (2015) Retroillumination photography analysis enhances clinical definition of severe Fuchs’ corneal dystrophy. Cornea. 34(12):1623–1626
Maamari RN, Ausayakhun S, Margolis TP, et al (2014) Novel telemedicine device for diagnosis of corneal abrasions and ulcers in resource-poor settings. JAMA Ophthalmol 132:894–895
He L, Myung D, Pershing S, Chang R (2014) iPhone photography of eye pathology for remote triage. Invest Ophthalmol Vis Sci 55(13):4875
Koo TK, Li MY (2016) A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Moloney G, Petsoglou C, Ball M et al (2017) Descemetorhexis without grafting for Fuchs’ endothelial dystrophy-supplementation with topical Ripasudil. Cornea 36(6):642–648
Yen CH, Wang GQ, Lin TY, Liu CH (2019) Semi-permanent smartphone adapter for microscopes: Design demonstration and workflow testing using a slit-lamp biomicroscope. Taiwan J Ophthalmol 9(2):111–117
Funding
No funding was received for conducting this study. Allen O Eghrari is provided salary support by Research to Prevent Blindness Sybil B. Harrington Special Scholar Award and the Tolsma family. All other authors have no funding sources to disclose.
Author information
Authors and Affiliations
Contributions
Rohan P Bajaj (ORCID 0000–0002–3934-3444): data curation; formal analysis; conceptualization; methodology.
Michael J Fliotsos (ORCID 0000–0001–8429-0831): writing – original draft; writing – review & editing; visualization; project administration.
Tejus Pradeep (ORCID 0000–0002-0506-069X): formal analysis; writing – review & editing.
Allen O Eghrari (ORCID 0000–0003-2798-038X): supervision; conceptualization; methodology; validation; data curation.
Corresponding author
Ethics declarations
Conflict of interest
Allen O Eghrari has an ownership interest in Treyetech and LuckyVision, LLC. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Informed consent / ethical approval
This study was reviewed by the Johns Hopkins University School of Medicine Institutional Review Board (IRB) under study identifier IRB00217273. All study activities adhered to the Declaration of Helsinki. The study was deemed exempt by the IRB under the United States Department of Health and Human Services (DHHS) regulations as a retrospective review of images and data existing in the medical record. The IRB, acting as a HIPAA (Health Insurance Portability and Accountability Act) Privacy Board, reviewed and approved the HIPAA consent waiver via the expedited mechanism as “secondary research” under 45 CFR 46.104(d)(4)(iii) of DHHS regulations.
Sources of support
A. O. Eghrari is supported by the Research to Prevent Blindness Sybil B. Harrington Special Scholar Award and the Tolsma family.
The remaining authors have no funding disclosures to report.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig. 1
Receiving operating characteristics curve for peripheral:central ratio of guttae. (JPG 35 kb).
Rights and permissions
About this article
Cite this article
Bajaj, R.P., Fliotsos, M.J., Pradeep, T. et al. Peripheral-to-central ratio of Guttae: validity and reliability of an objective method to characterize severity of Fuchs endothelial corneal dystrophy. Graefes Arch Clin Exp Ophthalmol 259, 685–690 (2021). https://doi.org/10.1007/s00417-020-04985-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04985-1