Abstract
Purpose
To investigate microvascular abnormalities in diabetic patients without conventional clinical signs of diabetic retinopathy (DR).
Methods
In this cross-sectional observational cohort study, the study group included randomly chosen participants of a community-based cohort with diabetes type 2 without DR, and the control group consisted of non-diabetic individuals from a population-based study. All participants underwent optical coherence tomographic angiography (OCTA).
Results
Upon OCTA, 118 (40.4%) eyes of the study group (n = 292 eyes) showed microvascular abnormalities including foveal avascular zone erosion (95 (32.5%) eyes), non-perfusion areas in the superficial and deep retinal layers (39 (13.4%) eyes and 19 (6.5%) eyes, respectively), and microaneurysms in the superficial and deep retinal layers (22 (7.5%) eyes and 31 (10.6%) eyes, resp.). None of these abnormalities was detected in the control group (n = 80). The study group showed a lower vessel density in the superficial retinal vascular layer in all regions except for the foveal region (P < 0.001), and higher vessel density in the parafoveal region in the deep retinal vascular layer (P = 0.01). Higher diabetes prevalence was associated with lower superficial retinal vascular density (P = 0.005) in multivariable analysis. A lower radial peripapillary capillary flow density was correlated (regression coefficient r, 0.62) with higher fasting blood concentration of glucose (P < 0.001) in multivariable analysis.
Conclusions
OCTA revealed microvascular abnormalities in 40% of eyes of diabetic patients without ophthalmoscopically detectable diabetic fundus changes in a community-based population. The early stage of DR may be re-defined upon OCTA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus affects approximately more than 366 million people worldwide and diabetic retinopathy (DR) is one of the severe sequels of diabetes [1,2,3,4,5]. Although DR has clinically so far been defined by ophthalmoscopically detectable characteristic morphological abnormalities of the ocular fundus, such as retinal microaneurysms, recent studies have suggested that microvascular changes can develop before the classical features of DR can be seen upon ophthalmoscopy [6,7,8,9,10,11,12,13,14,15,16,17,18]. It may challenge the status of conventional ophthalmoscopy or the assessment of conventionally taken fundus photographs as the gold standard technique for the definition of DR. Fluorescein angiography has been the second pillar for the diagnosis of DR; it is, however, an invasive technique with the potential risk of adverse effects including an anaphylactic shock. Fluorescein angiography is thus not suitable as a routinely performed screening method for the early detection of DR.
Optical coherence tomography angiography (OCTA) has recently been introduced as new imaging technology into the clinical practice and allows the visualization of the microvasculature of the retina, stratified between different layers, and of the choriocapillaris. Several studies have demonstrated its usefulness to illustrate microvascular changes in the retina in patients with ophthalmoscopically detectable DR [6, 19,20,21,22,23,24,25,26,27]. Relatively few investigations, however, have so far been focused on quantitative measurements of early retinal changes in patients with diabetes without ophthalmoscopically detected DR [6,7,8,9,10,11,12,13,14,15,16,17,18]. In view of the general importance of diabetes mellitus in clinics and public health, since the early detection of a disease or the early detection of a clinically important marker of an advanced stage of a systemic disease is warranted, and since previous pilot studies had limitations such as a relatively small number of study participants and a hospital-based recruitment of the study participants, we conducted this study to explore the OCTA-based detectability of microvascular changes in the superficial and deep vascular layers of the retina in diabetic patients without conventional clinical signs of DR. To obtain data on the general feasibility of OCTA for the detection of early, ophthalmoscopically undetected, retinal diabetic changes, we chose a community-based recruitment of the study participants, in attempt to reduce the risk of a referral bias.
Methods
The community-based observational cross-sectional study included patients with diabetes type 2 who participated in the Beijing Community Diabetes Study and who were recruited between May 2018 and June 2018. They formed the study group. The individuals of the control group were participants of the Kailuan Eye Study. The study protocol was approved by the Medical Ethics Committee of the Beijing Tongren Hospital and written informed consent was obtained from all study participants.
The Beijing Communities Diabetes Study (BCDS) is a prospective study on the relationship between different types of treatment of diabetes mellitus and the development of complications of the disease in the urban population of Beijing [28]. The study included 15 communities with their health care centers selected by a multistage random sampling approach of five districts in the urban region of Beijing. Patients with diabetes mellitus type 2, whose age ranged between 20 and 80 years and who had lived in the community for at least 5 years, were recruited between August 2008 and July 2009 and followed-up with yearly systemic and ophthalmic examinations. Diabetes mellitus type 2 was defined by a random plasma glucose concentration of ≥ 200 mg/dL (11.1 mmol/L) or a fasting plasma glucose concentration of ≥ 126 mg/dL (7.0 mmol/L) or a 2-h posttest plasma glucose concentration of ≥ 200 mg/dL (11.1 mmol/L) after a 75-g oral glucose load [28]. We added OCTA as new examination technique to the series of examinations performed for the participants of two communities in the study period from May 2018 to June 2018. This group of participants with OCTA examinations eventually included 179 patients with diabetes and had an age ranging between 36 and 84 years. The control subjects were recruited from the ongoing and repeatedly performed Kailuan Eye Study which included a total of 101,510 employees and retirees of the Kailuan Group company [29]. From March 2017 to June 2017, randomly selected participants underwent an OCTA examination, out of whom, 80 individuals fulfilled the inclusion criteria for the control group. Inclusion criteria for the study group were the diagnosis of diabetes mellitus type 2 made at least 1 year prior to the enrollment into the study, a normal appearance of the anterior segment upon slit lamp-based biomicroscopy, an unremarkable posterior segment of the eye, no clinical signs of DR as examined upon assessment of fundus photographs, and an intraocular pressure within the normal range. Exclusion criteria were any retinal microaneurysms and retinal hemorrhages, any retinal exudation and retinal neovascularization, and any other sign of DR as detectable on fundus photographs centered on the optic disc (Diabetic Retinopathy Study standard field 1) and on the macula (Diabetic Retinopathy Study standard field 2). Additional exclusion criteria were the history of any ocular diseases (except for age-related cataract), hypertensive retinopathy, and history of any ocular surgeries (except for cataract surgery). Exclusion criteria for the control group were history of any ocular diseases or ocular surgeries except for cataract surgery, and history of diabetes mellitus. Myopia, incipient cataract, and pseudophakia not affecting the quality of the OCTA images were no exclusion criteria.
The study participants underwent an ophthalmoscopical examination including measurement of visual acuity, slit lamp-based biomicroscopy of the anterior segment, tonometry, and ocular biometry applying optical low-coherence reflectometry (Lenstar 900 Optical Biometer; Haag-Streit, Koeniz, Switzerland) for measurement of the axial length, central corneal thickness, anterior chamber depth, and lens thickness. We took fundus photographs (Hybrid Digital Mydriatic Retinal Camera CX-1; Canon Inc., Tokyo, Japan) and performed a spectral-domain optical coherence tomography (OCT) of the posterior segment with a 6 mm × 6 mm raster scan (ReVue software, version 2017.1; Optovue Inc., Fremont, CA, USA). In those eyes, the pupil of which was too small for obtaining fundus images in sufficient quality, we medically induced a mydriasis by applying eye drops containing 0.5% tropicamide and 0.5% phenylephrine hydrochloride.
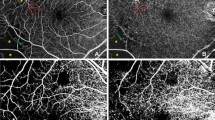
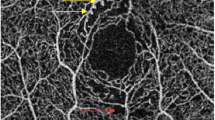
We performed an OCTA using the RTVue XR Avanti device (ReVue software, version 2017.1; Optovue Inc., Fremont, CA, USA) with the Angio Retina mode (3 × 3 mm and 6 × 6 mm) and the Angio Disc (4.5 × 4.5 mm) mode. The principle of OCTA and the imaging processing technique have been described in detail recently [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. The macular region was automatically stratified into four layers: the superficial retinal vascular layer extending from the inner limiting membrane to the inner plexiform layer with an offset of 10 mm, the deep retinal vascular layer reaching from the inner plexiform layer with an offset of 10 mm to the outer plexiform layer with an offset of 10 mm, the outer retinal layer extending from the outer plexiform layer to Bruch’s membrane, and the choriocapillaris layer. Measurements of the foveal avascular zone (FAZ) were generated based on the Retina slab (inner limiting membrane to outer plexiform layer + 10 μm), with automated detection of the FAZ boundary by the AngioVue software (Fig. 1). The acircularity index (AI) was calculated as the ratio of the FAZ perimeter divided by the perimeter of a circle with equal area. The foveal vascular density (FD-300) was determined as vessel density within a ring of a width of 300 μm surrounding the FAZ. The foveal area density in the 300-μm ring (FD-300 length density) was calculated by dividing the number of vessel pixels by the total number of pixels then multiplied by 100%. The vascular density was calculated in the superficial retinal and deep retinal layer separately in five regions (fovea, temporal, superior, nasal, and inferior). The radial peripapillary capillaries (RPC) were measured based on the RPC slab which extended from the inner limiting membrane to the posterior border of the retinal nerve fiber layer. The automatically performed segmentations of all examinations were interactively checked before any measurements were performed. Exclusion criteria were a poor image quality based on the criteria of a low-quality index (< 5), presence of blink artifacts, motion or doubling artifacts caused by poor fixation, and media opacities obscuring the view of the vasculature. Two readers evaluated the presence of the morphological features of FAZ erosion, microaneurysms, tissue non-perfusion, vascular loops, and capillary tortuosity on the OCTA images in the superficial and deep retinal layers (Fig. 2). To assess the intraobserver and interobserver variability, OCTA images of 50 participants with diabetes were randomly selected. For the determination of the intraobserver variability, each picture was evaluated three times by each reader, making the diagnosis of a “pathologic OCTA finding” versus “a normal OCTA finding.” The intraclass correlation coefficient (ICC) was 0.959 and 0.949, respectively. For the determination of the interobserver variability, the images were evaluated once by both readers, and we calculated Cohen’s kappa coefficient, which was 0.915.
Optical coherence tomographic angiographic image of the macula. Measurements of the foveal avascular zone (FAZ) were generated based on the Retina slab (inner limiting membrane to outer plexiform layer + 10 μm), with automated detection of the FAZ boundary by the AngioVue software (Fig. 1). The acircularity index (AI) was calculated as the ratio of the FAZ perimeter divided by the perimeter of a circle with equal area. The foveal vascular density (FD-300) was determined as vessel density within a ring of a width of 300 μm surrounding the FAZ
Optical coherence tomographic angiographic (OCTA) images (3 × 3 mm) of eyes of diabetic patients without ophthalmoscopically detected microvascular retinal changes. a OCT angiogram of the deep retinal layer showing an erosion of the foveal avascular zone (green arrow). b OCTA of the superficial retinal layer showing a microaneurysm (red arrow) and an area with tissue non-perfusion (yellow arrow). c The microaneurysm can also be visualized in the deep retinal layer; the red stars show an abnormal enlargement of vessels in the deep retinal layer. d The superficial retinal layer shows a vascular loop (red circle)
The statistical analysis was performed using a commercially available statistical software program (SPSS for Mac, version 25.0; IBM/SPSS, Chicago, IL, USA). First, we calculated the means and standard deviations of the main outcome parameters, before we applied the Student t test for unpaired samples to evaluate the statistical significance of differences between the study group and the control group. A linear regression analysis was performed to examine potential associations between the ocular and general parameters. A logistic regression analysis was carried out to calculate odds ratios (ORs) and their 95% confidence intervals (CI). A two-tailed P value of < 0.05 was considered statistically significant.
Results
The study included 292 eyes from 179 patients with diabetes and 80 healthy eyes from 80 participants of the control group (Table 1). We excluded 66 eyes of diabetic subjects due to clinical signs of DR on the fundus photographs. Study group and control group did not differ significantly in gender (P = 0.06) or age (68.6 ± 8.2 years versus 66.8 ± 7.8 years; P = 0.08) (Table 1).
In the study group, OCTA demonstrated microvascular abnormalities in the superficial retinal layer and deep retinal layer in 118 (40.4%) of 292 eyes and in 76 (42.5%) out of the 179 patients with diabetes. These abnormalities included an erosion of the FAZ in 95 (32.5%) eyes, non-perfusion areas in 39 (13.4%) eyes and microaneurysms in 22 (7.5%) of 292 eyes in the superficial retinal layer, non-perfusion areas in 19 (6.5%) eyes and microaneurysms in 31 (10.6%) in the deep retinal layer, vascular loops in 3 (1%), and a capillary tortuosity in 1 (0.3%). None of these microvascular abnormalities was detected in the eyes of the control group, and the difference in their prevalence between the study group and control group was significant (all P < 0.001, except for the prevalence of vascular loops and capillary tortuosity).
The study group as compared with the control group showed a significantly (P < 0.001) lower vessel density in the superficial retinal vascular layer in all regions except for the foveal region (Table 2). Additionally, the vascular density in the 300-μm wide ring surrounding the FAZ (FD-300) was significantly (P = 0.001) lower in the study group than in the control group (Table 2). In the deep retinal vascular layer, the retinal vascular density in the parafoveal region (3 mm × 3 mm region) was higher (P = 0.01) in the study group than in the control group (Table 2). The size of the FAZ did not differ significantly (P = 0.33) between both groups.
If only one eye per study participant was included into the statistical analysis, similar results were obtained. The vessel density in the superficial retinal vascular layer in all regions except for the foveal region (whole field, 41.63 ± 5.15 versus 45.09 ± 4.32; P < 0.001) and the vascular density in the 300-μm wide ring surrounding the FAZ (FD-300) (46.28 ± 5.58 versus 48.82 ± 4.3, P < 0.001) were significantly lower in the study group as compared with the control group. In the deep retinal vascular layer, the retinal vascular density in the parafoveal region was higher (52.99 ± 4.05 versus 51.44 ± 5.43, P = 0.01) in the study group than in the control group. In a similar manner, the radial peripapillary vascular density was lower in the study group (whole image, 47.48 ± 4.97 versus 49.24 ± 3.12, P = 0.007).
After adjusting for age, gender, and systolic blood pressure, a higher prevalence of diabetes was significantly (P < 0.001) associated with a lower superficial retinal vascular density (Table 3). In a reverse pattern, the presence of diabetes was significantly associated with a lower superficial retinal vascular density (P = 0.005) after adjusting for older age (P < 0.001), female gender (P = 0.005), and systolic blood pressure (P < 0.001). In multivariable linear regression, a higher vascular density in the parafoveal deep retinal layer was significantly associated (regression coefficient r, 0.24) with the presence of diabetes (P = 0.01), younger age (P = 0.003), and female gender (P = 0.007) (Table 4). In multivariable linear regression analysis (regression coefficient r, 0.62), a lower RPC flow density was associated with a higher fasting blood concentration of glucose (P < 0.001) after adjusting for older age (P < 0.001), longer axial length (P < 0.001), and higher blood concentration of creatinine (P < 0.001) (Table 5).
Discussion
Our study revealed that 118 (40%) out of 292 of eyes of diabetic patients without ophthalmoscopically detected signs of conventionally defined DR showed microvascular abnormalities as assessed by OCTA. These microvascular abnormalities included an erosion of the FAZ, presence of non-perfusion areas and microaneurysms in the superficial retinal layer and deep retinal layer, vascular loops, and capillary tortuosity. Correspondingly, the retinal vessel density in the superficial retinal vascular layer in all regions (except for the foveal region) was significantly lower in the study group than in the control group, while the retinal vessel density in the deep retinal vascular layer in parafoveal region was higher in diabetic patients. As a corollary, a higher prevalence of diabetes was associated with a lower superficial retinal vascular density in a multivariable analysis. A lower radial peripapillary capillary flow density was associated (r, 0.62) with a higher fasting blood concentration of glucose (P < 0.001) in multivariable analysis.
The findings obtained in our study agree with observations made in previous investigations [6,7,8,9,10,11,12,13,14,15,16,17,18]. To cite an example, Simonett and colleagues found a significant (P < 0.001) lower parafoveal vessel density in the eyes without DR of patients with diabetes mellitus type 1 as compared with non-diabetic controls, while there was however no significant difference between the groups in the parafoveal vessel density in the superficial and deep capillary plexus or in the FAZ area [9]. It also agrees with previous investigations which examined eyes with DR. Durbin and associates, evaluating 50 eyes from 26 participants with diabetes and 50 healthy eyes from 25 non-diabetic participants, reported that the retinal vessel density in the superficial retinal vascular layer had the highest area under the receiver operating characteristic curve to separate both groups, followed by the perfusion density in the superficial retinal layer, the FAZ area, and finally the vessel density in the deep retinal layer [24]. In Durban’s study, a lower visual acuity was associated with a lower retinal vessel density in the superficial retinal layer and with the severity of DR. In the study by Lee and colleagues on 56 eyes of 34 subjects with DR and 21 age-matched controls of 12 individuals, a wide range of vascular changes in eyes with DR were identified upon OCTA, including retinal microaneurysms, vascular loops, non-perfusion areas, neovascularization, an erosion of the FAZ, and venous beading within multiple distinct capillary beds. In the study by Tang and coworkers, examining 434 eyes of 286 patients with diabetes, the severity of DR was associated with an increase in the FAZ and a decrease in the FAZ circularity [12].
Our study confirming the results of the previous investigations extends their qualitative observations to quantitative measurements of the vascular density in the deep vascular plexuses and of the FAZ area. Unexpectedly, our study revealed that the eyes of diabetic patients without ophthalmoscopically detected DR had a significantly increased vessel density in the deep vascular layer in the parafoveal region (Table 3). If future studies confirm this finding, it would go along with the theory formulated by Spaide that, at a later stage of DR, a reduction in the vascular density in the deep retinal vascular layer may lead to a decrease in the re-absorption of intraretinal fluid in that layer and secondarily to an intraretinal edema [30].
The results of our study also indicated that eyes without ophthalmoscopically detected DR of patients with diabetes showed a lower density of the radial peripapillary capillaries as compared with healthy controls, and this reduction in the vascular density was correlated with a higher fasting blood glucose concentration. This finding goes along with the observations made in the other retinal vascular layers and may be of interest to explain the development of cotton-wool spots as microinfarcts in the retinal nerve fiber layer in later stages of DR. The finding that the reduction in the radial peripapillary capillaries density was associated with an increased blood creatinine concentration as surrogate for an impaired kidney function furthermore supports the notion that OCTA is able to detect microvascular changes in patients with diabetes in association with other vascular sequelae of diabetes mellitus. Future studies may explore whether the OCTA-detected changes in the retinal and optic nerve head microvascular may serve as a rough surrogate for the impairment of the kidney function in patients with diabetes.
Our study revealed that OCTA demonstrated a wide range of microvascular changes seen in both the superficial retinal layer and deep retinal layer in 40% of 292 eyes without conventional signs of DR. Based on these findings, one may raise the question whether the definition of DR should be re-formulated, with ophthalmoscopically detectable retinal microaneurysms being no longer the first microvascular sign of DR.
When the results of our study are discussed, its limitations should be considered. First, we did not exclude non-capillary blood vessels from the analysis, so that the inclusion of the large retinal vessels usually not showing marked changes in the early or mediate advanced stage of DR might have reduced the sensitivity to detect differences. Despite this limitation, however, the differences between the study and control group in the retinal vessel density were statistically significant strengthening the conclusions drawn. Second, although we could reduce a part of the projection of the superficial retinal vasculature by the projection artifact removal algorithm, the artifact might still have affected the assessment of the deep retinal vascular density. Third, our study had a cross-sectional study design so that associations between parameters cannot automatically be considered to be causal relationships. Fourth, the study population consisted of Chinese so that the results of our study may not directly be transferred to other ethnicities. Fifth, we assessed only the central region of the retina so that changes in the peripheral area of the retina remained undetected. The diabetic changes in the retinal periphery maybe examined in future studies applying wide-field OCTA [31, 32].
In conclusions, OCTA revealed typical microvascular abnormalities in 40% of eyes without ophthalmoscopically detectable diabetic fundus changes. OCTA-assessed retinal vascular density decreased with higher prevalence of diabetes and higher fasting blood glucose concentration. The early stage of DR may be defined upon OCTA.
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2•7 million participants. Lancet 378:31–40
NCD Risk Factor Collaboration (2016) Worldwide trends in diabetes since 1980: pooled analysis of 751 population-based measurement studies with over 4.4 million participants. Lancet 387:1513–1530
Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease (META-EYE) Study Group (2012) The global prevalence and major risk factors of diabetic retinopathy and vision-threatening retinopathy in persons with diabetes. Diabetes Care 35:556–564
Leasher JL, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study (2016) Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care 39:1643–1649
Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK, Mitchell P, Klein R, Cheung CMG, Wong TY (2019) Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol 7:140–149
Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH (2016) Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57:362–370
Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, Witkin AJ, Duker JS, Fujimoto JG, Waheed NK (2016) Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol 134:644–650
Bandello F, Corbelli E, Carnevali A, Pierro L, Querques G (2016) Optical coherence tomography angiography of diabetic retinopathy. Dev Ophthalmol 56:107–112
Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, Varano M, Frontoni S, Parravano M (2017) Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol 95:e751–e755
Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K (2017) Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci 58:190–196
Soares M, Neves C, Marques IP, Pires I, Schwartz C, Costa MÂ, Santos T, Durbin M, Cunha-Vaz J (2017) Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br J Ophthalmol 101:62–68
Tang FY, Ng DS, Lam A, Luk F, Wong R, Chan C, Mohamed S, Fong A, Lok J, Tso T, Lai F, Brelen M, Wong TY, Tham CC, Cheung CY (2017) Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci Rep 7:2575
Chen Q, Ma Q, Wu C, Tan F, Chen F, Wu Q, Zhou R, Zhuang X, Lu F, Qu J, Shen M (2017) Macular vascular fractal dimension in the deep capillary layer as an early indicator of microvascular loss for retinopathy in type 2 diabetic patients. Invest Ophthalmol Vis Sci 58:3785–3794
Scarinci F, Picconi F, Giorno P, Boccassini B, De Geronimo D, Varano M, Frontoni S, Parravano M (2018) Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol 96:e264–e265
Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H (2018) Early detection of diabetic retinopathy. Surv Ophthalmol 63:601–608
Rosen RB, Andrade Romo JS, Krawitz BD, Mo S, Fawzi AA, Linderman RE, Carroll J, Pinhas A, Chui TYP (2019) Earliest evidence of preclinical diabetic retinopathy revealed using OCT angiography (OCTA) perfused capillary density. Am J Ophthalmol 203:103–115
Li Z, Wen X, Zeng P, Liao Y, Fan S, Zhang Y, Li Y, Xiao J, Lan Y (2019) Do microvascular changes occur preceding neural impairment in early-stage diabetic retinopathy? Evidence based on the optic nerve head using optical coherence tomography angiography. Acta Diabetol 56:531–539
Zeng Y, Cao D, Yu H, Yang D, Zhuang X, Hu Y, Li J, Yang J, Wu Q, Liu B, Zhang L (2019, 2019) Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2018-313582
Miwa Y, Murakami T, Suzuma K, Uji A, Yoshitake S, Fujimoto M, Yoshitake T, Tamura Y, Yoshimura N (2016) Relationship between functional and structural changes in diabetic vessels in optical coherence tomography angiography. Sci Rep 6:29064
Bhanushali D, Anegondi N, Gadde SG, Srinivasan P, Chidambara L, Yadav NK, Sinha Roy A (2016) Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57:OCT519–OCT525
Balaratnasingam C, Inoue M, Ahn S, McCann J, Dhrami-Gavazi E, Yannuzzi LA, Freund KB (2016) Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology 123:2352–2367
Lee J, Moon BG, Cho AR, Yoon YH (2016) Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology 123:2368–2375
Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, Hsu J, Ho AC (2017) Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology 124:235–244
Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, Neves C, Cunha-Vaz J (2017) Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol 135:370–376
Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, Shi Y, Wang RK (2017) Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res 60:66–100
Hirano T, Kitahara J, Toriyama Y, Kasamatsu H, Murata T, Sadda S (2019) Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br J Ophthalmol 103:216–221
Parravano M, De Geronimo D, Scarinci F, Virgili G, Querques L, Varano M, Bandello F, Querques G (2019) Progression of diabetic microaneurysms according to the internal reflectivity on structural optical coherence tomography and visibility on optical coherence tomography angiography. Am J Ophthalmol 198:8–16
Xu J, Wei WB, Yuan MX, Yuan SY, Wan G, Zheng YY, Li YB, Wang S, Xu L, Fu HJ, Zhu LX, Pu XL, Zhang JD, Du XP, Li YL, Ji Y, Gu XN, Li Y, Pan SF, Cui XL, Bai W, Chen YJ, Wang ZM, Zhu QS, Gao Y, Liu DY, Ji YT, Yang Z, Jonas JB (2012) Prevalence and risk factors for diabetic retinopathy: the Beijing Communities Diabetes Study 6. Retina (Philadelphia, Pa) 32:322–329
Han QL, Wu SL, Liu XX, An SS, Wu YT, Gao JS, Chen SH, Liu XK, Zhang Q, Mao RY, Shang XM, Jonas JB (2016) Ideal cardiovascular health score and incident end-stage renal disease in a community-based longitudinal cohort study: the Kailuan Study. BMJ Open 6:e012486
Spaide RF (2016) Retinal vascular cystoid macular edema: review and new theory. Retina (Philadelphia, Pa) 36:1823–1842
Hirano T, Kakihara S, Toriyama Y, Nittala MG, Murata T, Sadda S (2018) Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. Br J Ophthalmol 102:1199–1203
Sawada O, Ichiyama Y, Obata S, Ito Y, Kakinoki M, Sawada T, Saishin Y, Ohji M (2018) Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 256:1275–1280
Acknowledgements
The authors would like to thank the support from the Xinjiekou and Jinsong Community Health Service Centers for their assistance of recruiting diabetes participants and the Beijing Communities Diabetes Study for contributing systemic parameters.
Funding
This study was financially supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (code: ZYLX201307), National Natural Science Foundation of China (Nr. 81272981), Beijing Natural Science Foundation (Nr. 7151003), Advanced Health Care Professionals Development Project of Beijing Municipal Health Bureau (No. 2014-2-003), The priming scientific research foundation for the junior research in Beijing Tongren Hospital Capital Medical University (2017-YJJ-ZZL-009 and NO. 2018-YJJ-ZZL-046), and Beijing Municipal Administration of Hospitals Incubating Program (Code: PX2019008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Medical Ethics Committee of the Beijing Tongren Hospital, and written informed consent was obtained from all study participants, and all methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J.Y., Wang, Q., Yan, Y.N. et al. Microvascular retinal changes in pre-clinical diabetic retinopathy as detected by optical coherence tomographic angiography. Graefes Arch Clin Exp Ophthalmol 258, 513–520 (2020). https://doi.org/10.1007/s00417-019-04590-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04590-x