Abstract
Purpose
To compare the clinical characteristics of Vogt-Koyanagi-Harada (VKH) disease patients with and without anti-retinal antibodies (ARAs) that are frequently detected in autoimmune retinopathy.
Methods
Using immunoblot analyses, serum autoantibodies for recoverin, carbonic anhydrase II, and α-enolase were examined in 20 treatment-naïve patients with VKH disease. Clinical factors before and after systemic corticosteroid therapy, including best-corrected visual acuity (BCVA) and macular outer retinal morphology, were statistically compared between patients with VKH disease with and without ARAs.
Results
Serum ARAs were detected in 50.0% of patients with VKH disease. There were no significant differences in clinical factors between the two groups, including final BCVA, frequency of uveitis recurrence, and recovery of the macular ellipsoid zone after systemic corticosteroid therapy.
Conclusions
Our results suggest that the detected ARAs did not influence visual outcomes, the chronicity of uveitis, or outer retinal morphology in patients with VKH disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vogt-Koyanagi-Harada (VKH) disease is a multisystemic syndrome thought to be caused by autoimmunity against melanocytes [1]. In the eye, VKH disease initially targets the uvea, especially the choroid [2]. Visual impairment results from outer retinal disorder with serous retinal detachment (SRD) [3]. The presence of choroiditis in VKH disease is marked by the combination of increased thickness and decreased blood flow velocity of the choroid during the acute stage [4, 5].
Numerous experimental studies have indicated that the pathogenesis of VKH disease involves activation of CD4+ T lymphocyte-mediated cellular immunity against melanocyte-associated proteins that is triggered by a viral infection [3, 6,7,8,9]. These observations suggest that cellular immunity mainly drives the pathogenesis of this disease; however, the infiltration of B lymphocytes and plasma cells into the vitreous of VKH disease patients has been observed, suggesting the association of humoral immunity as well [10]. On the other hand, previous immunohistochemical results revealed that antibodies against the photoreceptor outer segments and Müller glial cells were positive in the sera of all the seven patients with this disease, and the positive rate and immunoreactivity were higher in patients with VKH disease than in those with Behcet’s disease [11]. Moreover, it is known that anti-retinal antibodies (ARAs) were also commonly detected in the sera of uveitis patients with different entities, but there was no relationship between the presence of ARAs and the clinical characteristics of uveitis [12, 13].
The activation of humoral immunity for retinal antigens may cause autoimmune retinopathy (AIR) following production of ARAs [14, 15]. Generally, patients with AIR tend to have progressive deterioration of visual function despite administration of systemic immunosuppressive agents [16,17,18]. AIR is diagnosed based on the clinical features and symptoms, in addition to the presence of ARAs on immunoblot and/or enzyme-linked immunosorbent assays, especially if the patients have antibodies against recoverin or α-enolase [15]. Major causative retinal antigens include α-enolase, carbonic anhydrase II, and recoverin seen in the majority of patients with ARAs-positive AIR [19]. Experimental studies have demonstrated that these antibodies have pathogenicity for retinal tissue, including photoreceptors [14, 20,21,22]. We recently reported that retinal pigment epithelium is also a possible target in Japanese patients with anti-α-enolase antibody-positive AIR, especially in a subtype with multiple drusen: namely, anti-enolase drusen [18].
Specific retinal antigens causative for AIR have yet to be identified in patients with VKH disease. Moreover, whether ARAs affect clinical characteristics of VKH disease remains unclear. The aim of the present study was to determine the positive rate of the three representative ARAs (i.e., α-enolase, carbonic anhydrase II, and recoverin) in patients with VKH disease and compare clinical features between VKH disease patients with and without the ARAs, in order to examine the association of ARAs with the pathogenesis of this disease.
Methods
Patients and diagnosis
This retrospective observational case series included 20 patients (40 eyes) with treatment-naïve VKH disease who visited Hokkaido University Hospital from July 2012 to August 2015. Inclusion criteria were patients with acute-stage VKH disease; sera collected before initiation of systemic corticosteroid therapy for detection of ARAs; and more than 6 months of follow-up after treatment. Exclusion criteria were convalescent-stage VKH disease with sunset glow fundus at the initial visit. VKH disease was diagnosed according to the criteria of Sugiura [23] and the VKH Disease Committee [24]. The presence of serum auto-antibodies against recoverin, carbonic anhydrase II, and α-enolase was examined in patients with VKH disease using immunoblot analyses (Fig. 1). Patients with VKH disease were divided into the ARAs group and the ARAs-negative group according to the presence (one or more) or absence of ARAs, respectively, and baseline characteristics and post-treatment outcomes were compared between the two groups. The current study was approved by the ethics committee of Hokkaido University Hospital (#015-0376) and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from each subject after the nature and potential consequences of the study had been explained.
Immunoblotting results in a patient with Vogt-Koyanagi-Harada disease whose serum tested positive for anti-recoverin (RCVN) antibody (case 10). Immunoblot analysis revealed predicted protein bands of approximately 49 kDa [recombinant human recoverin (23 kDa)-fusion glutathione S-transferase (GST; 26 kDa) protein] in response to anti-RCVN antibody (right lane) and the patient’s serum (left lane) but not in the control serum (middle lane)
Ophthalmologic examinations
At their initial visit, patients underwent comprehensive ophthalmic examinations, including measurement of decimal best-corrected visual acuity (BCVA), indirect ophthalmoscopy, color fundus photography, fluorescein angiography, indocyanine green angiography, spectral domain optical coherence tomography (OCT), and enhanced depth imaging OCT (EDI-OCT) (RS-3000 Advance; Nidek, Gamagori, Japan). During follow-up, EDI-OCT measurements were performed every week for up to 4 weeks after treatment and every month thereafter. Fundus photography was taken 6 months or later after the initiation of systemic corticosteroid therapy up to 15 months. Two investigators (Y.H., K.M.) routinely determined the onset of sunset glow fundus based on previously reported criteria [5] and peripapillary atrophy using funduscopic photographs taken after treatment. There was no significant difference in the mean period from the initiation of treatment until fundus picture taking between the ARAs (8.7 ± 3.0 months; range, 6–14 months) and the ARAs-negative (8.9 ± 3.2 months; range, 6–15 months) groups (P = 0.94). At the final visit, foveal outer retinal morphology was investigated using a horizontal B-scan image of EDI-OCT through the fovea.
Systemic treatment
Patients received either high-dose or pulse corticosteroid regimens according to the timing of their first visit. In five patients visiting before April 2014 (three ARAs patients, two ARAs-negative patients), intravenous prednisolone was administered and tapered from 200 mg/day (high-dose therapy). The tapering schedule for prednisolone required intravenous administration at 200 mg/day, 150 mg/day, and 100 mg/day, with each dose administered over a 2-day period. After the first 6 days of intravenous administration, tapering was continued using oral doses according to the following schedule: 4 days at 60 mg/day, 10 days at 40 mg/day, 14 days at 30 mg/day, 1 month at 20 mg/day, 1 month at 15 mg/day, 1 month at 10 mg/day, 1 month at 7.5 mg/day, and 1 month at 5 mg/day [4]. In the remaining 15 patients visiting from April 2014 onwards (seven ARAs patients, eight ARAs-negative patients), intravenous methylprednisolone was initially administered at 1000 mg/day for 3 consecutive days (pulse therapy). Oral prednisolone was then initiated and tapered per the following schedule: 10 days at 40 mg/day, 10 days at 30 mg/day, 10 days at 25 mg/day, 1 month at 20 mg/day, 1 month at 15 mg/day, 1 month at 10 mg/day, 2 months at 5 mg/day, and 1 month at 5 mg/every other day.
Oral prednisolone was temporarily increased or restarted if there were anterior and/or posterior recurrences of VKH disease. Oral cyclosporine was also administered at and after the second recurrence concurrently with oral prednisolone. In the present study, recurrences were defined as the recurrence of anterior chamber cells and/or posterior segment lesions (i.e., SRD and/or choroidal folds), based on criteria reported previously [25, 26].
Immunoblot analyses for recoverin, carbonic anhydrase II, and α-enolase
The methods used for production and purification of glutathione S-transferase (GST) fusion recoverin protein and immunoblot analyses were described previously [27]. Recombinant human carbonic anhydrase II and α-enolase proteins were purchased from Biovision (Milpitas, CA, USA) and ATGen (Gyeonggi-do, South Korea), respectively. Antibodies for protein detection were patient and negative control sera (1/2000 dilution), anti-recoverin antibody (1/20,000, Merck Millipore, Billerica, MA, USA), anti-α-enolase antibody (1/2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-carbonic anhydrase II antibody (1/2000, Abcam, Cambridge, MA, USA). Preliminary results showed the positive rate (14.3% in total) of antibodies against recoverin (4.8%), carbonic anhydrase II (0%), and α-enolase (9.5%) in 21 patients with idiopathic epiretinal membrane [18]. In concert with the previously reported rate (9%) in normal subjects [20], these results confirmed the reliability of our detection system, given the non-autoimmune etiology of idiopathic epiretinal membrane.
Statistical analyses
Values were expressed as the mean ± standard deviation. Decimal BCVA was converted to the logarithm of the minimum angle of resolution (logMAR). The Mann–Whitney U test or Fisher’s exact test was used to compare patient demographics, clinical characteristics, and logMAR values of BCVA at the baseline and final visits between VKH disease patients with and without ARAs. For all tests, P < 0.05 was considered to be statistically significant.
Results
Positive rate of three ARAs in patients with VKH disease
Serum ARAs were positive in 10 (50.0%) patients with VKH disease, with one (5.0%), one (5.0%), and eight patients (40.0%) positive for antibodies against recoverin, carbonic anhydrase II, and α-enolase, respectively.
Baseline clinical parameters
Baseline patient characteristics in the ARAs and ARAs-negative groups are summarized in Table 1. Before treatment, SRD was detected at the macular area (Fig. 2a, e) in 16 of 20 eyes with ARAs and 15 of 20 eyes without ARAs. No statistically significant difference was detected between the two groups in any of the following parameters examined: age, sex, follow-up period, refractive error, duration from onset to treatment, pleocytosis, height of SRD, and logMAR value of BCVA (Table 2).
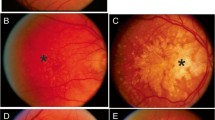
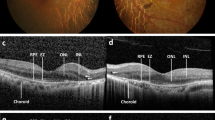
Ocular findings at the initial visit (a, c, e) and after the initiation of systemic corticosteroid therapy (b, d, f) in the patient with anti-recoverin antibody (Fig. 1). a, b Fundus photographs showing macular serous retinal detachment (SRD) (a) and resolution of SRD without sunset glow fundus 30 months after the initiation of systemic corticosteroid therapy (b). c, d Late-phase indocyanine green angiography showing numerous hypofluorescent dark dots (c) and resolution of the dots 1 month after treatment (d). e, f Enhanced depth imaging optical coherence tomography demonstrating macular SRD (e) and resolution of the SRD with recovery of the macular ellipsoid zone 30 months after treatment (f)
Post-treatment parameters in patients with VKH disease
SRD disappeared within 3 months after the initiation of treatment in all eyes with SRD (Fig. 2b, f). EDI-OCT at the final visit showed complete recovery of the ellipsoid zone in the fovea in all eyes, even in the patient who tested positive for anti-recoverin antibody (Fig. 2f). The final BCVA was more than 1.0 in almost all eyes. Post-treatment clinical parameters were compared between the ARAs group and the ARAs-negative group (Table 2). There were no significant differences in the logMAR values of the final BCVA, the rates of the onset of sunset glow fundus or peripapillary atrophy, recovery of the foveal ellipsoid zone, or uveitis recurrence. Moreover, when patients with anti-α-enolase antibody alone and none of the 3 ARAs were compared, there were still no significant differences between the two groups in the pre- and post-treatment parameters (Table 3).
Discussion
The present study showed for the first time that the positive rate of specific serum ARAs commonly detected in AIR was 50% in treatment-naïve patients with VKH disease. However, there were no significant differences in pre- and post-treatment clinical parameters including visual outcomes between patients with and without ARAs.
The three serum ARAs, examined in this study, were shown to be pathogenic for retinal tissues [14, 20,21,22]. A previous immunohistochemical study showed that serum-derived antibodies for photoreceptors and Müller cells were positive in all of seven patients with VKH disease [11]. However, the results did not determine whether the antibodies detected were pathogenic for retinal cells, because the study did not identify antibodies for specific retinal antigens. Therefore, the present study is the first to show that antibodies for retinal antigens are concretely detected in patients with VKH disease. However, the frequency of total antibodies for retinal antigens is still unknown in patients with VKH disease, because we examined only the three ARAs, and other unproven antibodies could be present.
The current results may contradict the consensus regarding the possible mechanism that causes this disease: activation of CD4+ T lymphocyte-mediated cellular immunity against melanocytes [3]. In patients with AIR, visual function and retinal morphology generally worsen progressively despite initiation of systemic immunosuppressive treatment [16, 17]. In our study, there were no significant differences in post-treatment outcomes including final BCVA, frequencies of sunset glow fundus, peripapillary atrophy, and uveitis recurrence, and recovery of the macular ellipsoid zone between VKH disease patients with or without ARAs. These results suggest that detected ARAs did not influence post-treatment visual outcomes, the chronicity of uveitis, and the recovery of macular photoreceptor morphology in patients with VKH disease. Therefore, we speculate that retinal disorders caused by choroiditis resulted in secondary production of ARAs, because autoimmunity for not only T cells but also B cells was activated in patients with VKH disease [10]. Recent studies revealed that a relationship between clinical parameters and ARAs in patients with uveitis was elusive [12, 13], consistent with our hypothesis. This may be possibly because the difference in the epitope of each individual ARA leads to the presence or absence of the pathogenicity of the corresponding ARAs [28].
This study has some limitations. First, we could not examine the prevalence of ARAs other than the three antibodies examined in the present study. It would be difficult to compare the positive rate of ARAs in patients with VKH disease between the present study and a previous study [11], because detection methods of ARAs differ between these studies. Next, in the present study, the major antiretinal antibody detected was anti-α-enolase antibody; however, cases with other antibodies showed no distinct features (Table 1) and there were still no significant differences between patients with anti-α-enolase antibody alone and none of the three ARAs in pre- and post-treatment parameters, (Table 3), which showed no significant correlation with the initial logMAR BCVA or SRD height (data not shown). Finally, the present study did not evaluate titers of the ARAs quantitatively, leaving open the possibility that titer difference between patients with VKH disease and AIR may lead to the absence or presence of pathogenicity, respectively.
In conclusion, serum antibodies against the three major retinal antigens generally causative for AIR was detected in 50% of patients with VKH disease. There were no significant differences in visual outcomes, uveitis recurrences, and recovery of macular ellipsoid zone after systemic corticosteroid therapy between VKH disease patients with or without ARAs. These results suggest that the ARAs did not influence visual prognosis or persistence of uveitis after treatment in patients with VKH disease. ARAs produced in patients with VKH disease were theorized to have no pathogenicity for the retina; they may be secondarily produced following exposure of retinal antigens to activated immune cells commonly seen in VKH disease.
References
Greco A, Fusconi M, Gallo A, Turchetta R, Marinelli C, Macri GF, De Virgilio A, de Vincentiis M (2013) Vogt-Koyanagi-Harada syndrome. Autoimmun Rev 12:1033–1038
Rao NA (2007) Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol 27:81–85
Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, Hirata CE, Yamamoto JH (2016) Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis 11:29
Hirooka K, Saito W, Namba K, Takemoto Y, Mizuuchi K, Uno T, Tagawa Y, Hashimoto Y, Ishida S (2013) Relationship between choroidal blood flow velocity and choroidal thickness during systemic corticosteroid therapy for Vogt-Koyanagi-Harada disease. Graefes Arch Clin Experiment Ophthalmol 253:609–617
Hirooka K, Saito W, Namba K, Mizuuchi K, Iwata D, Hashimoto Y, Ishida S (2017) Early post-treatment choroidal thickness to alert sunset glow fundus in patients with Vogt-Koyanagi-Harada disease treated with systemic corticosteroids. PLoS One 12:e0172612
Sakamoto T, Murata T, Inomata H (1991) Class II major histocompatibility complex on melanocytes of Vogt-Koyanagi-Harada disease. Arch Ophthalmol 109:1270–1274
Yamaki K, Gocho K, Hayakawa K, Kondo I, Sakuragi S (2000) Tyrosinase family proteins are antigens specific to Vogt-Koyanagi-Harada disease. J Immunol 165:7323–7329
Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, Sugamoto Y, Futagami Y, Itoh K, Mochizuki M (2006) Ocular infiltrating CD4+ T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Invest Ophthalmol Vis Sci 47:2547–2554
Sugita S, Takase H, Kawaguchi T, Taguchi C, Mochizuki M (2007) Cross-reaction between tyrosinase peptides and cytomegalovirus antigen by T cells from patients with Vogt-Koyanagi-Harada disease. Int Ophthalmol 27:87–95
Perry HD, Font RL (1977) Clinical and histopathologic observations in severe Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 83:242–254
Chan CC, Palestine AG, Nussenblatt RB, Roberge FG, Benezra D (1985) Anti-retinal auto-antibodies in Vogt-Koyanagi-Harada syndrome, Behcet's disease, and sympathetic ophthalmia. Ophthalmology 92:1025–1028
Ten Berge JC, Schreurs MW, Vermeer J, Meester-Smoor MA, Rothova A (2016) Prevalence and clinical impact of antiretinal antibodies in uveitis. Acta Ophthalmol 94:282–288
Ten Berge JC, van Rosmalen J, Vermeer J, Hellström C, Lindskog C, Nilsson P, Qundos U, Rothova A, Schreurs MW (2016) Serum autoantibody profiling of patients with paraneoplastic and non-paraneoplastic autoimmune retinopathy. PLoS One 11:e0167909
Adamus G, Machnicki M, Seigel GM (1997) Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest Ophthalmol Vis Sci 38:283–291
Heckenlively JR, Ferreyra HA (2008) Autoimmune retinopathy: a review and summary. Semin Immunopathol 30:127–134
Chan JW (2003) Paraneoplastic retinopathies and optic neuropathies. Sur Ophthalmol 48:12–38
Rahimy E, Sarraf D (2013) Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Surv Ophthalmol 58:430–458
Ando R, Saito W, Kanda A, Kase S, Fujinami K, Sugahara M, Nakamura Y, Eguchi S, Mori S, Noda K, Shinoda K, Ishida S (2018) Clinical features of Japanese patients with anti-α-enolase antibody-positive autoimmune retinopathy: novel subtype of multiple drusen. Am J Ophthalmol 196:181–196
Adamus G (2009) Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev 8:410–414
Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA (1996) The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol 78:120–129
Ren G, Adamus G (2004) Cellular targets of anti-a-enolase autoantibodies of patients with autoimmune retinopathy. J Autoimmun 23:161–167
Adamus G, Webb S, Shiraga S, Duvoisin RM (2006) Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J Autoimmun 26:146–153
Sugiura S (1978) Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol 22:9–35
Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, Pivetti-Pezzi P, Tessler HH, Usui M (2001) Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 131:647–652
Tagawa Y, Namba K, Mizuuchi K, Takemoto Y, Iwata D, Uno T, Fukuhara T, Hirooka K, Kitaichi N, Ohno S, Ishida S (2016) Choroidal thickening prior to anterior recurrence in patients with Vogt-Koyanagi-Harada disease. Br J Ophthalmol 100:473–477
Takemoto Y, Namba K, Mizuuchi K, Iwata D, Uno T, Ohno S, Hirooka K, Hashimoto Y, Saito W, Sugiyama K, Ishida S (2016) Choroidal circulation impairment during the anterior recurrence of Vogt-Koyanagi-Harada disease confirmed with indocyanine green angiography and laser speckle flowgraphy. Acta Ophthalmol 94:e629–e636
Saito M, Saito W, Kanda A, Ohguro H, Ishida S (2014) A case of paraneoplastic optic neuropathy and outer retinitis positive for autoantibodies against collapsin response mediator protein-5, recoverin, and α-enolase. BMC Ophthalmol 14:5
Adamus G, Amundson D, Seigel GM, Machnicki M (1998) Anti-enolase-alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun 11:671–677
Acknowledgements
We thank Mrs. Ikuyo Hirose (Hokkaido University) for her technical assistance.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
Statement of human rights
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical approval retrospective studies
The current study was approved by the ethics committee of Hokkaido University Hospital (#015-0376).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashimoto, Y., Saito, W., Namba, K. et al. Comparison of clinical characteristics in patients with Vogt-Koyanagi-Harada disease with and without anti-retinal antibodies. Graefes Arch Clin Exp Ophthalmol 257, 1751–1758 (2019). https://doi.org/10.1007/s00417-019-04330-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04330-1