Abstract
Purpose
To evaluate the clinical characteristics of patients with acute zonal occult outer retinopathy (AZOOR), according to the presence or absence of anti-retinal antibodies (ARAs) that are frequently detected in autoimmune retinopathy.
Methods
Retrospective observational case series. This study included 33 patients with acute-stage AZOOR who had been followed up for more than 6 months after the initial visit. The median follow-up period was 26 months. Immunoblot analyses were used to detect autoantibodies for recoverin, carbonic anhydrase II, and α-enolase in serum from these patients. Main outcome measures comprised clinical factors at the initial and final visits, including best-corrected visual acuity, mean deviation on Humphrey perimetry, and retinal morphology, which were statistically compared between patients with AZOOR who exhibited ARAs and those who did not.
Results
At least one serum ARA was detected in 42% of patients with AZOOR. There were no significant differences in clinical factors between the two groups, including follow-up period, best-corrected visual acuity and mean deviation at the initial and final visits, a-wave amplitude on single-flash electroretinography at the initial visit, and frequencies of improvement of the macular ellipsoid zone and AZOOR recurrence.
Conclusions
Our findings suggest that the presence of ARAs did not influence visual outcomes or outer retinal morphology in patients with AZOOR.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute zonal occult outer retinopathy (AZOOR) is an idiopathic disease characterized by the development of a zonal area of acute outer retinal impairment, despite normal funduscopic retinal appearance at the initial stage [1, 2]. The outer retinal impairment is demonstrated by diminished responses on full-field or multifocal electroretinography [3] and disrupted ellipsoid zone (EZ) detected with spectral domain optical coherence tomography (OCT) [4].
In Japanese patients with AZOOR, spontaneous improvement of visual function with good visual prognosis is more frequent, and the development of late-onset retinal degeneration corresponding to the zone of visual loss is less frequent than in Caucasian ones [2, 5,6,7]. These observations suggest that clinical features of AZOOR differ according to ethnicity and the Japanese patients have better visual prognosis. Strategies for the management of AZOOR are not clearly established thus far. However, several reports have suggested that systemic corticosteroid therapy is effective for patients with progressive visual impairment [5, 8, 9].
Autoimmune retinopathy (AIR) is a syndrome caused by the development of anti-retinal antibodies (ARAs), which result in retinal degeneration. Autoantibodies against retinal antigens, especially α-enolase, carbonic anhydrase II, and recoverin, are present in the majority of patients with AIR who exhibit ARAs [10]. These antibodies are pathogenic for retinal tissues including photoreceptors [11,12,13,14]. Visual function generally worsens in a progressive manner over a period of weeks to months in patients with AIR who exhibit anti-recoverin antibody [15, 16], whereas it takes over a period of years to worsen in patients with anti-α-enolase antibody [17, 18]. Patients with AIR tend to have poor visual prognosis, despite administration of systemic immunosuppressive agents [15, 18]. AIR is diagnosed on the basis of the clinical features described above, as well as the presence of ARAs on immunoblot analysis; therefore, an important consideration for each patient is whether antibodies against recoverin and/or α-enolase are present [19].
Thus far, two major hypotheses have been proposed regarding the mechanism underlying outer retinal impairment in patients with AZOOR [20]. The first hypothesis comprises the choroidal impairment theory [8, 21], on the basis of the prerequisite that the choriocapillaris supplies oxygen and nutrition to the outer retinal layers. Indocyanine green angiography has revealed hypofluorescence in areas related or unrelated to AZOOR lesions [5, 8, 22, 23]. Notably, subfoveal choroidal thickness significantly decreases and choroidal blood flow velocity increases, in combination with improvements in visual function and outer retinal morphology [8, 24]. Moreover, changes in choroidal thickness are negatively correlated with changes in sensitivity on perimetry [24]. These observations suggest a relationship between choroidal circulatory impairment and AZOOR pathogenesis. However, the choroidal impairment in patients with AZOOR might result from primary damage to photoreceptors [4, 21].
The second hypothesis comprises the ARA theory, on the basis of the observation that the features with normal funduscopic appearance, along with photoreceptor impairment, in the early stages of AZOOR are similar to features present in patients with AIR. Immunoblot analyses have revealed non-specific ARAs in serum samples of all patients with AZOOR [25, 26]. In addition, anti-α-enolase antibody was detected in the serum of 26% of patients with AZOOR; anti-carbonic anhydrase II antibody was detected in the serum of 17% of these patients [27]. Based on these results, some clinicians have proposed that ARAs are involved in AZOOR pathogenesis [25, 26]. However, to the best of our knowledge, there remains no clear evidence of the relationship between ARAs and clinical characteristics of AZOOR [25,26,27,28]. Therefore, ARAs may be non-pathogenic for the retina if they are secondarily produced following exposure of retinal antigens at the onset of AZOOR [29]. Here, we hypothesized that visual prognosis in patients with AZOOR is worse in those with ARAs than in those without ARAs. To examine the association of ARAs with the pathogenesis of AZOOR, the aims of the present study were to determine the rates of detection regarding the three representative ARAs frequently associated with AIR (i.e., autoantibodies for recoverin, carbonic anhydrase II, and α-enolase) in patients with AZOOR, and to evaluate clinical features of patients with AZOOR according to ARA status.
Methods
Patients
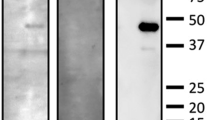
This retrospective observational case series included 33 patients (45 eyes) with AZOOR who visited Hokkaido University Hospital from October 2004 to March 2015. Patients were included if they had acute-stage AZOOR and had been followed up for more than 6 months after the initial visit. Patients were excluded if they had convalescent AZOOR at the initial visit or if they had AZOOR in combination with any disease of AZOOR complex such as multiple evanescent white dot syndrome. The presence of serum autoantibodies against recoverin, carbonic anhydrase II, and α-enolase was examined in patients with AZOOR by using immunoblot analyses (Supplemental Fig. 1). Patients with AZOOR were divided into the ARA and non-ARA groups according to the presence or absence of ≥ 1 ARA, respectively. Characteristics including visual function at the initial and final visits were compared between the two groups. This study was approved by the ethics committee of Hokkaido University Hospital (approval number 020–0236) and was performed in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from each patient after the nature and potential consequences of the study had been explained.
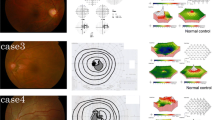
Images of the left eye in a 43-year-old patient with acute zonal occult outer retinopathy (AZOOR) with anti-α-enolase antibody (case 14). a Fundus photograph at the initial visit shows no abnormal findings, except peripapillary atrophy. b Multifocal electroretinography shows reduced amplitudes corresponding to the retinal site with a visual field defect (d). c Horizontal image through the fovea on enhanced depth imaging optical coherence tomography (EDI-OCT) at the initial visit shows loss of the ellipsoid zone (EZ) (arrowheads), corresponding to the AZOOR lesion site. d, e On Humphrey perimetry, a blind spot enlargement observed at the initial visit (d) shrunk at the final visit (e). f On EDI-OCT, the area of EZ loss at the final visit was shortened, compared with the corresponding area at the initial visit
Diagnosis
AZOOR was diagnosed for patients who fulfilled all of the following criteria [2, 5]: (i) acute visual field or vision loss, typically with concurrent photopsia; (ii) ≥ 1 visual field defect regions that could not be explained by the results of funduscopic examination or fluorescein angiography; (iii) reduced multifocal electroretinography responses corresponding to retinal sites with visual field defects; (iv) negative screening results for infection, including syphilis and tuberculosis; in addition to (i)-(iv), (v) beginning in 2007, outer retinal morphologic abnormalities, including absence or discontinuity of the EZ and/or interdigitation zone on OCT (available in 30 of 33 patients). A representative case is shown in Fig. 1.
Ophthalmologic examinations
At their initial visit, patients underwent comprehensive ophthalmic examinations, including decimal best-corrected visual acuity (BCVA) measurement, indirect ophthalmoscopy, color fundus photography, fluorescein angiography, spectral domain and/or enhanced depth imaging OCT (OCT Ophthalmoscope C7, RS-3000, or RS-3000 Advance; both from Nidek, Gamagori, Japan), and 20 J scotopic single-flash electroretinography (LE-3000, Tomey, Nagoya, Japan), followed several days later by visual field testing (Goldmann perimetry and/or the Humphrey 30–2 Swedish interactive threshold algorism standard test) and multifocal electroretinography (VERIS; EDI, San Mateo, CA, USA). BCVA assessment, Humphrey perimetry, fundus photography, and OCT were performed during follow-up. Two investigators (Y.H. and W.S.) routinely evaluated the development of late-onset zonal retinal atrophy by comparing fundus photographs taken at the initial visit and more than 6 months later.
Treatment
Patients were not treated if they exhibited no central visual acuity loss at the initial visit with no clinical progression thereafter. Patients with progressive central visual acuity loss received systemic corticosteroid therapy including corticosteroid pulse therapy, as previously described [8].
Blood samples
Blood samples were obtained at the initial visit in patients with AZOOR and were centrifuged at 3000 rpm for 10 min at 4 ̊C to collect the serum. The serum was then carefully transferred into a polypropylene tube and stored at − 80 ̊C. Between November and December 2015, all the samples were thawed and immediately processed for immunoblot analyses.
Immunoblot analyses for recoverin, carbonic anhydrase II, and α-enolase
The methods used for production and purification of glutathione S-transferase (GST) fusion recoverin protein and immunoblot analyses were described previously [30]. Recombinant human carbonic anhydrase II and α-enolase proteins were purchased from Biovision (Milpitas, CA, USA) and ATGen (Gyeonggi-do, South Korea), respectively. Antibodies for protein detection were patients’ and normal sera (1/2000 dilution), anti-recoverin antibody (1:20,000 dilution, Merck Millipore, Billerica, MA, USA), anti-α-enolase antibody (1:2000 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), and anti-carbonic anhydrase II antibody (1:2000 dilution, Abcam, Cambridge, MA, USA). The normal sera used as negative serum controls were previously proven to be seronegative for ARAs [18, 30, 31], and GST was electrophoresed as a negative antigen control followed by each antibody or serum application [30, 31]. Preliminary results regarding the rates of detection (14% in total) for antibodies against recoverin (5%), carbonic anhydrase II (0%), and α-enolase (10%) were determined in a study of 21 patients with idiopathic epiretinal membrane [18]. Given the non-autoimmune etiology of idiopathic epiretinal membrane, these results were similar to the previously reported detection rate (9% for α-enolase) in normal healthy individuals [12], confirming the reliability of our detection system.
Statistical analyses
Values were expressed as the mean ± standard deviation. Decimal BCVA was converted to the logarithm of the minimum angle of resolution (logMAR). The Mann–Whitney U test or Fisher’s exact test was used to compare patient demographics and clinical characteristics (e.g., logMAR values of BCVA and mean deviation [MD] values on Humphrey perimetry) at the initial and final visits between ARA and non-ARA groups. For all tests, P < 0.05 was considered statistically significant. Significant improvement and deterioration in visual functions during follow-up were defined as logMAR BCVA change of ≥ 0.3 and MD value change of ≥ 30%.
Results
Rates of ARA detection in patients with AZOOR
At least one serum ARA was detected in 14 (42%) of 33 patients with AZOOR with 3 (9%), 4 (12%), and 10 patients (30%) having antibodies against recoverin, carbonic anhydrase II, and α-enolase, respectively (Supplemental Fig. 1).
Baseline clinical parameters
Clinical characteristics of patients in the ARA and non-ARA groups are summarized in Tables 1 and 2. Regarding baseline parameters, no statistically significant differences were detected between the two groups in any of the following parameters: age, follow-up period, refractive error, bilaterality, photopsia, a-wave amplitude of single-flash electroretinography, logMAR BCVA, and MD value at the initial visit; however, sex and blind spot enlargement significantly differed between groups (Table 3). Moreover, when patients with anti-α-enolase antibody alone were compared with patients who had no ARAs, only spherical equivalent significantly differed between the two groups (Supplemental Table 1).
Clinical parameters at the final visit
Parameters at the final visit did not significantly differ between the two groups with respect to logMAR BCVA or MD values; the frequencies of patients who received systemic corticosteroid therapy, who exhibited improvement of macular EZ, who exhibited development of zonal retinal atrophy, and who exhibited AZOOR recurrence were also similar between groups. Moreover, when patients with anti-α-enolase antibody alone were compared with patients who had no ARAs, there were no significant differences between the two groups in any of the parameters at the final visit (Supplemental Table 1).
Visual outcomes
Compared with initial values, the final logMAR BCVA improved in 3 eyes (17%) and 7 eyes (26%), remained unchanged in 15 eyes (83%) and 20 eyes (74%), and worsened in 0 eyes and 0 eyes in the ARA (N = 18) and non-ARA (N = 27) groups, respectively. The frequency of BCVA improvement did not significantly differ between the two groups (P = 0.716).
The mean MD values were significantly higher at the final visit than at the initial visit in both groups (Table 3, ARA group; P = 0.006, non-ARA group; P = 0.0003). Compared with initial values, the final MD values improved in 11 eyes (69%) and 12 eyes (57%), remained unchanged in 4 eyes (25%) and 8 eyes (38%), and worsened in 1 eye (6%) and 1 eye (5%) in the ARA (N = 16) and non-ARA (N = 21) groups, respectively. The frequencies of improvement or deterioration of the MD value did not significantly differ between the two groups (P = 0.515 and P = 1.000, respectively).
Discussion
The present study showed that the detection rate of ≥ 1 specific ARAs, which are frequently detected in patients with AIR, was 42% in the serum from patients with AZOOR. However, there were no significant differences in clinical parameters including photoreceptor function at the initial visit, visual functions (i.e., BCVA and visual field) at the initial and final visits, and the frequencies of improvement of macular EZ and AZOOR recurrence between patients with ARAs and those without ARAs.
We recently reported similar results in Vogt-Koyanagi-Harada (VKH) disease [31], the mechanism of which is presumed to differ from that of AIR. In VKH disease, choroiditis caused by activation of CD4 + T lymphocyte-mediated cellular immunity against melanocyte-associated proteins leads to outer retinal disorder with serous retinal detachment [32]. The detection rate of ≥ 1 of the three serum ARAs examined was 50% in patients with VKH disease. However, there were no significant differences in clinical parameters (e.g., visual outcomes, recovery of the macular EZ, and uveitis recurrences) after systemic corticosteroid therapy between patients with and without ARAs [31]. These results suggest that the ARAs produced did not influence visual prognosis, outer retinal morphology, and the chronicity of uveitis in patients with VKH disease and that these ARAs would reasonably be non-pathogenic for the retina. Although VKH disease is associated with activated cellular immunity, it may have such a high rate of ARA detection because autoimmunity for both T cells and B cells is activated during its acute stage.
In the present study, final logMAR BCVA was ≦0.0 in nearly all eyes of patients with AZOOR. Perimetric sensitivity at the final visit improved or remained unchanged in > 90% of eyes in both groups, compared with sensitivity at the initial visit. These results suggest that Japanese patients with AZOOR have good visual prognosis, regardless of the presence or absence of ARAs. Moreover, there were no significant differences in clinical parameters including visual outcomes, improvement of macular morphology, and AZOOR recurrence between patients with AZOOR who exhibited ARAs and those who did not. Therefore, the current results suggest that the ARAs detected did not influence visual outcomes or AZOOR disease activity and were thus non-pathogenic for the retina in patients with AZOOR, similar to the findings in patients with VKH disease. This is presumably because differences in the ARA epitopes cause the diverse range of pathogenicity (often little or no) depending on the corresponding ARAs [33]. Therefore, our results could not validate the causative relationship of ARAs with AZOOR pathogenesis in terms of clinical outcomes. Notably, our conclusions are supported by evident differences in many clinical parameters, such as age, sex ratio, refraction, binocularity, and BCVA at the initial and final visits between patients with AZOOR and those with AIR, both of whom exhibited anti-α-enolase antibodies (Table 4).
Approximately 30% of patients with AZOOR have a history of systemic autoimmune diseases, such as Hashimoto disease [2]. The results of several studies have suggested the effectiveness of systemic corticosteroids and adalimumab [5, 8, 9, 22, 34]. Based on these observations, the previously reported high rates of ARA seropositivity [25,26,27,28], and our present results, we presume that an autoimmune mechanism other than ARAs (e.g., activation of T cells) might be involved in AZOOR pathogenesis. Further studies are needed to investigate the more detailed mechanisms involved in AZOOR.
ARAs reportedly have been detected in patients with various retinal diseases such as retinitis pigmentosa, age-related macular degeneration, uveitis, and diabetic maculopathy [35,36,37,38,39]. However, the previous studies revealed no relationships between clinical parameters and the presence of ARAs in patients with various types of uveitis [37, 38] or in our patients with VKH disease [31], consistent with the present findings. Therefore, the association of ARAs with disease pathogenesis could not be determined simply by assessing the presence of ARAs.
This study has some limitations. First, it only examined the prevalence of three specific ARAs. It would be difficult to compare the rate of ARA detection in patients with AZOOR between the present study and previous studies because of differences regarding immunoblot methods [25, 26]. Second, in the present study, the major anti-retinal antibody detected was anti-α-enolase antibody; however, cases with other antibodies showed no distinct features (Table 1) and there were still no significant differences between patients with anti-α-enolase antibody alone and none of the three ARAs in parameters at the baseline and final visits (Supplemental Table 1). Third, the interval between serum collection and ARA detection varied depending on patients. The variation of interval among samples, even though stored at − 80 ̊C, may have caused the detection rate of ARAs to be underestimated. Finally, the number of female patients was higher in the ARA group than in the non-ARA group. This is presumably because, compared with men, women exhibit higher prevalence for systemic autoimmune diseases [40].
In conclusion, autoantibodies against three major retinal antigens frequently associated with the onset of AIR were detected in 42% of patients with AZOOR. There were no significant differences in clinical parameters, including visual outcomes and improvement of macular morphology, between patients with AZOOR who exhibited ARAs and those who did not. These results suggest that the ARAs detected did not influence visual prognosis or disease activity in patients with AZOOR. ARAs produced in patients with AZOOR are suggested to be non-pathogenic for the retina, and may be secondarily produced following immunologic exposure to retinal antigens caused by destruction of retinal tissues.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Gass JD (1993) Acute zonal occult outer retinopathy. Donders Lecture: The Netherlands Ophthalmological Society, Maastricht, Holland, June 19, 1992. J Clin Neuroophthalmol 13:79–97
Gass JD, Agarwal A, Scott IU (2002) Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol 134:329–339
Francis PJ, Marinescu A, Fitzke FW, Bird AC, Holder GE (2005) Acute zonal occult outer retinopathy: towards a set of diagnostic criteria. Br J Ophthalmol 89:70–73
Spaide RF, Koizumi H, Freund KB (2008) Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol 146:111–120
Saito S, Saito W, Saito M, Hashimoto Y, Mori S, Noda K, Namba K, Ishida S (2015) Acute zonal occult outer retinopathy in Japanese patients: clinical features, visual function, and factors affecting visual function. PLoS One 10:e0125133
Nakao S, Kaizu Y, Yoshida S, Iida T, Ishibashi T (2015) Spontaneous remission of acute zonal occult outer retinopathy: follow-up using adaptive optics scanning laser ophthalmoscopy. Graefes Arch Clin Exp Ophthalmol 253:839–843
Mrejen S, Khan S, Gallego-Pinazo R, Jampol LM, Yannuzzi LA (2014) Acute zonal occult outer retinopathy: a classification based on multimodal imaging. JAMA Ophthalmol 132:1089–1098
Saito M, Saito W, Hashimoto Y, Yoshizawa C, Shinmei Y, Noda K, Ishida S (2014) Correlation between decreased choroidal blood flow velocity and the pathogenesis of acute zonal occult outer retinopathy. Clin Experiment Ophthalmol 42:139–150
Kitakawa T, Hayashi T, Takashina H, Mitoola K, Gekka T, Tsuneoka H (2012) Improvement of central visual function following steroid pulse therapy in acute zonal occult outer retinopathy. Doc Ophthalmol 124:249–254
Adamus G (2009) Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev 8:410–414
Maeda T, Maeda A, Maruyama I, Ogawa KI, Kuroki Y, Sahara H, Sato N, Ohguro H (2001) Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci 42:705–712
Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA (1996) The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol 78:120–129
Ren G, Adamus G (2004) Cellular targets of anti-a-enolase autoantibodies of patients with autoimmune retinopathy. J Autoimmun 23:161–167
Adamus G, Karren L (2009) Autoimmunity against carbonic anhydrase II affects retinal cell functions in autoimmune retinopathy. J Autoimmun 32:133–139
Braithwaite T, Vugler A, Tufail A (2012) Autoimmune retinopathy. Ophthalmologica 228:131–142
Suimon Y, Saito W, Hirooka K, Kanda A, Kitai H, Sakakibara-Konishi J, Ishida S (2017) Improvements of visual function and outer retinal morphology following spontaneous regression of cancer in anti-recoverin cancer-associated retinopathy. Am J Ophthalmol Case Rep 5:137–140
Weleber RG, Watzke RC, Shults WT, Trzupek KM, Heckenlively JR, Egan RA, Adamus G (2005) Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol 139:780–794
Ando R, Saito W, Kanda A, Kase S, Fujinami K, Sugahawa M, Nakamura Y, Eguchi S, Mori S, Noda K, Shinoda K, Ishida S (2018) Clinical features of Japanese patients with anti-α-enolase antibody-positive autoimmune retinopathy: novel subtype of multiple drusen. Am J Ophthalmol 196:181–196
Heckenlively JR, Ferreyra HA (2008) Autoimmune retinopathy: a review and summary. Semin Immunopathol 30:127–134
Saito W, Ishida S (2019) Acute zonal occult ocular retinopathy. In: Yu HG (ed) Inflammatory and infectious ocular disorders. Springer Nature, Singapore, pp 45–50
Fagan XJ (2014) A new insight into an old mystery. Clin Exp Ophthalmol 42:103–104
Saito A, Saito W, Furudate N, Ohno S (2007) Indocyanine green angiography in a case of punctate inner choroidopathy associated with acute zonal occult outer retinopathy. Jpn J Ophthalmol 51:295–300
Monson DM, Smith JR (2011) Acute zonal occult outer retinopathy. Surv Ophthalmol 56:23–35
Hashimoto Y, Saito W, Saito M, Hasegawa Y, Takita A, Mori S, Noda K, Ishida S (2017) Relationship between choroidal thickness and visual field impairment in acute zonal occult outer retinopathy. J Ophthalmol 2017:2371032
Tagami M, Matsumiya W, Imai H, Kusuhara S, Honda S, Azumi A (2014) Autologous antibodies to outer retina in acute zonal occult outer retinopathy. Jpn J Ophthalmol 58:462–472
Qian CX, Wang A, DeMill DL, Jayasundera T, Branham K, Abalem MF, Khan N, Heckenlively JR (2017) Prevalence of anti-retinal antibodies in acute zonal occult outer retinopathy (AZOOR): a comprehensive review of 25 cases. Am J Ophthalmol 176:210–218
Adamus G (2011) Is Zonal occult outer retinopathy an autoimmune disease? J Clin Exp Ophthalmol 2:104e
Zeng HY, Liu Q, Peng XY, Cao K, Jin SS, Xu K (2019) Detection of serum anti-retinal antibodies in the Chinese patients with presumed autoimmune retinopathy. Graefes Arch Clin Exp Ophthalmol 257:1759–1764
Forooghian F (2017) Prevalence of antiretinal antibodies in acute zonal occult outer retinopathy: a comprehensive review of 25 cases. Am J Ophthalmol 179:210–211
Saito M, Saito W, Kanda A, Ohguro H, Ishida S (2014) A case of paraneoplastic optic neuropathy and outer retinitis positive for autoantibodies against collapsin response mediator protein-5, recoverin, and α-enolase. BMC Ophthalmol 14:5
Hashimoto Y, Saito W, Namba K, Mizuuchi K, Iwata D, Noda K, Ishida S (2019) Comparison of clinical characteristics in patients with Vogt-Koyanagi-Harada disease with and without anti-retinal antibodies. Graefes Arch Clin Exp Ophthalmol 257:1751–1758
Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, Hirata CE, Yamamoto JH (2016) Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis 11:29
Adamus G, Amundson D, Seigel GM, Machnicki M (1998) Anti-enolase-alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun 11:671–677
Neri P, Ricci F, Giovannini A, Arapi I, De Felici C, Cusumano A, Mariotti C (2014) Successful treatment of an overlapping choriocapillaritis between multifocal choroiditis and acute zonal occult outer retinopathy (AZOOR) with adalimumab (HumiraTM). Int Ophthalmol 34:359–364
Heckenlively JR, Jordan BL, Aptsiauri N (1999) Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol 127:565–573
Kubicka-Trząska A, Wilańska J, Romanowska-Dixon B, Sanak M (2012) Circulating antiretinal antibodies predict the outcome of anti-VEGF therapy in patients with exudative age-related macular degeneration. Acta Ophthalmol 90:e21–e24
Ten Berge JC, Schreurs MW, Vermeer J, Meester-Smoor MA, Rothova A (2016) Prevalence and clinical impact of antiretinal antibodies in uveitis. Acta Ophthalmol 94:282–288
Ten Berge JC, van Rosmalen J, Vermeer J, Hellström C, Lindskog C, Nilsson P, Qundos U, Rothova A, Schreurs MW (2016) Serum autoantibody profiling of patients with paraneoplastic and non-paraneoplastic autoimmune retinopathy. PLoS One 11:e0167909
Yoshitake S, Murakami T, Suzuma K, Yoshitake T, Uji A, Morooka S, Dodo Y, Fujimoto M, Shan Y, Fort PE, Ito S, Tsujikawa A, Yoshimura N (2019) Anti-fumarase antibody promotes the dropout of photoreceptor inner and outer segments in diabetic macular oedema. Diabetologia 62:504–516
Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM (2012) Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 38:J109-119
Acknowledgements
We would like to thank Mrs. Ikuyo Hirose (Hokkaido University) for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The current study was approved by the ethics committee of Hokkaido University Hospital (#020–0236).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Hashimoto, Y., Saito, W., Kanaizumi, S. et al. Comparison of clinical characteristics in patients with acute zonal occult outer retinopathy according to anti-retinal antibody status. Graefes Arch Clin Exp Ophthalmol 259, 2967–2976 (2021). https://doi.org/10.1007/s00417-021-05198-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05198-w