Abstract
Purpose
To evaluate the diagnostic efficacy of optical coherence tomography (OCT) angiography, in patients with pre-perimetric open-angle glaucoma.
Methods
Thirty-eight consecutive eyes of 26 patients with pre-perimetric open-angle glaucoma and 48 normal eyes of 32 controls were included in this prospective study. We evaluated OCT angiography, spectral-domain OCT and visual fields parameters. Differences between controls and patients and between OCT angiography parameters and traditional glaucoma measurements were evaluated. Receiver operating characteristic curves for OCT angiography parameters were assessed.
Results
The flow index (p = 0.021) and vessel density (p = 0.001) were significantly lower in pre-perimetric glaucoma eyes versus normal eyes. Ganglion cell complex parameters were significantly correlated to OCT angiography parameters (p < 0.05). Neither retinal nerve fiber layer (RNFL) nor visual field parameters correlated with OCT angiography parameters.
Conclusions
OCT angiography can be considered a reliable, easy-to-perform method with which to evaluate microvascular changes of optic nerve head for the early diagnosis and follow-up of glaucoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is the second leading cause of blindness worldwide, affecting more than 60 million people. An early diagnosis and close monitoring of glaucoma are important to avoid irreversible nerve damage associated with vision loss. Visual field testing remains the gold standard for the diagnosis of this glaucoma, but it has substantial variability, and poor reproducibility in some cases [1,2,3]. Elevated intraocular pressure (IOP) was long believed to be the only cause of neural tissue loss at the optic nerve head and progressive degeneration of the retinal ganglion cells and their axons, subsequently resulting in visual field loss [4]. Evidence now suggests that vascular factors play a critical role in the development of glaucoma. For example, blood flow is diminished in the retinal and choroidal circulation in primary open-angle glaucoma [5,6,7,8].
Retinal and disc blood flow can be rapidly and accurately evaluated with optical coherence tomography (OCT) angiography, combined with split-spectrum amplitude–decorrelation angiography. This is a non-invasive technique that does not require the injection of any dye and provides near-automatic quantification of disc perfusion [9]. OCT angiography can visualize reduced peripapillary retinal perfusion in glaucomatous eyes, and calculate the peripapillary flow index and vessel density [10].
The purpose of this prospective study was to evaluate peripapillary retinal perfusion in glaucomatous pre-perimetric eyes, and to explore the possible correlation between disc perfusion, neural structure and visual field loss using OCT angiography.
Materials and methods
In this prospective study we evaluated 38 consecutive eyes of 26 patients presenting pre-perimetric open-angle glaucoma enrolled from October 2015 to February 2016 in the Eye Clinic of the University of Naples “Federico II”. Each patient underwent evaluation of best-corrected visual acuity (BCVA) according to the Early Treatment of Diabetic Retinopathy Study (ETDRS), Goldman applanation tonometry, gonioscopy, slit-lamp biomicroscopy, fundus examination with a + 90 D lens, standard visual field testing (perimetry), spectral-domain (SD)-OCT and OCT angiography. Pre-perimetric glaucomatous eyes were defined as those with optic nerve head changes such as localized rim thinning, increased cupping (a vertical cup-disc ratio of >0.7), and inter-eye cup asymmetry >0.2 but without visual field loss [11]. All patients had ocular hypertension and were undergoing therapy with timolol 0.5% (baseline IOP 22.2 ± 1.3 mmHg). Forty-eight normal eyes of 32 patients with a normal ophthalmic examination, no family history of glaucoma in a first-degree relative, and no history of intraocular surgery or retinal pathologic features served as control group. Normal eyes had a BCVA of 20/40 or better, intraocular pressure below 21 mmHg, a normal-appearing optic nerve head and normal visual field tests with normal glaucoma hemifield test results and a normal mean deviation (MD) and pattern standard deviation (PSD). Exclusion criteria were clinically relevant opacities of the optic media and low-quality images obtained with OCT, unreliable visual field results, history or evidence of intraocular surgery, and evidence of vitreoretinal disease, uveitis, diabetic retinopathy, or other diseases that could cause visual field loss or optic disc abnormalities.
The study was approved by our institutional review board, and informed consent was obtained from all individuals. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Spectral-domain optical coherence tomography
The mean circumpapillary retinal nerve fiber layer (cpRNFL) and ganglion cell complex (GCC) thickness were evaluated, after pupil dilation (minimum diameter 5 mm), with SD-OCT (RTVue-100 OCT, Optovue Inc., Fremont, CA, USA; software version/processing algorithm A5, 1, 0, 90) which captures 26,000 axial scans (A-scans) per second and provides a 5-μm depth resolution in tissue. The optic nerve head map protocol was used to evaluate the cpRNFL. This protocol generates a cpRNFL thickness map based on measurements obtained around a circle 3.45 mm in diameter centered on the optic disc. The GCC scan was centered 1 mm temporal to the fovea and covered a square grid (7 × 7 mm) on the central macula, and GCC thickness was measured from the internal limiting membrane to the outer boundary of the inner plexiform layer. Only high-quality images, as defined by a signal strength index above 50, were accepted. The examiner rejected scans that had motion artifacts, poor centration, incorrect segmentation, or poor focus [12].
Visual field
Visual field perimetry (Humprey Field analyzer with Swedish interactive thresholding algorithm (SITA) standard 30–2 test program (Carl Zeiss Meditec, Dublin, CA, USA) was considered reliable when fixation losses were less than 20%, and false-positive and false-negative errors were less than 15%. The perimeter software was used to calculate mean deviation (MD) and pattern standard deviation (PSD) [13].
Optical coherence tomography angiography
We obtained OCT angiography images with the Optovue Angiovue System (Optovue Inc., Fremont, CA, USA) which is based on split-spectrum amplitude decorrelation angiography. The instrument has an A-scan rate of 70,000 scans per seconds, with a tissue axial resolution of 5 μm and a 15-μm beam width. Each B-scan contained 304 A-scans. Two consecutive B-scans were captured at a fixed position before proceeding to the next sampling location. Size volumes were recorded, and the B-scan images were compared with each other to calculate decorrelation in the images [14]. Blood flowing through vessels causes a change in reflectance over time, and results in localized areas of flow decorrelation between frames. The spectrum of the light source was split into multiple component parts to decrease the noise present in the image; each part was used to perform the decorrelation step, and the results of all the split spectra were averaged. In any given region of tissue, the projection image can be viewed to obtain an image of the contained blood flow [15]. The peripapillary region was defined as a 700-μm-wide elliptical annulus extending from the optic disc boundary; the disc boundary for each subject was automatically delineated along the neural canal by OCT software thanks to reflectance images.
Cross-sectional registered reflectance intensity images and flow images were summarized and viewed as an en-face maximum flow projection from the inner limiting layer to the retinal epithelial pigment. The disc flow index was defined as the average decorrelation value within the disc, and is a dimensionless parameter between 0 and 1. As a result of the nonlinear relationship between decorrelation and flow velocity, the flow index mainly measured the capillaries area and the blood velocity. Peripapillary vessel density was defined as the percentage area occupied by the large vessels and microvasculature in the peripapillary region [9]. The flow index and vessel density in the peripapillary region were automatically calculated using the AngioVue software: (1) for the flow index, the software identified the optic nerve area and calculated the vessel area in mm2, and (2) for the vessel density, the specific tool was able to measure the percentage of vascular areas on en face angiograms [16]. Poor-quality images with a signal strength index less than 50 or image sets with residual motion artifacts (discontinuous vessel pattern or discontinuous disc boundary) were excluded from the analysis.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (Version 20.0 for Windows; SPSS Inc., Chicago, Ill, USA). The Mann–Whitney U test was used to evaluate differences in the GCC, RNFL, and visual field parameters between controls and patients with pre-perimetric glaucoma, and to evaluate differences in flow index and vessel density calculated with OCT angiography between controls and patients. Differences between OCT angiography parameters and traditional glaucoma measurements of function and structure such as GCC, RNFL, and visual field were evaluated with Spearman’s correlation. Finally, receiver operating characteristic (ROC) curves were obtained to assess the ability of flow index and vessel density to detect glaucomatous changes among patients. A p value of <0.05 was considered statistically significant.
Results
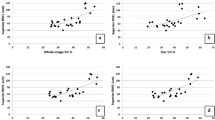
Twenty-six patients (12 females, 14 males, mean age 65.05 ± 11.54 years) for a total of 38 eyes examined were included in this prospective study. Mean BCVA was 0.16 ± 0.21 LogMAR and mean IOP was 15.68 ± 1.66 mmHg. The control group was constituted by 32 individuals (18 females, 14 males, mean age 59.20 ± 8.93 years) for a total of 48 eyes examined. There were no significant age differences between patients and controls. Both GCC and RNFL parameters were lower in patients than in controls. Similarly, flow index (U = 134, p = 0.021) and vessel density (U = 90, p = 0.001) were significantly lower in eyes affected by pre-perimetric glaucoma than in normal eyes. (Figs. 1 and 2) Visual field parameters did not differ between the two groups (Table 1). Ganglion cell complex parameters were significantly correlated with OCT angiography parameters (p < 0.05) (except in the case of flow index versus inferior GCC). No correlation was found between RNFL and OCT angiography parameters. Similarly, neither MD nor PSD was correlated with OCT angiography parameters. (Table 2)
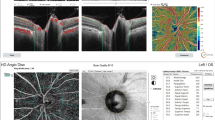
A 61-year-old man with preperimetric glaucoma in the left eye. a Color fundus image. b An OCT angiography scan of the optic disc shows a reduction of vessel density. c GCC significance map shows a small reduction of GCC thickness. The significance map is color-coded: green represents values within normal range (P 5–95%); yellow indicates borderline results (P < 5%), and red represents outside normal limits (P < 1%). d the cpRNFL thickness map shows normal values in all sectors. e No alterations were found at perimetry
Lastly, we calculated the area under receiver operating curve to assess the ability of OCT angiography to detect glaucoma in pre-perimetric patients. As shown in Fig. 3, the areas under the curve (AUCs) for vessel density (AUC 0.803, CI 0.551–0.861) and flow index (AUC 0.706, CI 0.647–0.931) best differentiated between control and pre-perimetric glaucoma eyes.
Discussion
In this prospective study, we used OCT angiography to detect peripapillary retinal perfusion changes in pre-perimetric glaucoma, and evaluated the relationship between optic disc perfusion and OCT and visual field parameters. This is the first study to use OCT angiography to analyze peripapillary vascular perfusion in patients affected by pre-perimetric glaucoma. Several previous studies have analyzed OCT angiography parameters in patients affected by various types of glaucoma [4, 9, 10, 17, 18], while the study conducted by Jia et al. [19] reported only a reduction of flow index and vessel density in pre-perimetric glaucoma patients but did not determine the relationship between these measurements and OCT and visual field parameters.
Here, we report that flow index and vessel density significantly discriminated pre-perimetric glaucoma patients from controls. We also found significant correlations between OCT angiography and GCC parameters. These results show the relationship between reduced blood flow and structural loss in glaucoma, and confirm the vascular physiopathology of glaucoma [5, 8, 18,19,20,21,22]. According to the vascular theory, glaucomatous optic neuropathy is a consequence of reduced ocular blood flow due to increased IOP or vascular risk factors (vascular dysregulation, local vasospasm, hypertension, and nocturnal hypotension) that lead to axonal ischemia [20, 23, 24]. Burgoyne et al. hypothesized that IOP exerts direct and indirect effects on axonal nutrition, and that axonal ischemia can result from either occlusion of the laminar capillaries (direct effect) or decreased diffusion of nutrients (indirect effect), or both [25].
An important anatomic result of our study is that reduced peripapillary blood flow significantly correlates with neural structure loss in pre-perimetric glaucoma patients. This finding suggests that perfusion and structural measurements are the first parameters that change in early glaucoma, while the absence of significant correlations between OCT angiography and visual field parameters suggests that in pre-perimetric glaucoma patients, ONH perfusion might be reduced before the manifestation of functional defects. These results, in agreement with previous studies, are consistent with the concept that visual field changes are less apparent than anatomical damage in the early stage of glaucoma [12, 26].
Our study has several limitations. The patient sample was relatively small, the effects of anti-glaucoma medication might have affected the results and, lastly, we performed only one OCT examination for each parameter.
In conclusion, perfusion and structural measurements may provide complementary information for the diagnostic evaluation of glaucoma. OCT angiography is a novel technology that detects blood flow by using intrinsic motion contrast within the microcirculatory network and does not need any intravenous dye. It is a practical method for the clinical evaluation of microvascular perfusion changes of the optic nerve head, to investigate the role of the microvasculature in the pathophysiology of glaucoma, and may be useful in early diagnosis. Further studies are needed to evaluate the exact potential of OCT-A in early diagnosis and in monitoring the progression of glaucoma.
References
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90(3):262–267
Congdon NG, Friedman DS, Lietman T (2003) Important causes of visual impairment in the world today. JAMA Ophthalmol 290(15):2057–2060
Gillespie BW, Musch DC, Guire KE, Mills RP, Lichter PR, Janz NK, Wren PA, CIGTS (Collaborative Initial Glaucoma Treatment Study) Study Group (2003) The Collaborative Initial Glaucoma Treatment Study: baseline visual field and test–retest variability. Invest Ophthalmol Vis Sci 44(6):2613–2620
Wang X, Jiang C, Ko T, Kong X, Yu X, Min W, Shi G, Sun X (2015) Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 253(9):1557–1564
Emre M, Orgül S, Gugleta K, Flammer J (2004) Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol 88(5):662–666
Chung HS, Harris A, Kagemann L, Martin B (1999) Peripapillary retinal blood flow in normal tension glaucoma. Br J Ophthalmol 83(4):466–469
Schmidl D, Garhofer G, Schmetterer L (2011) The complex interaction between ocular perfusion pressure and ocular blood flow—relevance for glaucoma. Exp Eye Res 93(2):141–155
Cennamo G, Finelli M, Iaccarino G, de Crecchio G, Cennamo G (2012) Choroidal thickness in open-angle glaucoma measured by spectral-domain scanning laser ophthalmoscopy/optical coherence tomography. Ophthalmologica 228(1):47–52
Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, Kraus MF, Fujimoto JG, Huang D (2014) Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 121(7):1322–1332
Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, Davis E, Morrison JC, Huang D (2015) Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol 133(9):1045–1052
Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, Varma R, Huang D (2009) Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 116(12):2305–2314
Hirashima T, Hangai M, Nukada M, Nakano N, Morooka S, Akagi T, Nonaka A, Yoshimura N (2013) Frequency-doubling technology and retinal measurements with spectral-domain optical coherence tomography in preperimetric glaucoma. Graefes Arch Clin Exp Ophthalmol 251(1):129–137
Anderson DR, Patella VM (1999) Automated static perimetry, 2nd edn. Mosby, St. Louis, pp 10–35
Chhablani J, Rao HB, Begum VU, Jonnadulla GB, Goud A, Barteselli G (2015) Retinal ganglion cells thinning in eyes with non-proliferative idiopathic macular telangiectasia type 2A. Invest Ophthalmol Vis Sci 56(2):1416–1422
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D (2012) Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 20(4):4710–4725
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M (2016) Optical coherence tomography angiography using the optovue device. Dev Ophthalmol 56:6–12
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, Yousefi S, Belghith A, Saunders LJ, Medeiros FA, Huang D, Weinreb RN (2016) Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci 57(9):451–459
Lévêque PM, Zéboulon P, Brasnu E, Baudouin C, Labbé A (2016) Optic disc vascularization in glaucoma: value of spectral-domain optical coherence tomography angiography. J Ophthalmol 2016:6956717
Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, Lu CD, Choi W, Fujimoto JG, Huang D (2013) Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 3(12):3127–3137
Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefánsson E (2002) The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 21(4):359–393
Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A (2000) Vascular risk factors for primary open angle glaucoma: the Egna–Neumarkt study. Ophthalmology 107(7):1287–1293
Petrig BL, Riva CE, Hayreh SS (1999) Laser Doppler flowmetry and optic nerve head blood flow. Am J Ophthalmol 127(4):413–425
Harris A, Kagemann L, Ehrlich R, Rospigliosi C, Moore D, Siesky B (2008) Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can J Ophthalmol 43(3):328–336
Resch H, Garhofer G, Fuchsjäger-Mayrl G, Hommer A, Schmetterer L (2009) Endothelial dysfunction in glaucoma. Acta Ophthalmol 87(1):4–12
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT (2005) The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 24(1):39–73
Cennamo G, Montorio D, Romano MR, Cardone DM, Minervino C, Reibaldi M, Cennamo G (2016) Structure–functional parameters in differentiating between patients with different degrees of glaucoma. J Glaucoma 25(10):884–888
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationship, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cennamo, G., Montorio, D., Velotti, N. et al. Optical coherence tomography angiography in pre-perimetric open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 255, 1787–1793 (2017). https://doi.org/10.1007/s00417-017-3709-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3709-7