Abstract
Purpose
To determine the molecular mechanisms underlying ocular ischemic syndrome (OIS). This study uses a rat model to evaluate the role of the RhoA/MEK1/ ERK1/2/iNOS pathways in response to OIS-associated oxidative and nitrosative stress, with a long-term goal of identifying therapeutic targets for OIS.
Methods
Rats were randomly allocated to one of three groups: bilateral occlusion of the common carotid artery (BOCCA), sham surgery control, or unoperated control (n = 8/group). Three months after the procedure, retinas were analyzed anatomically, using immunohistochemistry and by enzyme-linked immunosorbent assay for RhoA, MEK1, ERK1, ERK2, iNOS. Retinal injury was assessed using TUNEL. Levels of superoxide dismutase (SOD) and malondialdehyde (MDA) were measured by WST-1 and TBA methods, respectively.
Results
In BOCCA rats, occlusion of the bilateral common carotid artery induced degeneration of retinal ganglion cells, which was not observed in either control group. Retinal levels of RhoA, MEK1, ERK1, ERK2, iNOS, NOX2, and MDA were elevated in the BOCCA group, but not in either control group. In comparison, retinal levels of SOD were reduced in SOD animals. By immunofluorescent staining, RhoA was elevated in all retinal layers, while the increased levels of MEK, ERK1/1, and NOX were restricted to the INL, and that of ERK1/2 and NOX inner nuclear layer; iNOS elevations were observed in both the inner and outer nuclear layers. TUNEL labeling results showed that BOCCA group is higher staining than sham and control group.
Conclusions
OIS elevates activity of the RhoA/MERK1/ERK1/2/iNOS pathways throughout the retina, likely reflecting a response to oxidative and nitrosative stress. Retinal thickness was reduced in BOCCA rats, reflecting a loss of retinal ganglion cells following the reduced blood flow to the eye. These results indicate that drugs that inhibit these pathways may be effective treatments for OIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocular Ischemic Syndrome (OIS) is a disease encountered in cardiology, ophthalmology, neurology, and neurosurgery clinics. In general, patients with stenosis and carotid artery obstruction have a high incidence of OIS [1], reflecting involvement of the ocular or cerebral vasculatures. Ocular manifestations include transient amaurosis, impaired pupillary light reflex, and decreased visual sensitivity.
To understand the pathophysiological processes involved in OIS, Lavinsky et al. [2] developed an animal model based on bilateral occlusion of the common carotid arteries (BOCCA). This model induces the beginning of chronic retinal ischemia, but does not impact intraocular pressure or retinal artery perfusion. According to experiments before [2], the available evidence indicates that retinal dysfunction starts to present after a 2- to 3-month period of BOCCA as a result of strong compensatory ability in rats such as ganglion cells starting lost, although the underlying pathogenic mechanisms have not been fully characterized. It is known that apoptosis of retinal ganglion cells leading to visual defect. We suppose that there may have some potential way involving in OIS retina injury.
Rho, a critical member of the Rho family of more than 20 small GTPases, regulates many cellular activities. For example, Rho plays a role in cell adhesion, cell division, and morphogenesis [3–5]. RhoA regulates cytoskeleton organization, contraction, migration, and proliferation [6, 7]. Along with RhoA, a key effector in the RhoA downstream signaling pathway is Rho-kinase [8]. The RhoA/Rho-kinase pathway [8] has been implicated in cellular hypertension and ischemia/reperfusion (I/R) injury [9–11]. RhoA/Rho-kinase signaling may also play an important role in inflammatory responses: increased production of nitric oxide (NO) and up-regulation of adhesion molecules and nicotinamide adenine dinucleotide phosphate (NADPH) can elevate production of reactive oxygen species (ROS). By regulating NO and NADPH, RhoA can regulate these cellular events [10, 11].
Dynamic changes of mitogen-activated protein kinase (MAPK) signal transduction and its upstream signals are associated with inflammatory and cerebral ischemia secondary to subarachnoid hemorrhage [12, 13]. MAPK-induced expression of inflammatory mediators such as mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)/inducible nitric oxide synthase (iNOS) pathways also increase NO production [14–16]. Sari and co-workers [17] reported that RhoA regulates the MEK1/ERK1/2/iNOS pathways and downstream production of NADPH as well as the formation of peroxynitrite (ONOO-), which can result in the production of NO and proinflammatory cytokines.
Since Rho and the associated proteins have been shown to play a vital role in the inhibition of neuron axons after injury, we tried to make educated guesses that Rho may participate in OIS retinal injury. Furthermore, Rho kinase (ROCK) has been proven to play a vital role in the pathogenesis of ischemia/reperfusion (I/R)-induced retinal injury, but there is no more information between ROCK and its downstream factors in chronic retinal ischemia in OIS [18]. As ROCK and its downstream factors have been proven to participate in inflammatory and cell proliferation, we hypothesized that this signaling pathway may be involved in chronic retina ischemia injury. To date, the involvement of the RhoA/ MEK1/ERK1/2/iNOS pathway and NADPH oxidase in the BOCCA model of OIS has not been examined. In view of the many roles of this signaling pathway, we examined the role of RhoA in regulating the expression of the MEK1/ERK1/2/iNOS pathway and the production of NADPH oxidase. The results demonstrate their importance in the response to oxidative/nitrosative stress and inflammation associated with this model of retinal ischemia injury.
Materials and methods
All protocols were in accordance with the Guide for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China, and all animal procedures were approved by the Institutional Animal Care and Use Committee of Capital Medical University (Beijing, China). Brown Norway (BN; 200–250 g) rats were obtained from Capital Medical University. Animals were reared under a 12:12 light cycle, and room temperatures were maintained at 22 °C [2].
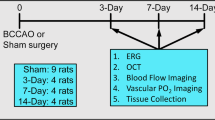
Rats were randomly allocated to one of three groups (n =8 per group): OIS, sham OIS, and control. OIS animals underwent a BOCCA procedure, under anesthesia induced by intraperitoneal injection of a 7 % solution of chloral hydrate (Sigma Chemical, St Louis MO, USA) at a dose of 0.42 mg/g body weight. After subcutaneous injection of 5 % procaine, the common carotid arteries were visualized through a ventral midline incision, separated from the carotid sheath and vagus nerve, and occluded with 3–0 silk suture. Sham OIS animals underwent all procedures, except occlusion of the common carotid arteries. After OIS or sham OIS surgery, the wound was closed and disinfected by medical grade alcohol, after which animals recovered in their home cage. After this initial recovery, animals were placed in a warm (36 °C) humidified container. Control animals did not undergo any surgical procedures. Experimental animals were followed for a 3-month period [2]. At this time point, animals were sacrificed by overdose with 10 % chloral hydrate, after which one eye was used for paraffin embedded and the other for biochemistry.
Histopathology and morphology of retina
Eyes were fixed in 4 % PFA for 1 h at 4 °C, and were then embedded in paraffin. Sections were cut at a thickness of 7 μm along the horizontal/vertical meridian through the optic disc. After staining with hematoxylin and eosin, we measured the number of cells in the ganglion cells(RGCs), and the thickness of the inner plexiform (IPL), inner nuclear (INL), outer plexiform (OPL), outer nuclear (ONL) layers. All measures were made at 400X magnification, approximately 200 μm from the optic disc using a light microscope (Leica DM6 B, Heidelberg, Germany). For each eye, five sections for each eye were measured and then averaged.
Double immunofluorescent staining
We double-labeled PFA-fixed retinal sections and obtained images using a confocal Laser Scanning microscope (Leica TCS SP8, Heidelberg, Germany). For RhoA and iNOS imaging, sections were incubated with a rabbit monoclonal antibody against rat RhoA (1:300 dilution, Abcam) and a rabbit polyclonal antibody against rat iNOS(1:100 dilution, Abcam) at 37 °C for 1 h, and subsequently with an Alexa Fluor 594 goat anti-rabbit IgG (1:200 dilution, Abcam) and an Alexa Fluor 488 goat anti-rabbit (1:200 dilution, Abcam) at 37 °C for 30 min. The procedure of MER1/ERK1/2 and NOX2/ERK1/2 were the same with RhoA/iNOS. The sections were incubated with a rabbit polyclonal antibody against rat MEK1(1:100, Cell Signaling Technology), a rabbit monoclonal antibody against rat ERK1/2(1:50, Cell Signaling Technology), a rabbit polyclonal antibody against rat NOX2 (1:100, Abcam) and a rabbit monoclonal antibody against rat ERK1/2(1:50, Cell Signaling Technology) respectively.
Biochemical analyses
Retinas were isolated from enucleated eyes in cold PBS. Prior to biochemical study, isolated retinas were disrupted using a tissue homogenizer.
In situ TUNEL labeling
Terminal deoxynucleotidyl transferase-mediated dUTP (2’-deoxyuridine 5’-triphosphate) nick-end labeling (TUNEL) assay was used to evaluate apoptosis of the retinal injury after treatment. The eyes were enucleated at a predetermined time point, and retinal paraffin sections were prepared as previously described. Apoptosis of the retinal tissue was assessed using the In Situ Cell Death Detection Kit (Merck, Germany) according to the manufacturer’s instructions. The sections were examined using light microscopy under × 400 magnification. TUNEL-positive cells were counted in six microscopic fields in each eye with three adjacent areas on both sides of the optic nerve head (1 mm from the optic nerve head). The obtained scores were averaged.
Enzyme-linked immunosorbent assay (ELISA) test
RhoA/MEK1/ ERK1/2/iNOS and NOX2 activities were measured by ELISA according to the manufacturer’s instructions using the following kits (all from Cloud-Clone Corp, Houston, TX, USA): RhoA Assay Kit, MEK1 ELISA Kit, ERK1/2 ELISA Kit, iNOS ELISA Kit, and NOX2 ELISA Kit. ELISA measures were made at 450 nm using a Multiskan™ FC (Thermo Fisher Scientific Inc, CA, USA), following the manufacturer’s guidelines.
Measurement of malondialdehyde (MDA) and superoxide dismutase (SOD)
MDA activity was depended on the measurement of pink color produced by interaction of barbituric acid with MDA caused by lipid peroxidation, using the MDA Assay Kit (Nanjing Jiancheng Institute of Biological Engineering, China). SOD activity was measured by using SOD Assay Kit (Nanjing Jiancheng Institute of Biological Engineering, China). Levels of SOD and MDA were measured by WST-1 and TBA methods, respectively.
Statistical analysis
Results are expressed as the median (interquartile range) deviation. Statistical analyses were performed by Mann–Whitney U test followed for between-group comparison using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). Differences were considered statistically significant at P < 0.05.
Results
Histological analysis of retinas
Figure 1a compares representative retinal sections obtained from an animal in each experimental group. While no difference was noted between the overall thickness of control and sham OIS retinas, nor of any cellular or plexiform layer, the overall retinal thickness of the OIS retina was significantly thinner (Fig. 1b). This thinning did not reflect a change in the ONL, which was not different in the OIS retina as compared to control (Fig. 1f). Instead, this thinning reflects a significant reduction in the thickness of the INL, IPL, and OPL in the OIS retina (Fig. 1c-e), along with a significant reduction in the number of cells within the RGC layer (Fig. 1g).
Retina histopathology and morphology results. Retina histopathology and morphology of Control group, Sham OIS group, OIS group (a). Scale bar: 50 nm. Comparison of retinal thickness after 3 months (b-f), the number of cells within the RGC layer Bars (g), all indicate median (interquartile range) (n = 8). * p < 0.01 vs. Sham group; # p < 0.01 vs. Control group; ∆ p > 0.05 vs. Control group
Effect of OIS on RhoA/MEK1/ERK1/2/iNOS protein and NOX2 activities
To understand the basis of these anatomical changes, we measured levels of RhoA, MEK1, ERK1, ERK2, iNOS, and NOX2 in OIS and control retinas (Fig. 2). In comparison to control and OIS sham retinas, which did not differ (all p > 0.05), the levels of these proteins were significantly elevated in OIS retinas (Fig. 2a-f).
The effect of the RhoA/MEK1/ERK1/2/iNOS signaling pathway and NOX2 in OIS rat retinas. Activities of RhoA (a), MEK1 (b), ERK1 (c), ERK2 (d), iNOS (e), NOX2 (f) in rat retinas of each group. The figure shows that the RhoA/MEK1/ERK1/2/iNOS pathway and NOX2 was highly expressed in OIS rat retina model compared with Control group and Sham OIS group. Bars indicate median (interquartile range) (n = 8). * p < 0.01 vs. Sham group; # p < 0.01 vs. Control group; ∆ p > 0.05 vs. Control group
Confocal laser immunohistochemistry
We further examined the distribution of these proteins by immunofluorescence analysis by confocal microscopy, sections 4 μm thick were cut in the vertical meridian through the optic disc. In the control retina, levels of iNOS and RhoA were quite low (Fig. 3). In comparison, iNOS and RhoA labeling was elevated in all layers of the OIS retina (Fig. 3). When we examined the distribution of ERK1/2, we noted that it was present in all layers of the control retinas and was elevated in the OIS retina (Figs. 4 and 5). In comparison, MEK (Fig. 4) and NOX2 (Fig. 5) expression was seen at very low levels in the control retina, and was elevated in the OIS retina.
Apoptosis evaluation
To evaluate apoptosis, TUNEL staining (Fig. 6) was performed. Higher levels of apoptotic bodies were observed either in the inner nuclear layers (INL) and outer plexiform layers (ONL) of the retina and in retinal ganglion cells (RGC) of all BOCCA groups as compared to the control and sham groups (Fig. 6). In addition, we measured the IOD changes in retinas between three groups. The results showed that IOD/Area rate significantly increased in the model group compared with the sham group (p < 0.01).
Effect of MDA levels and SOD activity
To determine the effect of OIS in lipid peroxidation, we measured retinal levels of MDA. As shown in Fig. 7a, MDA levels were increased in the OIS retinas (p < 0.01; Fig. 7a) as compared with sham OIS and control groups. We also measured SOD, and noted that retinal levels were decreased in the OIS model (p < 0.01; Fig. 7b) as compared with either control group.
Discussion
While OIS is a recognized clinical entity, the cellular mechanisms underlying this condition have not yet been elucidated. In the present study, we use a rat model of OIS to examine the role of Rho/ROCK signaling, as these pathways have been reported to make an important contribution in I/R injury. The results of the present study indicate that overproduction of NO by the activation of the RhoA/MEK1/ERK1/2/iNOS pathway associate with overproduction of NO and lipid peroxidation to the retina injury in OIS rat model.
As OIS is recognized as a chronic retinal ischemic injury, early study has been performed and the results showed that there are no significant changes either in the rats’ retinal basic function or biochemical tests until 2–3 months after BOCCA, due to the rat compensatory function. We chose 3 months as a time point and compared different changes in each group.
In our study, we documented high expression of RhoA throughout the rat retina (Figs. 2 and 3). This expression pattern agrees with a study of the mouse retina by Mitchell and colleagues [19]. I/R injury is encountered in many tissues, and in many clinically relevant conditions. Across these, nitrosative stress has been shown to be a vital factor involved in I/R cellular damage. In this model, iNOS expression is up-regulated by I/R injury (Figs. 2 and 3), inducing overexpression of NO a biological signaling molecule which can generate superoxide anion (O2 −), creating peroxynitrite (ONOO−), which then leads to lipid peroxidation as well as cellular apoptosis and necrosis. Those secondary events involve tyrosine residues on target tissue proteins [20]. COX-2, induced by cytokines and growth factors, is a key protective factor against I/R injury and inflammation [21]. By binding to iNOS, COX-2 decreases prostaglandin formation [22], in a MAPK-dependent faction [23–25]. MAPKs, including RhoA, MEK1/2, ERK1/2, are important mediators of inflammatory damage. RhoA, a member of the Ras family, has two main stable states, bound either to guanosine diphosphate (GDP) or guanosine triphosphate (GTP). The active form of RhoA, RhoA-GTP, regulates multiple downstream targets, including Raf kinase, which phosphorylates MEK1/2, which in turn activates ERK1/2.This MEK/ERK pathway plays many biological roles, including modulation of iNOS [26]. In a hindlimb I/R injury model, Sariet al. [17] reported the roles of NO and superoxide in response to oxidative and nitrosative stress, although they did not evaluate the role of the RhoA/MEK1/ERK1/2/iNOS pathway.
In the present study, we also examined MAPK and Ras/Raf/MEK/ERK pathways, which plays important roles in mitosis [27]. In the OIS rat model, the up-regulation of MEK1/ERK1/2 may reflect its function in cell proliferation (Fig. 6). Along with chronic retina ischemia, INL cell apoptosis and activation of RhoA, downstream MEK1/ERK1/2 pathways were activated, and ERK1/2 were then transferred into the nucleus (Figs. 3, 4, and 5).
Since changes of nNOS are valuable to comprehensively understand the pathogenesis of retinal ischemia [28, 29], we tested the expression of iNOS in BOCCA rats in this study (Fig. 3). As NO, a catalyzed product of iNOS, is a controversial molecule. NO can exert a deleterious effect on cells by generating toxic oxynitride, it also meditates blood supply maintenance by dilating blood vessels. NO could react with superoxide, which mainly generated by NOX2 (NOX2; a superoxide generating NOX enzyme) and p47phox (NOXO2; organizer subunit of NOX2) to form peroxynitrite (a powerful oxidant and nitrating molecule), and could lead to nitrotyrosine formation by reacting with proteins subsequently. Folino A et al. and Han J et al. [26, 30] have proved that peroxynitrite got involved in NO-dependent pathogenic mechanism while body was enduring with ischemia/reperfusion or inflammatory. According to iNOS and NOX2 expression results (Figs. 3 and 5), we observed that NO significantly get involved in retinal ischemia injury.
According to the early research, selectively pharmacological blockage of MAPK pathway, drugs such as U0126 (a MAPK/ERK kinase inhibitor), can significantly prevent functional loss of the RGCs [29, 31]. In addition, another study showed that MAPK pathway get involved in iNOS production and result in retina ischemia injury during ischemia and reperfusion [28]. Since ERK/iNOS pathway has been once reported get involved in retina chronic ischemia, according to results above we could easily conclude that RhoA/MEK/ERK/iNOS pathway may participated in BOCCA rat model.
The results of this study indicated that OIS rat model caused a decrease in SOD and an increase in MDA (Fig. 7), as markers of oxidative stress and an index for lipid peroxidation, respectively. In OIS model retina would bring about large amount of free radical concentrating. In dealing with this abnormal, radical scavenging system auto-started to suppressed consumption and maintain homeostasis. It was known that oxidative stress uploaded secondary to acute ischemia and regulating ROCK expression. Therefore, these results suggest that activation of the RhoA/MEK1/ERK1/2/iNOS pathway associated with oxidative/nitrosative stress and inflammation contributes to OIS induced chronic ischemia injury in rat retinas (Fig. 8).
In the OIS retina, the result in consistent with the impression that RhoA plays many roles in the retina, from development and differentiation, but with a substantially decreased role in adulthood [19]. Szabadfi [32, 33] reported that retinal ischemia would activate tissue inflammatory responses and apoptosis (Fig. 6), resulting in neurodegeneration. Taken together, we propose that the active form of RhoA, namely GTPase bound RhoA, makes a greater contribution to transduce signals from extracellular and regulate cell growth.
Consistent with a prior report [13], the IPL, INL, ONL and GCL layers were significantly reduced in our OIS model (Fig. 1). These changes are likely secondary to the obstructed choroidal circulation achieved after BOCCA. The central retinal artery is the main vasculature to the inner retina. In the BOCCA model, blood flow through the central artery is greatly diminished, leading to inner retinal ischemia [34].
In this study, we did not determine whether the expression changes in the RhoA/MEK1/ERK1/2iNOS pathway were also altered in the brain, but note that Allen et al. [35] have shown that the BOCCA model is associated with brain damage due to the increased levels of oxidative stress and the decreased metabolic support. Using a model of middle cerebral artery occlusion (MCAO), Koh et al. [36] linked the brain injury to activity within the Raf/MEK1/ERK1/2/RSK/Bad pathway. While not specifically examined, we can assume that the BOCCA model used here will also increase activity of the RhoA/MEK1/ERK/1/2/iNOS pathway in the rat brain.
In conclusion, the present study provides evidence that BOCCA induced chronic retinal ischemia leads to inflammation via activation of the RhoA pathway resulting in increased levels of MEK1, ERK1/2, and iNOS expression, and subsequent development of oxidative and nitrosative stress. Figure 8 presents a diagram of this pathophysiological model. Although this model identifies a number of points at which the pathophysiological process may be ameliorated, the observed up-regulation of the RhoA/MEK1/ERK1/2/iNOS pathway may not be the only pathway underlying oxidative and nitrosative stress during OIS retina injury, and further research is needed to completely characterize this model. Nevertheless, the information provided here suggest that selective inhibitors or drugs could be beneficial in preventing retinal degeneration by restoration of expression and/or activity of the RhoA/ MEK1/ERK1/2/iNOS pathway as well as oxidant/antioxidant balance in the OIS rat model and point out a new method of dealing with OIS retina injury.
References
Terelak-Borys B, Skonieczna K, Grabska-Liberek I (2012) Ocular ischemic syndrome - a systematic review. Med Sci Monit 18:RA138–144
Lavinsky D, Arterni NS, Achaval M, Netto CA (2006) Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefes Arch Clin Exp Ophthalmol 244:199–204
Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–69
Heasman SJ, Ridley AJ (2008) Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9:690–701
Chi X, Wang S, Huang Y et al (2013) Roles of rho GTPases in intracellular transport and cellular transformation. Int J Mol Sci 14:7089–108
Amano M, Fukata Y, Kaibuchi K (2000) Regulation and functions of Rho-associated kinase. Exp Cell Res 261:44–51
Olson MF (2004) Contraction reaction: mechanical regulation of Rho GTPase. Trends Cell Biol 14:111–4
Ong TJ, Paine M, O’Day J (2002) Retinal manifestations of ophthalmic artery hypoperfusion. Clin Experiment Ophthalmol 30:284–91
Schofield AV, Bernard O (2013) Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol 48:301–16
Noma K, Kihara Y, Higashi Y (2012) Striking crosstalk of ROCK signaling with endothelial function. J Cardiol 60:1–6
Satoh K, Fukumoto Y, Shimokawa H (2011) Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol 301:H287–96
Dumont AS, Dumont RJ, Chow MM et al (2003) Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 53:123–33, discussion 133–5
Kaminska B (2005) MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754:253–62
Katada K, Bihari A, Badhwar A et al (2009) Hindlimb ischemia/reperfusion-induced remote injury to the small intestine: role of inducible nitric-oxide synthase-derived nitric oxide. J Pharmacol Exp Ther 329:919–27
Kuntscher MV, Kastell T, Altmann J et al (2002) Acute remote ischemic preconditioning II: the role of nitric oxide. Microsurgery 22:227–31
Li G, Labruto F, Sirsjo A et al (2004) Myocardial protection by remote preconditioning: the role of nuclear factor kappa-B p105 and inducible nitric oxide synthase. Eur J Cardiothorac Surg 26:968–73
Sari AN, Kacan M, Unsal D et al (2014) Contribution of RhoA/Rho-kinase/MEK1/ERK1/2/iNOS pathway to ischemia/reperfusion-induced oxidative/nitrosative stress and inflammation leading to distant and target organ injury in rats. Eur J Pharmacol 723:234–245. doi:10.1016/j.ejphar.2013.11.027
Song H, Gao D (2011) Fasudil, a Rho-associated protein kinase inhibitor, attenuates retinal ischemia and reperfusion injury in rats. Int J Mol Med 28:193–198. doi:10.3892/ijmm.2011.659
Mitchell DC, Bryan BA, Liu JP et al (2007) Developmental expression of three small GTPases in the mouse eye. Mol Vis 13:1144–53
Chun J, Choi RJ, Khan S et al (2012) Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-kappaB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol 14:375–83
Park JY, Pillinger MH, Abramson SB (2006) Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119:229–40
Kim SF, Huri DA, Snyder SH (2005) Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310:1966–70
Li XN, Su J, Zhao L et al (2013) The p38 MAPK inhibitor JLU1124 inhibits the inflammatory response induced by lipopolysaccharide through the MAPK-NF-kappaB pathway in RAW264.7 macrophages. Int Immunopharmacol 17:785–92
Shi Q, Cao J, Fang L et al (2014) Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int Immunopharmacol 20:298–306
Kim KN, Ko YJ, Yang HM et al (2013) Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-kappaB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem Toxicol 57:126–31
Folino A, Losano G, Rastaldo R (2013) Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. J Cardiovasc Pharmacol 62:567–75
Sebastian S, Settleman J, Reshkin SJ et al (2006) The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta 1766:120–39
Guo XJ, Tian XS, Ruan Z et al (2014) Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp Eye Res 125:156–163. doi:10.1016/j.exer.2014.06.003
Fang I-M, Yang C-M, Yang C-H (2015) Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp Eye Res 130:38–50. doi:10.1016/j.exer.2014.12.001
Han J, Shuvaev VV, Muzykantov VR (2012) Targeted interception of signaling reactive oxygen species in the vascular endothelium. Ther Deliv 3:263–76
Wheeler LA, Gil DW, WoldeMussie E (2001) Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv Ophthalmol 45(Suppl 3):S290–294, discussion S295–296
Szabadfi K, Danyadi B, Kiss P et al (2012) Preconditioning with volatile anaesthetic sevoflurane in ischemic retinal lesion in rats. J Mol Histol 43:565–9
Osborne NN, Casson RJ, Wood JP et al (2004) Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 23:91–147
Osborne NN, Safa R, Nash MS (1999) Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vis Res 39:3995–4002
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4:461–70
Koh PO (2015) Ferulic acid attenuates the down-regulation of MEK/ERK/p90RSK signaling pathway in focal cerebral ischemic injury. Neurosci Lett 588:18–23
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
National Science Foundation of China provided financial support in the form of quota subsidy funding (No. 81173412).
The sponsor had no role in the design or conduct of this research.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Jia-lin Wang and Yan-ling Wang contributed equally to this work presented and they are both corresponse to this manuscript.
Rights and permissions
About this article
Cite this article
Du, R., Wang, Jl. & Wang, Yl. Role of RhoA/MERK1/ERK1/2/iNOS signaling in ocular ischemic syndrome. Graefes Arch Clin Exp Ophthalmol 254, 2217–2226 (2016). https://doi.org/10.1007/s00417-016-3456-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3456-1