Abstract
Background
Ocular ischemic syndrome is a devastating eye disease caused by severe carotid artery stenosis. The reduction of blood flow produced by bilateral common carotid artery occlusion (BCCAO) of rats for 7 days induces events related to gliosis with no evident histological damage. However, retinal degeneration and cellular death occur after 90 days of BCCAO. Our purpose has been to investigate the effects of BCCAO for 30 days in the retina of adult rats.
Methods
Adult Wistar rats were submitted to BCCAO or sham surgery. Both direct and consensual pupillary light reflexes were investigated before and after surgery, everyday for the first week and weekly for 30 days. After 1 month, eyes were enucleated and embedded in paraffin. The retinal ganglion cell (RGC) density and thickness of the internal plexiform (IPL), internal nuclear, outer plexiform, and outer nuclear layers were estimated.
Results
Four rats of the BCCAO group (50%) lost the direct pupillary reflex in both eyes, three rats (37%) lost this reflex in one eye, and only one (13%) maintained it in both eyes. RGC density (cells/mm) was diminished in the BCCAO group, and a significant decrease was found in the total retina and IPL thickness; however, no changes were evident in the other layers. BCCAO pupillary-reflex-negative rats presented with a significant decrease in total retinal thickness and retinal ganglion cell density compared with the sham group. Both BCCAO pupillary-reflex-positive) and -negative rats showed a decrease in IPL compared with the sham group.
Conclusion
This study demonstrates that BCCAO for 30 days induces functional and morphological damage to the retina with loss of the pupillary reflex and a decrease in IPL thickness and RGC number. We suggest that this protocol might be used as a model for ocular ischemic syndrome in the rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocular ischemic syndrome (OIS) is a devastating disease elicited by chronic ischemia of the anterior and posterior segments of the eye. It seems to be caused by prolonged hypoperfusion secondary to carotid artery stenosis greater than 90% [9]. This syndrome is characterized by loss of visual acuity, inflammation of the conjunctiva, cornea and anterior uvea, iris neovascularization, mid-dilated poorly reactive pupil, cataract, neovascular glaucoma and mid-peripheral retinopathy with dot and blot hemorrhages, microaneurysms, retinal arteriolar narrowing, capillary non-perfusion, and optic disc neovascularization [3, 14, 16].
Beti et al. [5] have reported bilateral OIS as the sole manifestation of severe bilateral stenosis of the internal carotid arteries, and Costa et al. [6], in a case-controlled study, have demonstrated that patients with severe bilateral carotid artery occlusion had a greater risk of developing OIS [11]. Treatment options for this conditions are limited, with carotid endarterectomy, panretinal photocoagulation, and the control of intraocular pressure being the few current possibilities [14].
Slakter et al. [21] were amongst the first groups to develop an animal model for carotid artery occlusive disease. Experimental data demonstrated that bilateral common carotid artery occlusion (BCCAO) in the rat resulted in an incomplete retinal ischemia, leading to chronic ganglion cell loss, optic nerve, and other cerebral degeneration [22, 26]. Barnett and Osborne showed that the reduction of blood flow by BCCAO for 7 days induced events related to gliosis with increased levels of glial fibrillary acidic protein, although it did not cause evident histological damage [3, 4, 17]. However, the loss of the direct pupillary light reflex appears during this period in the majority of the animals [7, 22], and retinal degeneration, cellular death, and permanent depression of a- and b-wave amplitude on electroretinography occurs after 90 days of BCCAO [3, 7, 18].
Limited data are available from morphological studies of this model in the period between 15 days and 3 months after occlusion, a time at which retinal damage becomes evident [4, 22]. Therefore, our purpose has been to investigate the pupillary reflex and the histopathological effects of permanent BCCAO in rats for 30 days.
Materials and methods
Animals
Six-month-old male Wistar rats (n=15, from the Animal House of the Federal University of Rio Grande do Sul) were housed in a 12-h reverse light cycle with free access to food and water. All investigations and procedures were conducted in accordance with the principles of laboratory animal care (NIH publication no. 85-23, revised 1985), the OPRR Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 1986), and the US Animal Welfare Act.
Surgical procedure
Animals were randomly assigned to either BCCAO or sham surgery groups. The procedure was performed under anesthesia with an intraperitoneal injection of 7% chloral hydrate in saline (0.42 mg/g body weight) [8]. A ventral midline incision was made, and the common carotid arteries were bilaterally separated from the carotid sheath and vagus nerve. Each artery was rigidly occluded with 3–0 silk sutures. Animals assigned to sham surgery were submitted to the same procedure without occlusion of the arteries [22].
Pupillary light reflex
Each animal was examined for direct and consensual pupillary light reflexes with a beam of light from a direct ophthalmoscope before and after surgery, everyday for the first week and weekly for 30 days. The examiner was blind to whether animals had been submitted to BCCAO or sham surgery. Each animal was first adapted to darkness for 5 min, and then a beam of light was first directed to the right eye for evaluation of direct pupillary light reflex. Subsequently, the beam was quickly moved to the left eye to assess the consensual reflex. The rat was left in the dark for 1 min for readaptation, and then the same procedure was repeated for the left eye. Loss of the pupillary reflex was defined as the failure of pupil constriction after exposure to a beam of light [7, 22].
Tissue preparation and histology
After 1 month, animals were anesthetized with chloral hydrate and transcardially perfused with paraformaldehyde. The eyes were enucleated and immersed in 10% formalin for at least 24 h. Eyes were dehydrated, cleaned, and embedded in paraffin and then cut at a thickness of 7 μm by using a microtome. Slices were stained with hematoxylin-eosin. The number of cells in the retinal ganglion cell layer (RGCL), the thickness of the inner plexiform (IPL), inner nuclear (INL), outer plexiform (OPL), and outer nuclear (ONL) layers, and the total thickness were estimated in a masked fashion with a Nikon Eclipse E 600 (400×) microscope coupled to a pro-series high-performance charge-coupled device camera and image Pro Plus software 4.1 (Media Cybernetics, Silver Spring, Md., USA).
The standard area for measurement was at the central retina, approximately 200 μm from the optic disc. The number of cells in the RGCL was estimated as the liner cell density (cells/mm). In each retina, the number of cells and the thickness (μm) of the IPL, INL, OPL, and ONL were obtained as the mean value of five different measurements. No attempt was made to differentiate cell types in the RGCL [1, 2]
Statistical analysis
Data are expressed as the mean and standard error of the mean (SEM). Statistical analysis was performed with the Student's t-test and one-way ANOVA, followed by the Duncan multiple-range test, when indicated. Differences were considered significant when P was <0.05.
Results
Eight rats were submitted to BCCAO surgery and seven to sham procedure. Animal behavior was observed during the experiments; no differences were observed in motion or feeding between groups.
Pupillary light reflex
All animals exhibited the pupillary light reflex before surgery. Four rats from the BCCAO group lost the direct pupillary reflex in both eyes (50%), three lost in this reflex in one eye (37%), and only one maintained it in both eyes (13%). Loss of the pupillary reflex occurred in the first 3 days after surgery, and its status did not change during the subsequent days and weeks after surgery. The consensual reflex was preserved in those rats with unilateral direct pupillary loss. No animal from the sham group lost the pupillary reflex.
Histology
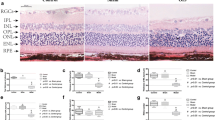
A total of 16 eyes from the BCCAO group and 14 eyes from sham group was analyzed. The retinal ganglion cell number from the BCCAO group was diminished (38.1±3.81) compared with that of the sham group (55.50±2.75; Figs. 1, 3a). There was a significant decrease in the thickness (μm) of the total retina (94.85±6.21×120.64±6.73) and IPL (35.47±3.16×52.12±3.36; P<0.05; Figs. 2, 3b, c) of the BCCAO group, but no evident changes in the other layers were demonstrated (Fig. 3d).
Photomicrographs of rat retina, stained with hematoxylin and eosin (H&E), showing the ganglion cell layer and inner plexiform layer from rats submitted to sham surgery (a) and to bilateral common carotid artery occlusion (BCCAO; b) for 30 days. The number of cells was diminished in the BCCAO group when compared to the sham group (P<0.05). Bar 50 μm
Photomicrographs stained with H&E showing a global view of the rat retina (RGCL retina ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, TRT total retina). a Sham group retina. b BCCAO retina. Asterisks Total retina, IPL thickness, and ganglion cell number were diminished in the BCCAO group (P<0.05). Bar 50 μm
Effect of bilateral common carotid artery occlusion (BCCAO) or sham surgery (SHAM) on retinal ganglion cell density (a), total retinal thickness (b), inner plexiform layer (c), and inner nuclear layer, outer plexiform layer, and outer nuclear layer (d). Retinal ganglion cell number and layer thickness are expressed as mean±SEM of cells per millimeter (cells/mm) and layer thickness (μm), respectively. *P<0.05; Student's t-test
Pupillary reflex and histology
We further divided the BCCAO group into eyes that had lost the pupillary reflex and those that had preserved it. Five eyes maintained the pupillary reflex and were classified as the BCCAO-PR+ group (pupillary-reflex-positive); 11 eyes had lost the pupillary reflex and were termed the BCCAO-PR− group (pupillary-reflex-negative). The same 14 eyes from the sham group were used as controls.
The BCCAO-PR− group presented with a significant decrease in total retinal thickness (87.16±6.94) compared with the sham group (120.64±6.73), but not with the BCCAO-PR+ group (111.75±6.21). However, the number of retinal ganglion cells was lower in the BCCAO-PR− group (34.45±4.02) than in either of the other groups (48.40±7.21 and 55.50±2.75). Interestingly, the thickness of the IPL was diminished in the BCCAO-PR+ (37.94±4.64) and BCCAO-PR− (34.35±4.17) groups with regard to the sham group (52.12±3.36). The ONL thickness showed a trend to decrease in the BCCAO-PR− rats (39.43±4.36×46.48±4.7×50.21±2.8; P=0.1), but no differences were identified in other retinal layers (Fig. 4).
Effect of BCCAO in the presence or absence of the pupillary reflex (BCCAO-PR+ or BCCAO-PR−) or of sham surgery (SHAM) on the retinal ganglion cell density (a), total retinal thickness (b), inner plexiform layer (c), and inner nuclear layer, outer plexiform layer, and outer nuclear layer (d). Retinal ganglion cell number and layer thickness are expressed as mean±SEM of cells per millimeter (cells/mm) and layer thickness (μm), respectively. *P<0.05 compared with sham group, **P<0.05 compared with the sham and BCCAO-PR+ groups; one-way ANOVA test
Discussion
BCCAO of rats produces retinal damage and functional deficits comparable with human retinal ischemic syndromes. Major cerebral arteries show many similarities between humans and rats, including anomalies in their general organization, the structure of these vessels at the light and electron microscope levels, and their morphological changes associated with cerebral vascular diseases. Consequently, the rat might be a suitable animal model for the study of human cerebral vascular diseases [13].
We have found that the inner retina suffers early damage after BCCAO; this supports results from other authors [7, 12, 22]. The inner retinal layers are nourished by branches of the central retinal artery, which, in the rats, is a direct branch of the posterior ciliary artery, which travels along the ventromedial aspect of the optic nerve [19]. Occlusion of the common carotid arteries probably decreases blood flow to this vessel causing severe hypoxia, at least for the first few days.
We have demonstrated that chronic hypoperfusion secondary to BBCAO for 30 days causes mainly a decrease in the number of cells in the RGCL and in the thickness of the IPL, with no other significant changes. Better histological analysis must be performed in order to conclude that the INL and OPL are not affected in animals submitted to BBCAO surgery, although the presented results are consistent with other findings [18]. These results resemble the consequences of central artery occlusion, a condition that causes complete inner retinal ischemia, while maintaining the structural integrity of the outer retina. We have also identified a trend to decrease in the ONL thickness in rats with pupillary reflex loss. Damage to the photoreceptors has been suggested to occur secondary to ganglions cell loss, which induces mydriasis allowing excessive light exposure to the eye thereby producing phototoxicity and neuronal death [18, 22].
The majority of the animals of our study presented loss of pupillary reflex in at least one eye. Takamatsu et al. [24] have demonstrated optic nerve atrophy after performing BCCAO in rats, and more recently, Davidson et al. [7] and Stevens et al. [22] have shown the loss of pupillary reflex and ONL damage using this model. This injury is probably secondary to acute ONL hypoxia/ischemia attributable to decreased blood flow on the first few days of BCCAO. After 14 days of BCCAO, the most severe white matter lesions were observed in the optic tract and other white matter areas [7, 15, 25, 26]. It is not clear whether the retinal pathology present in this model and its consequent pupillary reflex loss is secondary to direct retinal ischemia or retrograde degeneration caused by optic tract lesions [19].
BCCAO reduces cortical and hippocampal blood flow to 25%–50% of normal levels at 2.5 h post-occlusion, and 7 days later, the reduction in flow is approximately 60%–75% and remains at this level for at least several months [7, 20]. We have identified that most of the pupillary reflex loss occurs during the first 3 days after surgery; these animals show a significant increase in retinal damage when compared with those submitted to BCCAO with preserved pupillary reflex. However, some injury occurred in BCCAO-PR+ group, which showed a significant decrease in IPL thickness compared with the sham group. Since the surgical procedure was performed in the same manner for all rats, the variability present in this study may have occurred secondary to differences in the vascular bed of these rats [13]. Thus, pupillary reflex loss might indicate whethereffective and severe retinal damage is caused by an acute or chronic blood flow decrease.
Retinal pathology is an important consequence of ocular ischemic syndrome in humans presenting with venous dilation, retinal hemorrhage, microaneurysms, and an easily induced retinal artery pulsation. Moreover, ischemic changes include retinal arteriolar narrowing, retinal capillary non-perfusion, macular edema, and optic disk and retinal neovascularization [9, 10, 14, 16]. Atrophy of the optic nerve and dilated poorly reactive pupils have been seen in several of these patients and also in those suffering from retina ischemia secondary to other conditions [28]. These findings are similar to those in the BCCAO model, which probably shares at least some pathophysiology characteristics with the human disease.
We suggest that this rat model may be used in the search for new therapeutic strategies for OIS and other conditions caused mainly by chronic retinal ischemia/hypoperfusion. Optic nerve damage secondary to chronic cerebral and ONL hypoperfusion may be prevented by drugs such as the sodium channel blocker mexiletine [23]. Wakita et al. [27] have demonstrated that Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage, including damage to the optic nerve tract, after 14 days of BCCAO. Additionally, Drago et al. [8], also using the BCCAO model, have shown that animals with retinal ischemia induced by arterial occlusion present with an increase in lactate and a decrease in glucose and ATP, and that these biochemical changes are reversed with the use of Latanoprost and MK-801. As none of these studies have tested the pupillary reflex, it remains to be determined whether neuroprotection therapeutics may prevent or even reverse pupillary reflex loss in rats submitted to BCCAO. However, since the variability of retinal damage seems to be secondary to BCCAO, the use of this model for neuroprotection studies might require larger groups of animals in order to obtain conclusive data.
Ocular ischemic syndrome is a serious and potentially blinding eye disease. It is rare in the general population, making human studies difficult to perform. We suggest that BCCAO causes retinal ischemia in the rat comparable to OIS in humans. Consequently, this model may contribute to the understanding of the human condition and the development of new therapeutic strategies. Furthermore, we suggest that pupillary reflex may serve as a functional sign of retinal and optic nerve damage, being useful as an in vivo tool for the assessment of therapeutic success in experimental studies associated with subsequent histopathological analysis.
References
Adachi K, Kashii S, Masai H, Ueda M, Kaneda K, Kume T, Akaike A, Honda Y (1998) Mechanism of the pathogenesis of glutamate neurotoxicity in retinal ischemia. Graefe Arch Clin Exp Ophthalmol 236:766–774
Arteni NS, Salgueiro J, Torres I, Achaval M, Netto CA (2003) Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat. Brain Res 973:171–178
Bachman JA (1995) Exacerbation of ocular ischemic syndrome following cataract surgery. Clin Eye Vis Care 7:139–142
Barnett NL, Osborne NN (1995) Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res 61:83–90
Beti NF, Mateo I, La Calle VD, Ruiz J, Beldarrain MG, Garcia-Monco JC (2003) The ocular ischemic syndrome. Clin Neurol Neurosurg 106:60–62
Costa VP, Kuzniec S, Molnar LJ, Cerri GC, Puech-Leao P, Carvalho CA (1997) Clinical findings and hemodynamic changes associated with severe occlusive carotid artery disease. Ophthalmology 104:1994–2002
Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA (2000) Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res 859:96–103
Drago F, Valzelli S, Emmi I, Marino A, Scalia CC, Marino V (2001) Latanoprost exerts neuroprotective activity in vitro and in vivo. Exp Eye Res 72:479–486
Dugan JD Jr, Green WR (1991) Ophthalmologic manifestations of carotid occlusive disease. Eye 5:226–238
Jacobs NA, Ridway AEA (1985) Syndrome of ischaemic ocular inflammation: six cases and a review. Br J Ophthalmol 69:681
Karakose S, Karabacakoglu A, Solak H (2002) Bilateral common carotid occlusion without neurological deficit. Australas Radiol 46:412–415
Kobayashi M, Kuroiwa T, Shimokawa R, Okeda R, Tokoro T (2000) Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol 44:235–244
Lee RM (1995) Morphology of cerebral arteries. Pharmacol Ther 66:149–173
Malhotra R, Gregory-Evans K (2000) Management of ocular ischaemic syndrome. Br J Ophthalmol 84:1428–1431
Miyamoto E, Nakao S, Tomimoto H, Wakita H, Yamada M, Masuzawa M, Takahira K, Sakamoto S, Shingu K (2004) Ketamine attenuates hypocapnia-induced neuronal damage in the caudoputamen in a rat model of chronic cerebral hypoperfusion. Neurosci Lett 354:26–29
Mizaner JB, Podhajsky P (1997) Ocular ischemic syndrome. Ophthalmology 104:859–864
Osborne NN, Block F, Sontag KH (1991) Reduction of ocular blood flow results in glial fibrillary acidic protein (GFAP) expression in rat retinal Muller cells. Vis Neurosci 7:637–639
Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J (2004) Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 23:91–147
Osborne NN, Safa R, Nash MS (1999) Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vis Res 39:3995–4002
Otori T, Katsumata T, Katayama Y, Terashi A (1997) Measurement of regional cerebral blood flow and glucose utilization in rat brain under chronic hypoperfusion conditions following bilateral carotid artery occlusion. Analyzed by autoradiographical methods. Nippon Ika Daigaku Zasshi 64:428–439
Slakter JS, Spertus AD, Weissman SS, Henkind P (1984) An experimental model of carotid artery occlusive disease. Am J Ophthalmol 97:168–172
Stevens WD, Fortin T, Pappas BA (2002) Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke 33:1107–1112
Stys PK, Lesiuk H (1996) Correlation between electrophysiological effects of mexiletine and ischemic protection in central nervous system white matter. Neuroscience 71:27–36
Takamatsu J, Hirano A, Levy D, Henkind P (1984) Experimental bilateral carotid artery occlusion: a study of the optic nerve in the rat. Neuropathol Appl Neurobiol 10:423–428
Wakita H, Tomimoto H, Akiguchi I, Kimura J (1995) Protective effect of cyclosporin A on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke 26:1415–1422
Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M (2003) Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Res 992:53–59
Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, McGeer PL (2002) Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res 924:63–70
Wilhelm H (1989) Tonic pupil caused by ischemia. Fortschr Ophthalmol 86:380–382
Acknowledgements
Financial support for this study was provided by PIBIC/CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavinsky, D., Sarmento Arterni, N., Achaval, M. et al. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefe's Arch Clin Exp Ophthalmo 244, 199–204 (2006). https://doi.org/10.1007/s00417-005-0006-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0006-7