Abstract
Objective
To evaluate safety and efficacy of 0.1 mg/ml versus 0.2 mg/ml mitomycin-C (MMC), applied for 1 min subconjunctivally, during trabeculectomy for primary adult glaucoma in previously un-operated eyes.
Materials and methods
This is a randomised controlled, non-inferior, clinical trial consisting of 50 consecutive POAG or CPACG patients uncontrolled on maximal hypotensive therapy, meeting all inclusion criteria. Patients were randomized into two groups and underwent a standard limbus-based trabeculectomy with MMC: Group I, 0.1 mg/ml and Group II, 0.2 mg/ml. The pre-operative and post-operative intraocular pressure (IOP), bleb morphology, and visual acuity were recorded every 6 months for 2 years. Complete success (primary outcome) was defined as IOP ≤ 15 mmHg without any additional medications at the end of 2 years.
Results
The average age of patients was 62.6 ± 9.8 years and 61.2 ± 8.1 years in Group 1 and 2, respectively; p = 0.57. The mean preoperative IOP was 22.5 ± 1.4 mmHg and 23.3 ± 1.8 mmHg; p = 0.10. The mean IOP at 2 years was 11.1 ± 1.6 mmHg and 10.8 ± 2.8 mmHg, a mean reduction in IOP of 50.6 ± 1.23 %, and 53.7 ± 2.25 % in Group I and II, respectively. The complete success was 92.0 % and 91.7 % in the two groups, respectively (p = 0.99), and there was one failure (Group II, post trauma). A wider bleb extent and larger areas of thin, transparent conjunctiva over the bleb were seen with the 0.2 mg/ml MMC group (p < 0.001) and in PACG eyes; p < 0.04.
Conclusion
A 1-min subconjunctival application of low dose 0.1 mg/ml MMC is non-inferior to 0.2 mg/ml and is probably a safer alternative, as thinning of the bleb is significantly less frequent in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is a common cause of irreversible blindness, for which medical therapy has to be continued lifelong, is expensive, and may not lead to an appropriate ‘target’ intraocular pressure (IOP). Glaucoma filtering surgery is frequently required to prevent progression of the optic neuropathy, and antifibroblastic agents, especially mitomycin-C (MMC), are used to increase success rates as well as achieve lower IOPs [1–7].
There is considerable inter-individual variability in the response to the surgery itself, to the application of MMC, and also long-term success. MMC has been applied in different concentrations, at different tissue planes, and for varying durations since its introduction [6–8]. Complications commonly associated with the use of MMC are thin walled, avascular blebs and extreme hypotony, with or without maculopathy, which appear to be dependent on dose and exposure time. A longer duration of application and higher concentrations of MMC have been shown to be associated with more complications [6, 9, 10]. The application of MMC, therefore, needs to be titrated and assessed to provide the best results with least complications.
This study was designed to compare the non-inferiority of low doses of MMC: 0.1 mg/ml versus 0.2 mg/ml, applied only for 1 min subconjunctivally, in adult primary glaucoma trabeculectomy, over a period of at least 2 years.
Materials and methods
Study design
A randomized, prospective study was conducted in which 50 eyes that met all criteria, were enrolled from the glaucoma clinic of our centre. A total of 93 consecutive adult patients having a primary adult glaucoma referred for a trabeculectomy were screened over 6 months. Out of this, 16 patients had associated ocular pathology, nine patients had very poor vision, and 18 had undergone a prior ocular surgery. Fifty eyes were assigned randomly into two parallel groups to use 0.1 mg/ml (Group I) or 0.2 mg/ml (Group II) of MMC during trabeculectomy, by a computer generated random number table using block randomisation with variable block size.
The inclusion criteria for this study were patients ≥50 years having a primary adult glaucoma, either primary open angle glaucoma (POAG if open angles on gonioscopy) or primary angle closure glaucoma (PACG- ‘occludable’ angle with peripheral anterior synechiae extending over at least 180° on indentation/manipulative gonioscopy); diagnosed if IOP > 21 mmHg on three different occasions (applanation tonometry) with characteristic optic nerve head and visual field changes suggestive of moderate glaucomatous optic neuropathy. Exclusion criteria included a visual acuity of less than 6/18, media opacities, any other ocular pathology, history of an attack of acute angle closure or secondary glaucoma, prior surgery, and uncooperative or unreliable patients. Patients were initially treated with topical hypotensive therapy. If ‘target' IOP was not achieved with maximally tolerated topical medications, trabeculectomy was performed. The study was approved by our institutional ethics committee and adhered to the tenets of the Declaration of Helsinki. Informed written consent was taken from each enrolled patient.
Study protocol
Patients underwent a thorough clinical examination which included Snellen visual acuity, slit-lamp biomicroscopy, fundus examination using +90D lens, and gonioscopy (one masked observer). Applanation tonometry and perimetry (Humphrey® Field Analyzer /HFA™ II-i Series) were also performed by a separate masked observer.
Postoperatively, patients were reviewed at 1 week, 1 month, and thereafter examined at least every 6 months for best corrected visual acuity, IOP, anterior chamber depth, bleb morphology and leaks, ocular hypotensive medications, perimetry, and any complications. Removal of releasable sutures was done between 1 and 2 weeks in all cases.
The blebs were graded by a single glaucomatologist who was masked to IOP values, according to the Indiana Bleb Appearance Grading Scale (IBAGS). Additionally, the presence of transparent, thin areas (TA) over the bleb, were noted in clock hours. The IBAGS uses standards consisting of slit lamp images for grades of bleb height (H0–H3), extent (E0–E3), vascularity (V0–V4), and leakage with Seidel test (S0–S2). Each bleb is then assigned a grade for each of the features assessed and is reported as HxExVxSx [11, 12].
The source of mitomycin-C was Kyowa Hakko pharmaceuticals, Japan. The two doses of MMC were prepared to make the two different concentrations in the same volume by a trained and masked operation theatre assistant.
Surgical technique
A standard trabeculectomy was performed in all patients by a single surgeon (RS). Under a peribulbar block, a 7–8 mm high, limbus based conjunctival flap was raised and a partial thickness scleral flap, 5 × 4 mm, was made. A 5 × 4 mm merocel sponge soaked in MMC was applied subconjunctivally for 1 min. MMC concentration was 0.1 mg/ml in Group I and 0.2 mg/ml in Group II. Thereafter, MMC was vigorously washed with at least 10 ml of balanced salt solution. A full thickness ostium, 2 × 1 mm, was excised and a broad basal iridectomy performed. The scleral flap was re-apposed with 10–0 nylon interrupted sutures at each corner. Two limbal releasable sutures were applied. The conjunctival flap was closed with 8–0 polyglactin continuous sutures.
Postoperatively, patients used topical antibiotic-steroid drops and ointment for 6–8 weeks and tropicamide eye drops OD for 4 weeks.
Success criteria
The trabeculectomy was considered a complete success when an IOP of 6–15 mmHg was achieved at the last follow-up without any medications, and a qualified success if achieved with medications [13]. Failure was defined as IOP > 15 mmHg with medications at the last follow-up.
Data analysis and statistical methods
Sample size for the study has been calculated for Non-inferiority trial with complete success (Yes/No) as the primary study outcome. The anticipated complete success in 0.2 mg/ml MMC (control arm) was 80 % and in 0.1 mg/ml MMC (study arm) was 85 %, with non-inferiority margin of 20 % (Δ = −20). To detect this difference with 95 % confidence level and 80 % power, we require 23 evaluable eyes in each of the groups. Considering about 10 % dropouts during the follow-up, we enrolled 50 patients in the study (25 patients per arm). Unit of enrolment was patient and unit of analysis was one eye.
Statistical analysis was performed using Stata 11.0 (College Station, TX, USA). Snellen BCVA measurements were converted to logarithm of the minimum angle of resolution (logMAR) equivalents for the purpose of data analysis. No patients were lost to follow-up, and 25 patients in each group were included in the analysis. Baseline categorical variables and continuous variables were compared between the groups using Chi-square test and Student’s t test for independent samples/Wilcoxon rank sum test, respectively. The difference in the complete/qualified success and bleb grading between the groups was tested using Fisher’s exact test. The IOP over a period of 24 months were analysed between the groups using repeated measure ANOVA. The BCVA and number of medications were analysed between/within the groups using Wilcoxon rank-sum/Wilcoxon signed rank test since they were not following normal distribution. A p-value of <0.05 was considered statistically significant.
Results
There were no significant differences in the baseline parameters between the two groups in terms of age, IOP, and best corrected visual acuity (BCVA). Various baseline and postoperative parameters are presented in Table 1. All patients were on a prostaglandin analogue, beta blockers, brimonidine, and systemic acetazolamide, at the time of surgery.
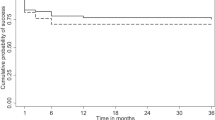
The pre-operative IOP in Group I was 22.52 ± 1.36 mmHg, which significantly reduced to 11.10 ± 1.60 mmHg at 24-month follow-up (p = 0.001). Similarly, in Group II, IOP significantly reduced from 23.28 ± 1.81 mmHg to 10.80 ± 2.80 mmHg at 24-month follow-up (p < 0.001). The mean percentage reduction of IOP in Group I was 50.62 ± 1.23 % and Group II was 53.73 ± 2.25 % (p = 0.23, independent t-test) from preoperative levels. There was a gradual decrease in IOP seen in both groups over the 24 months of follow-up (Fig. 1). Two years after surgery, ocular hypotensive medications were being used in two cases in Group I and three cases in Group II: timolol in four eyes and both timolol and latanoprost in one eye in Group II that incurred a trauma on the second postoperative day, leading to a cut-through of a scleral suture, which was re-sutured. In this patient, the medications were added at 3 weeks postoperatively and the IOP at 24-month follow-up was 16 mmHg on two medications. In the rest of the patients, medications were added at 3 months postoperatively. Complete success at 24 months in Group I was seen in 23 (92.0 %) eyes and in 22 (88.0 %) of Group II eyes, and there was no significant difference in the two groups (p = 0.99). The complete success in Group I was more than 4 % as compared to Group II (92 vs. 88 %; 95%CI, −10.2 to 18.2) with the lower 95 % CI being −10.2 %, which is greater than the pre-defined non-inferiority margin (Δ = −0.20). Qualified success in Group I was 2 (8.0 %) and Group II was 2 (8.0 %); p = 0.99, and there was one failure in group II (postoperative trauma). The bleb parameters were evaluated according to the IBAGS, shown in Tables 2 and 3.
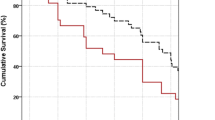
Box plot representation of IOP values over 24 months of follow-up in Group I (black) and Group II (white), respectively. Median value (dark lines), 25/75 boxes, 5/95 percentiles (bars), and outliers (black circles). The horizontal red dashed line drawn at IOP of 15 mmHg represents success criteria. Therefore, this represents success over time, which is similar from 6-month follow-up to last follow-up of 24 months. The blue star depicts the addition of ocular hypotensive medications in either group between 3 weeks to 3 months
Analyzing POAG (20/50) and PACG (30/50) eyes in the two groups shows higher IOP in POAG eyes, (p = 0.02) at 6 months and 1 year, but the IOP achieved at 2 years was similar; p = 0.24. The mean IOP at 2-year follow-up was 11.3 ± 2.4 mmHg in PACG and 10.5 ± 2.1 mmHg in POAG eyes. Absolute success in POAG patients was noted in 20 (100 %) eyes and in PACG patients was 25 (83.3 %) eyes; p = 0.054. Qualified success was seen in five (16.7 %) PACG eyes: two in Group I (0.1 mg/ml MMC) and three in Group II (0.2 mg/ml MMC). Both POAG and PACG eyes showed more thinning of the bleb with 0.2 mg/ml MMC used for 1 min (p < 0.005, Table 4). Also, bleb thinning was more common in PACG eyes when 0.2 mg/ml of MMC was used (p = 0.04). However, there were no significant difference in any other bleb parameters. There was no correlation between the concentration of MMC used and bleb vascularity or bleb height.
Visual fields showed no change within the 2 years of the study with either dose of MMC. No bleb leaks, choroidal detachment, hypotony, or blebitis were seen on follow-up. No other patient required any further surgical intervention, such as a needling or re-surgery.
Discussion
Many patients having a moderate or advanced glaucomatous optic neuropathy need surgery to achieve low ‘target’ IOPs, and trabeculectomy augmented with MMC has been the most commonly performed surgery in such eyes. A Cochrane review has shown that MMC application in trabeculectomies lowers the IOP significantly in previously un-operated eyes [6]. The dose of MMC used in literature has varied from 0.1 to 0.5 mg/ml and in duration from 0.5 to 5 min, with reported complications such as hypotony, bleb leaks, blebitis, and a significant loss of vision. A higher dose of MMC has been reported to lead to lower IOPs and a higher success of the trabeculectomy, but also more frequent complications. There are few randomized studies comparing doses or duration of application of MMC [6, 14].
In this prospective, randomized study we compared the non-inferiority of two low concentrations of MMC, 0.1 mg/ml and 0.2 mg/ml, applied subconjunctivally for a short duration of 1 min in the long term, in moderate adult primary glaucoma eyes. Complete success (IOP of ≤ 15 mmHg) was seen in 92.0 % after the use of 0.1 mg/ml MMC and a similar 91.7 %, using 0.2 mg/ml MMC at 2-year follow-up. Two eyes in each group required one ocular hypotensive medication to reach an IOP of ≤ 15 mmHg, and one eye needed two medications. The IOP achieved with both doses of MMC decreased progressively up to 6 months, and stabilized thereafter over 2 years (Fig. 2). Kim et al. reported a 0.5 to 1 min application of 0.5 mg/ml MMC to be optimal [9]. Robin et al. studied four groups: placebo, 0.2 mg/ml MMC for 2 min, 0.2 mg/ml MMC for 4 min, and 0.4 mg/ml MMC for 2 min, achieving an IOP < 18 mmHg in 78.2 %, 90.5 %, 90.7 %, and 91.8 %, respectively, at 1 year, and concluded that a possible dose response relationship exists between efficacy and the concentration and duration of exposure to mitomycin [10]. Mégevand et al. reported that 2-min and 5-min intraoperative applications of 0.2 mg/ml mitomycin C were similarly effective [14]. Errico et al. used 0.1 mg/ml MMC, for 3 min below the Tenon’s capsule and for 1–2 min below the scleral flap reporting complete success in 93 % of patients and a qualified success in 100 % of patients at 2 years [15]. Alwitry et al. in a retrospective, nonrandomized study used 0.1 mg/ml MMC for 1 min in eyes for a ‘low risk, primary trabeculectomy’, and found 83.1 % had an IOP <16 mmHg at 1-year follow-up [16]. Lee et al., in a retrospective review, showed that eyes where 0.1, 0.2, and 0.4 mg/ml of MMC were used for 5 min had a similar qualified success, but absolute success was only 40 % with 0.1 mg/ml [17]. Kitazawa et al. studied 0.02 and 0.2 mg/ml MMC in primary trabeculectomy and found a 63.6 and 100 % success respectively. He suggested that the appropriate dose was between these two [18]. Laube et al. used 0.1, 0.2, and 0.4 mg/ml of MMC for 2.5 min, and found 0.2 mg/ml to work best [19]. Sacu et al. used 0.1 mg/ml MMC for 5 min and found a complete success rate (IOP < 21 mmHg) of 87.7 % at 1 year [20]. Costa used MMC 0.2 mg/ml for 3 min or placebo in patients with uncontrolled POAG or PACG with a success rate of 75.7 % in the MMC group and 28.5 % in the control group [21]. Annen and Stürmer used 0.2 mg/ml of MMC for 1 min and noted an IOP of <21 mmHg in 88 % at about 1 year, with 8.8 % developing an avascular bleb [22]. Ben Simon et al. used 0.4 mg/ml of MMC for a shorter duration of 15 s and recorded a complete success rate (IOP < 21 mmHg) of 73 % [23].
a & b Trabeculectomy filtering bleb 2 years after the use of MMC 0.1 mg/ml applied for 1 min subconjunctivally. The bleb is diffuse and relatively avascular with small microcystic spaces at the limbus (arrow). c & d Trabeculectomy filtering bleb 2 years after the use of MMC 0.2 mg/ml applied for 1 min subconjunctivally. The bleb is defined, avascular, and has large areas of thinning with transparency over the bleb (arrow)
Mégevand et al. reported that a similar complication rate with MMC used for 2 or 5 min [14], with three eyes developing a bleb-related infection and two eyes late endophthalmitis. He concluded that a shorter duration of MMC application in a trabeculectomy appears to be both efficacious and safe. Robin et al. found no difference in complications with 0.2 mg/ml MMC for 2 or 4 min, and 0.4 mg/ml MMC for 2 min, and reported cataract in 18.1 %, choroidal detachment in 4.4 % and macular folds in 6.4 % [10]. Alwitry et al. noted a bleb leak in 27.1 % of eyes [16]. Lee et al. noted postoperative hypotony only in the 0.4 mg/ml MMC group [17]. Kitazawa et al. reported cataract progression in 18 % eyes in 0.2 mg/ml MMC group [18]. Ben Simon et al. found that 11 % required further surgical intervention, needling, or a repeat trabeculectomy [23]. In our study, one patient sustained direct injury to the operated eye on the second day after surgery, leading to a cut through of a scleral flap suture, which was re-sutured. Over 2 years, none of the other eyes had a bleb leak, hypotony, blebitis, or a significant fall in visual acuity. We did not specifically image for cataract status, but there was no significant change in BCVA in either groups postoperatively. The large disparity in the number of complications reported by us and other studies could be attributed to the lower dose and duration of MMC utilized.
There was a significantly greater limbal extent of the bleb seen in the 0.2 mg/ml MMC group in our study, with no difference in bleb vascularity or bleb height. The IBAGS classification does not specifically identify transparent, thin areas (TA) that are not leaking, but these could be more prone to infection, trauma or lead to microleaks in the future. This study found that such transparent, thin areas of the bleb, extending over more than two clock hours, were significantly larger and seen more often after the use of 0.2 mg/ml MMC (Fig. 2). Mégevand et al. reported cystic blebs in 60 % as compared to 76 % eyes in which 0.2 mg/ml MMC was used for 2 min or 5 min, respectively [14]. Lee et al. found no significant difference in bleb grading [17].
We also performed a subanalysis in POAG and PACG eyes, and found the IOP response and IBAGS bleb characteristics to be similar in both types of primary adult glaucoma. Both POAG and PACG eyes showed more thinning of the bleb with 0.2 mg/ml MMC used for 1 min, with thinning of the bleb more common in PACG eyes when 0.2 mg/ml of MMC was used. This could not be explained, as the medications used in the long term were similar in both groups. Pilocarpine was used prior to laser iridotomy only. To the best of our knowledge, there are no reports comparing different concentrations of MMC in PACG and POAG. An earlier study in the same population, reported that trabeculectomy without the use of MMC lead to a similar overall success, i.e., an IOP < 21 mmHg in 94 % eyes with or without medications at 5 years [24].
An in vitro study of Tenon’s capsule fibroblast cultures found that a 1-min exposure of 0.4 mg/ml of MMC inhibited 77 % of 3H-Thymidine uptake, as compared to 90 % inhibition after exposure for 5 min [25]. Yamamoto et al. studied the effect of MMC on rabbit fibroblasts and showed a dose dependent effect of the MMC, with lower doses and shorter duration of application having a reversible effect, as compared to the irreversible effect on cell proliferation [26]. Seah et al. reported no correlation between sponge size, time of MMC exposure and aqueous MMC level [27].
The major limitation of our study was the small sample size, but this was because we wanted a tight cohort with regard to age and exclusion criteria, ensuring that the difference in MMC concentration was the only variable. This may also have lead to better success rates than reported in literature. Other limitations were the absence of a control group where MMC was not used, and also the absence of lens imaging to record subtle lens changes over time.
Glaucoma patients require a lifelong reduction in IOP with minimal short- or long-term complications. This study has shown that the application of lower doses of MMC for a short duration are effective in achieving low ‘target’ IOPs in primary adult trabeculectomies in the long term, and have fewer complications.
In conclusion, 0.1 mg/ml MMC is non-inferior to 0.2 mg/ml MMC in achieving low ‘target’ IOPs in primary adult trabeculectomies and is probably a safer alternative, as thinning of the bleb is significantly less frequent in the long term.
References
Edmunds B, Thompson JR, Salmon JF (2001) The National Survey of Trabeculectomy.II. Variations in operative technique and outcome. Eye 15:441–448
Bindlish R, Condon GP, Schlosser JD et al (2002) Efficacy and safety of mitomycin-C in primary trabeculectomy: five year follow up. Ophthalmology 109:1336–1341
Lama PJ, Fechtner RD (2003) Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol 48:314–346
Siriwardena D, Edmunds B, Wormald RP, Khaw PT (2004) National survey of antimetabolite use in glaucoma surgery in the United Kingdom. Br J Ophthalmol 88(7):873–876
Joshi AB, Parrish RK, Feuer WF (2005) 2002 survey of the American Glaucoma Society: practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma 14:172–174
Wilkins M, Indar A, Wormald R (2005) Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev 19, CD002897
Jampel HD, Solus JF, Tracey PA et al (2012) Outcomes and bleb related complications of trabeculectomy. Ophthalmology 119:712–722
Lee SJ, Paranhos A, Shields MB (2009) Does titration of mitomycin C as an adjunct to traneculectomy significantly influence the intraocular pressure outcome? Clin Ophthalmol 3:81–87
Kim YY, Sexton RM, Shin DH et al (1998) Outcomes of primary phakic trabeculectomies without, versus with 0.5- to 1-minute versus 3- to 5-minute mitomycin C. Am J Ophthalmol 126:755–762
Robin AL, Ramakrishnan R, Krishnadas R, Smith SD, Katz J, Selvaraj S, Skuta GL, Bhatnagar R (1997) A long-term dose–response study of mitomycin in glaucoma filtration surgery. Arch Ophthalmol 115(8):969–974
Cantor LB, Mantravadi A, WuDunn D et al (2003) Morphologic classification of filtering blebs after glaucoma filteration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma 12:266–271
Wells AP, Crowston JG, Marks J et al (2004) A pilot study of a system for grading of drainage blebs after glaucoma surgery. J Glaucoma 13:454–460
Heuer DK, Barton K, Grehn F, Sharaway T, Sherwood M (2009) Consensus on definition of success. Text Book of guidelines on design and reporting of glaucoma surgical trials. World Glaucoma Association. Kugler publications, The Netherlands. p.17
Mégevand GS, Salmon JF, Scholtz RP, Murray AD (1995) The effect of reducing the exposure time of mitomycin C in glaucoma filtering surgery. Ophthalmology 102:84–90
Errico D, Scrimieri F, Riccardi R et al (2011) Trabeculectomy with double low dose of mitomycin C - two years of follow-up. Clin Ophthalmol 5:1679–1686
Alwitry A, Abedin A, Patel V et al (2009) Primary low-risk trabeculectomy augmented with low-dose mitomycin-C. Eur J Ophthalmol 19:971–976
Lee JJ, Park KH, Youn DH (1996) The effect of low-and high-dose adjunctive mitomycin C in trabeculectomy. Korean J Ophthalmol 10:42–47
Kitazawa Y, Suemori-Matsushita H, Yamamoto T, Kawase K (1993) Low-dose and high-dose mitomycin trabeculectomy as an initial surgery in primary open-angle glaucoma. Ophthalmology 100:1624–1628
Laube T, Ritters B, Selbach M, Hudde T (2003) Clinical experiences and results of mitomycin C in trabeculectomy. Klin Monbl Augenheilkd 220:618–624
Sacu S, Rainer G, Findl O et al (2003) Correlation between the early morphological appearance of filtering blebs and outcome of trabeculectomy with mitomycin C. J Glaucoma 12:430–435
Costa VP, Comegno PE, Vasconcelos JP et al (1996) Low dose mitomycin C trabeculectomy in patients with advanced glaucoma. J Glaucoma 5:193–199
Annen DJ, Stürmer J (1995) Follow-up of a pilot study of trabeculectomy with low dosage mitomycin C (0.2 mg/ml for 1 minute). Independent evaluation of a retrospective nonrandomized study. Klin Monbl Augenheilkd 206:300–302
Ben Simon GJ, Glovinsky Y (2006) Trabeculectomy with brief exposure to mitomycin C. Clin Exp Ophthalmol 34:765–770
Sihota R, Gupta V, Agarwal HC (2004) Long-term evaluation of trabeculectomy in primary open angle glaucoma and chronic primary angle closure glaucoma in an Asian population. Clin Exp Ophthalmol 32(1):23–28
Jampel HD (1992) Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon’s capsule fibroblasts. Ophthalmology 99:1471–1476
Yamamoto T, Varani J, Soong HK, Lichter PR (1990) Effects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology 97(9):1204–1210
Seah SK, Prata JA Jr, Minckler DS et al (1993) Mitomycin-C concentration in human aqueous humour following trabeculectomy. Eye (Lond) 7:652–655
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 48 kb)
Rights and permissions
About this article
Cite this article
Sihota, R., Angmo, D., Chandra, A. et al. Evaluating the long-term efficacy of short-duration 0.1 mg/ml and 0.2 mg/ml MMC in primary trabeculectomy for primary adult glaucoma. Graefes Arch Clin Exp Ophthalmol 253, 1153–1159 (2015). https://doi.org/10.1007/s00417-015-3028-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3028-9