Abstract
Purpose
To evaluate changes in macular sensitivity, as measured with microperimetry, among patients with maculopathy and stable visual acuity (VA).
Methods
Macular sensitivity was assessed using the Spectral OCT/SLO™ microperimetry (OCT/SLO, Optos Plc., Dunfermline, UK) in 25 eyes (16 patients) with maculopathy and stable VA (<5 letters change in ETDRS score) at two consecutive clinic visits. To take the limits of the test–retest repeatability of the OCT/SLO into account, coefficient of repeatability (CoR) was employed to estimate the probability of the sensitivity changes being secondary to measurement noise.
Results
The point sensitivity changes were statistically significant (Wilcoxon signed-rank test, P < 0.001). Seventy-seven points (11 %) out of a total of 700 sensitivity points had a genuine sensitivity change, with a mean increase of 8.6 ± 2.6 dB in 35 points and a mean decrease of 7.9 ± 2.2 dB in 42 points.
Conclusions
Point-to-point change in macular sensitivity can be used as a biomarker of changes in disease activity in patients with maculopathy, and can be more accurate than either mean sensitivity or BCVA in detection of changes in macular function. The measurement variability should be considered when observing the local sensitivity changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Visual acuity (VA), the gold standard for assessing macular function in clinical practice, may not fully portray the macular function [1]. Besides VA, several validated vision function measurements such as Amsler grid testing, contrast sensitivity [2], fixation stability test [3], and reading speed [4] have been utilized additionally to access macular function. However, these examinations provide an overall measure of macular function and do not allow fine spatially localized testing in the macular area [5].
A relatively new technology, microperimetry, was introduced to overcome some of these limitations. Microperimetry determines light sensitivity at discrete points in the macula while allowing for simultaneous visualization of fundus. It enables an exact correlation between functional deficits and corresponding morphologic features [6]. Since its invention, there has been an increasing interest in applying microperimetry in analyzing characteristics of various macular disorders [7, 8] and in evaluating outcomes of various pharmacological therapies [9, 10] and surgical procedures [11].
The disparity between VA and light sensitivity was frequently seen in these studies. Decreased retinal sensitivity was observed in patients with early AMD and normal VA [12]. In patients with central serous chorioretinopathy, reduced light sensitivity was documented even after months of restoration of VA and anatomical resolution of the macular fluid [13]. Lack of significant correlation between macular sensitivity and VA was also observed in patients with DME [14]. Theoretically, there is a possibility that the retinal sensitivity may change during a stable VA period, but no such longitudinal study has been conducted to verify this hypothesis.
The aim of our study was to further investigate if macular sensitivity may change during a stable VA period in patients with maculopathies. The macular sensitivity changes were evaluated not only with the mean sensitivity but also with the individual point sensitivity, as the main advantage of microperimetry is the ability to measure retinal function at individual retinal locations.
Participants and methods
Participants
In this prospective study, adult patients with maculopathy who were managed at the Wilmer Eye Institute of the Johns Hopkins Hospital between August 2012 and November 2012 were eligible for participation. The inclusion criteria were patients with maculopathy who had best-corrected visual acuity (BCVA) changes < 5 letters in ETDRS score at two consecutive clinic visits. The type of macular disease was not specified as a criterion; thus, maculopathy may have included diabetic macular edema, non-neovascular age-related macular degeneration, idiopathic macular hole, uveitis, etc. If the patient had macular edema, the variations in central macular thickness (CMT) should be no more than 20 μm on spectral-domain optical coherence tomography (SD-OCT) at two consecutive visits. The variation of macular thickness was calculated from the mean change of central 3 mm of macular on SD-OCT, as it is the corresponding testing area in the microperimetry examination.
The exclusion criteria included significant media opacities that precluded fundus examination and inability to cooperate during microperimetry assessment. To ensure reliability of the microperimetry tests, results with more than 10 % of false-positive or false-negative answers of tests were excluded [15]. All the microperimetry tests were performed by a single experienced examiner (HL).
The study followed the tenets of the Declaration of Helsinki and was approved by the Johns Hopkins Institutional Review Board/Ethics Committee. Written informed consent was obtained from each participant.
Methods
Ophthalmology examination
All patients underwent a complete ophthalmology examination that included best-corrected visual acuity (BCVA) measurement, biomicroscopic examination, fundus examination, optical coherence tomography evaluation (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany), and microperimetry examination (Spectral OCT/SLO; Optos Inc, Scotland, UK) at two consecutive clinic visits. All patients had experience in having perimetry or microperimetry test (at least once before the enrollment).
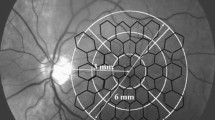
Spectral OCT/SLO and its microperimetry techniques have been previously described [16]. Test parameters are standardized as: Polar 3 Pattern, Goldman III stimulus size, 200 ms stimulus duration, a 1500 ms interval between stimulus presentations, and the 4–2 test strategy. At the second visit, a follow-up microperimetry test was performed over the same macular area using the built-in eye tracking system in the device. A sensitivity difference map was generated to compare visits (Fig. 1). Changes in macular sensitivity were evaluated with point sensitivity as well as mean sensitivity.
Evaluation of sensitivity changes in microperimetry
To take the measurement noise into account, the repeatability of spectral OCT/SLO in eyes with and without maculopathy was evaluated in a previous study (Jang HSK et al., ARVO 2013) in which the maculopathy group included 22 eyes from 13 subjects with a mean age of 57.1 (±19.9 years). The subjects completed three consecutive sessions of microperimetry within one day. In the maculopathy group, the CoR of OCT/SLO point sensitivity was 4.64 dB (95 % CI: 4.32, 5.01) and the CoR of mean sensitivity was 2.15 dB (95 % CI: 2.00, 2.32).
Proposed by the British Standards Institution and recommended by Bland and Altman, CoR was defined as the value beyond which the difference between the two measurements will lie with probability 0.95 [17]. The implication is that for a particular change of greater than CoR, the probability of the change being due to the intrinsic measurement noise is less than 5 %. In other words, on the basis of probability, such a difference would more probably represent a genuine change (probability is more than 95 %) rather than a measurement noise [18, 19]. The method of calculation of CoR was described in detail by Brutton et al [18, 20].
Hence, in our study, coefficient of repeatability (CoR) was applied to estimate the probability of the sensitivity differences being due to the measurement noise. Only sensitivity changes of greater than 5.01 dB for point sensitivity or 2.32 dB for mean sensitivity were considered to represent a genuine change.
Statistical analysis
Normal distribution of the macular sensitivity values was assessed using the Shapiro–Wilk test. Due to the non-normal distribution of data, the sensitivity values at the two visits were analyzed by Wilcoxon signed-rank test. P-values less than or equal to 0.05 were considered statistically significant. All analyses were performed using the SPSS statistical package (Version 19.0, SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Twenty-five eyes of 16 subjects (mean age ± SD: 54.0 ± 16.0 years, seven males, nine females) were enrolled in the study. The mean BCVA (mean ± SD) was 72 ± 15 ETDRS letters and the mean central macular thickness (mean ± SD) was 261 ± 30 μm. The mean interval (mean ± SD) of the two visits was 3.7 ± 1.8 months. At the first clinic visit, thirty-six eyes (25 subjects) with maculopathies were prospectively recruited with a reasonable expectation that VA might remain stable at the next visit, as per the experienced ophthalmologist. At the second visit, seven eyes (six subjects) were excluded due to changed VA or changed central macular thickness; four eyes (three subjects) were lost during follow up.
The maculopathies among the 16 enrolled subjects (25 eyes) consisted of diabetic macular edema (seven eyes), hydroxychloroquine maculopathy (five eyes), multifocal choroidopathy (three eyes), central serous retinopathy (two eyes), idiopathic macular hole (two eyes), pathologic myopia (two eyes), multiple sclerosis-associated macular edema (two eyes), idiopathic posterior uveitis (one eye), and non-neovascular age-related macular degeneration (one eye).
Point sensitivity changes
A total of 700 individual points of sensitivity were analyzed (28 test points per eye from all 25 eyes). In our study, 68.7 % of tested points (481 points) showed changes of macular sensitivity at the second visit, with a mean increase of 3.5 dB in 32.9 % of points (230 points) and a mean decrease of 3.4 dB in 35.9 % (251 points). Macular sensitivity remained unchanged in 31.2 % of points (219 points). The sensitivity differences are summarized in Table 1. The changes in point-to-point sensitivity between the two visits were statistically significant (Wilcoxon signed-rank test, P < 0.001).
When taking the previously reported repeatability limits of OCT/SLO (Jang HSK et al., ARVO 2013) into account, 11.0 % of points (77 points) had a change greater than 5.01 dB (upper limit CoR of OCT/SLO for point sensitivity) with a mean increase of 8.6 dB in 35 points and a mean decrease of 7.8 dB in 42 points. The changes in these 77 point-to-point sensitivities were statistically significant (Wilcoxon signed-rank test, P < 0.001). The results are summarized in Table 2 and Fig. 2.
Among the 77 points, there were 16 points located at the inner ring (about 16 % of tested points), 39 points at the middle ring (13 %), and 22 points at the outer ring (7.5 %). The changes in these 16 point-to-point sensitivities were statistically significant (Wilcoxon signed-rank test, P <0.001). Figure 3 shows the relative locations of changes.
Mean sensitivity changes
Twenty-four out of 25 eyes had mean sensitivity changes ranging from −2.85 dB to 1.57 dB. The mean macular sensitivity changes between two visits were not statistically significant (paired t-test, P = 0.8).
However, two eyes had mean sensitivity changes greater than 2.15 dB (CoR of the OCT/SLO for mean sensitivity). One eye was from a patient who had a 5-year history of non-proliferative diabetic retinopathy (moderate NPDR) with stable VA at 20/32 during the two visits (3-month interval). The mean macular sensitivity decreased from 8.1 dB to 5.3 dB. The fellow eye of the patient had a stable mean macular sensitivity with 11.1 dB at the first visit and 10.3 dB at the second visit.
The other eye was from a patient with hydroxychloroquine (Plaquenil) macular toxicity who took hydroxychloroquine 200 mg twice a day for over 15 years for systemic lupus erythematous, and had discontinued because of macular toxicity 3 years before being enrolled in our study. The VA was 20/25 during the two visits (9-month interval), while the macular sensitivity decreased from 9.6 dB to 7.2 dB. The fellow eye of the patient dropped two lines to 20/40 in VA at the second visit, and hence was excluded from this study.
Discussion
With the ability of measuring light sensitivity in discrete retinal locations and performing a precise test–retest over the same area, microperimetry is an advanced tool that could enable precise longitudinal observation of the changes in macular function. It can provide more comprehensive information in the various areas of the macula.
In any longitudinal observation, knowledge of the repeatability limits of a test is a prerequisite as it provides data that defines the significance of changes, i.e., whether due to a measurement noise, or an actual change. For example, clinically significant change in VA has been defined as the loss or gain of one or more lines on the ETDRS chart, which translates as more than 5 letters of measurement fluctuation. However, there was no similar convention for retinal sensitivity changes based on the repeatability of microperimetry. In some studies, ± 1 dB of mean sensitivity change has been accepted as a default cutoff as sensitivity values expressed in the form of integer [9, 21]. However, this was not based on previous studies that checked the repeatability limits of the devices used. Applying the same cutoff value in our cohort, about 70 % of points had changes in sensitivity at the second visit of more than 1 dB. However, several repeatability studies have indicated that the microperimetry measurement variances were more than 1 dB. For example, the short-term sensitivity variability of Rodenstock SLO 101(Rodenstock, Ottobrunn, Germany) was 2.0 ± 0.8 dB [15]. Seiple [22] reported the agreement between repeated tests was 2.0 dB for MP-1 and 2.5 dB for OCT/SLO with stimulus of Goldman III size. In addition, the previous studies were based on data from normal subjects, and used changes in mean sensitivity as the primary outcome variable. Repeatability indices are even worse in diseased subjects. For example, the mean (SD) of CoR of the MP-1 in subjects with maculopathy was 5.56 (0.86) dB for point sensitivity, and 1.81 (0.17) dB for the mean sensitivity [23]. In this study, we have used our previously reported repeatability limits of the OCT/SLO (Jang HSK et al., ARVO 2013). Despite being comparable to the MP-1, CoR of 4.64 dB (95 % CI: 4.32, 5.01) for point sensitivity and 2.15 (95 % CI: 2.00, 2.32) dB for mean sensitivity are different enough that the repeatability limits of different microperimeters cannot be used interchangeably and must be specific to the device used.
In our study, we found that despite the fact that there were no obvious changes in best-corrected visual acuity in the study eyes, macular sensitivity was significantly different, statistically, in about 70 % of tested points. However, statistical significance is not always equal to clinical significance.
When the limits of the test–retest repeatability of the OCT/SLO are applied, 11 % of the tested points show differences that are beyond such limits, which suggests a true progression or improvement of maculopathy rather than a measurement error.
These sensitivity changes indicate that deterioration or recovery of the macular function may occur during periods of stable VA, and that point sensitivity may be a good ancillary tool to monitor macular function and disease burden. In pooled testing points, 77 points showed changes in sensitivity, out of which 16 points were located within the foveal region. However, these points were scattered across different eyes, and may not cause significant visual acuity change in an individual eye.
Although mean sensitivity has no spatial information, it is a concise index as it gives an overall macular functional assessment. Chen et al. recommend using mean sensitivity for monitoring the macular function, as it has much less variance than point sensitivity [23].
Only two eyes (8 %) in our study showed changes in mean sensitivity beyond the limits of repeatability. Despite being widely used to monitor patients with maculopathy, mean sensitivity cannot be used alone to determine disease progression or improvement, as it is less susceptible to focal changes. Therefore, it is better used in conjunction with changes in point sensitivity to determine the spatial extent of change.
The main limitation of our study is that the maculopathy group is heterogeneous, and no disease-specific conclusion could be drawn. However, the aim of the study was not to document the progress of a certain disease. Instead, the current study has demonstrated the value of retinal sensitivity as an ancillary, perhaps as a complementary method to VA testing, while it also has emphasized the influence of measurement variance. Current microperimetry studies either focus on the repeatability itself or address the reduced retinal sensitivity in macular disorders. Our results recommend combining these two aspects and evaluating the longitudinal changes in point macular sensitivity.
Another limitation of our study is that we have used a change of sensitivity of 5 dB or more as our upper limit for clinical significance, based on the results of a previous study. However, that limit could be different for different disease groups, and further studies that are more disease-specific may be needed to elucidate more precision of repeatability limits. An ideal way to determine whether sensitivity changes are secondary to the microperimetry measurement noise or are actual changes would be to have a thorough individual assessment of repeatability at baseline visit. Also, physicians should always evaluate sensitivity changes in the context of clinical knowledge.
In conclusion, our results suggest that point-to-point change in sensitivity may be used as a biomarker of changes in disease activity in patients with maculopathy, and can be more accurate than BCVA in detection of changes in macular function. It also suggests that point-to-point sensitivity is more sensitive than mean sensitivity in detecting clinical changes.
Monitoring the macular function by using microperimetry is a good complement to VA limitations. Thus, with additional evaluations and studies, in the future, microperimetry may become part of the standard of care for patients with macular disorders. It is also important to recognize the relative high variance of point sensitivity measurement when accessing the local sensitivity changes.
References
Grenga P, Lupo S, Domanico D et al (2008) Efficacy of intravitreal triamcinolone acetonide in long standing diabetic macular edema: a microperimetry and optical coherence tomography study. Retina 28(9):1270–1275
Wong R, Khan J, Adewoyin T et al (2008) The ChromaTest, a digital color contrast sensitivity analyzer, for diabetic maculopathy: a pilot study. BMC Ophthalmol 8:15
Vujosevic S, Pilotto E, Bottega E et al (2008) Retinal fixation impairment in diabetic macular edema. Retina 28(10):1443–1450
Cacho I, Dickinson CM, Smith HJ et al (2010) Clinical impairment measures and reading performance in a large age-related macular degeneration group. Optom Vis Sci 87(5):344–349
Hartmann KI, Bartsch DU, Cheng L et al (2011) Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina 31(7):1323–1331
Rohrschneider K, Bultmann S, Springer C (2008) Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog Retin Eye Res 27(5):536–548
Kube T, Schmidt S, Toonen F et al (2005) Fixation stability and macular light sensitivity in patients with diabetic maculopathy: a microperimetric study with a scanning laser ophthalmoscope. Ophthalmologica 219(1):16–20
Sepah YJ, Hatef E, Colantuoni E et al (2012) Macular sensitivity and fixation patterns in normal eyes and eyes with uveitis with and without macular edema. J Ophthal Inflamm Infect 2(2):65–73
Rohrschneider K, Bultmann S, Gluck R et al (2000) Scanning laser ophthalmoscope fundus perimetry before and after laser photocoagulation for clinically significant diabetic macular edema. Am J Ophthalmol 129(1):27–32
Malagola R, Spinucci G, Cofone C et al (2013) Prospective microperimetry and OCT evaluation of efficacy of repeated intravitreal bevacizumab injections for persistent clinically significant diabetic macular edema. Int Ophthalmol 33(3):261–267
Baba T, Mizuno S, Tatsumi T et al (2012) Outer retinal thickness and retinal sensitivity in macula-off rhegmatogenous retinal detachment after successful reattachment. Eur J Ophthalmol 22(6):1032–1038
Parisi V, Perillo L, Tedeschi M et al (2007) Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina 27(7):879–890
Ozdemir H, Karacorlu SA, Senturk F et al (2008) Assessment of macular function by microperimetry in unilateral resolved central serous chorioretinopathy. Eye (Lond) 22(2):204–208
Hatef E, Colantuoni E, Wang J et al (2011) The relationship between macular sensitivity and retinal thickness in eyes with diabetic macular edema. Am J Ophthalmol 152(3):400–405, e402
Rohrschneider K, Becker M, Schumacher N et al (1998) Normal values for fundus perimetry with the scanning laser ophthalmoscope. Am J Ophthalmol 126(1):52–58
Landa G, Rosen RB, Garcia PM et al (2010) Combined three-dimensional spectral OCT/SLO topography and microperimetry: steps toward achieving functional spectral OCT/SLO. Ophthalmic Res 43(2):92–98
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Bartlett JW, Frost C (2008) Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 31(4):466–475
James L (2010) The statistical interpretation of the coefficient of repeatability. Other, Paper 1 http://arrow.dit.ie/otpomoth
Anne Bruton JHC, Holgate ST (2000) Reliability: what is it, and how is it measured. Physiotherapy 86(2):94–99
Richter-Mueksch S, Vecsei-Marlovits PV, Sacu SG et al (2007) Functional macular mapping in patients with vitreomacular pathologic features before and after surgery. Am J Ophthalmol 144(1):23–31
Seiple W, Rosen RB, Castro-Lima V et al (2012) The physics and psychophysics of microperimetry. Optom Vis Sci 89(8):1182–1191
Chen FK, Patel PJ, Xing W et al (2009) Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci 50(7):3464–3472
Acknowledgments
We gratefully acknowledge the valuable advice of Professor James Loughman, Optometry Department, Dublin Institute of Technology, Ireland, for his assistance in appropriately interpreting the coefficient of repeatability.
Financial disclosure(s)
The Johns Hopkins University and the University of Nebraska Medical Center have received research support from Optos, Inc. Optos had no role in the design or conduct of this research. The study was supported by a Wilmer Research Grant Award (HL) and Research to Prevent Blindness (QDN). Dr. Nguyen serves on the Scientific Advisory Board for Optos, Inc.
Conflict of interest
No conflicting relationship exists for any author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Bittencourt, M.G., Wang, J. et al. Retinal sensitivity is a valuable complementary measurement to visual acuity — a microperimetry study in patients with maculopathies. Graefes Arch Clin Exp Ophthalmol 253, 2137–2142 (2015). https://doi.org/10.1007/s00417-015-2974-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2974-6