Abstract
Purpose
To evaluate the short-term efficacy of aflibercept monotherapy for patients with treatment-naïve polypoidal choroidal vasculopathy (PCV).
Design
Prospective, consecutive case series.
Methods
Thirty-three consecutive eyes of 33 symptomatic PCV patients (17 men, 16 women, mean age 75 ± 8.7 years), not treated previously, received an intravitreal injection of 2.0 mg of aflibercept monthly for 3 months. Changes in best-corrected visual acuity (BCVA), optical coherence tomography (OCT) findings, and indocyanine green angiography (ICGA) findings 3 months after initial injection were evaluated.
Results
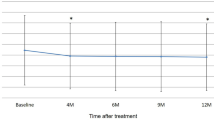
Compared with baseline, mean BCVA at 3-month visit significantly improved (0.40 ± 0.34 vs 0.22 ± 0.20 log minimum angle of resolution [logMAR] unit, P < 12 0.001). Eight eyes (24 %) showed improvement in BCVA ≥ 0.3 logMAR unit, and no eyes (0 %) showed a decrease in BCVA of ≥ 0.3 logMAR unit. Mean foveal thickness improved significantly (348 ± 184 μm at baseline vs 194 ± 32 μm at 3-month visit, P < 15 0.001). At 3-month visit, 31 eyes (97 %) achieved dry macula evaluated on OCT. Polypoidal lesions disappeared completely on ICGA in 16 eyes (48 %), and the number and/or the size of polypoidal lesions decreased in nine eyes (27 %). The remaining eight eyes (24 %) had unchanged polypoidal lesions. A branching vascular network remained and was unchanged in diameter in all 27 eyes in which it was detected at baseline.
Conclusion
Intravitreal aflibercept was well-tolerated in patients with treatment-naïve PCV over the short-term.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypoidal choroidal vasculopathy (PCV) is a variant of wet age-related macular degeneration (AMD) characterized [1] by an inner choroidal vascular network of vessels with terminating polypoidal structures observed clearly on indocyanine green angiography (ICGA) [2–5]. Several treatment strategies have been reported for PCV [6]. Among them, photodynamic therapy (PDT) [7] and intravitreal injection of anti-vascular endothelial growth factor (VEGF) antibodies, including ranibizumab (Lucentis; Genetech, Inc., South San Francisco, CA, USA) [8–10] and bevacizumab (Avastin; Genetech, Inc.) [11, 12] have shown favourable results. However, several problems have been reported with these treatments. Serious complications after PDT for PCV have been reported, including massive subretinal hemorrhage and retinal atrophy [13]. With regard to anti-VEGF monotherapy, even if performed by three monthly injections of ranibizumab, almost 65 % of eyes achieved a dry macula [8, 9], and only 20–30 % eyes obtained complete disappearance of polypoidal lesions [8–10]. Furthermore, it was reported that vascular abnormalities, such as PCV, may be hidden in wet AMD refractory to a course of anti-VEGF monotherapy [14, 15].
Aflibercept (VEGF-Trap Eye/Eylea; Regeneron, Tarrytown, NY, USA) is a recently approved anti-VEGF drug with greater binding activity to VEGF molecules than either ranibizumab or bevacizumab [16, 17]. The VIEW (VEGF-Trap Eye: Investigation of Efficacy and Safety in Wet AMD) 1 and VIEW 2 studies demonstrated that aflibercept given every 2 months after three initial loading doses was not inferior to monthly ranibizumab in maintaining vision in patients with treatment-naïve wet AMD over a 61-year period [18]. Several recent studies have shown that aflibercept monotherapy can reduce exudative changes and preserve visual acuity in AMD resistant to ranibizumab and/or bevacizumab monotherapy [19–23]. With regard to PCV, Miura et al. [24] reported that switching therapy to aflibercept was effective for patients with PCV who developed tachyphylaxis to ranibizumab. To our knowledge, there have been no reports regarding the efficacy of intravitreal aflibercept monotherapy in patients with treatment-naïve PCV. In the present study, we evaluated the short-term efficacy of aflibercept monotherapy in patients with treatment-naïve PCV.
Patients and methods
This was a prospective, consecutive case study. Thirty-three consecutive eyes of 33 patients with PCV who had progressive visual symptoms and had not undergone previous treatments received an intravitreal injection of 2.0 mg of aflibercept monthly for 3 months at the Kanazawa University Hospital from December 2012 to August 2013. The institutional review board approved the study protocol. PCV was diagnosed based on the fundus findings and ICGA findings. Diagnosis met at least one of the following criteria [25]: the presence of elevated orange-red lesions (excluding pigment epithelial detachment, choroidal hemangioma, and subretinal blood) observed by fundus examination, or characteristic polypoidal lesions on ICGA [2–5]. PCV was diagnosed in all eyes after confirmation of the presence of polypoidal lesions on ICGA. At baseline, all eyes had a complete ophthalmic examination, including best-corrected visual acuity (BCVA) measured by decimal visual acuity, digital simultaneous fluorescein angiography (FA) and ICGA using Heidelberg Retina Angiograph 2 (HRA-2; Heidelberg Engineering, Inc., Dossenheim, Germany) and optical coherence tomography (OCT) (3D-OCT 1000 MARK 2; software version 3.36, Topcon Corporation, Tokyo, Japan). The 3D-OCT 1000 MARK 2 scan protocols consisted of the radial scan (12 0.30-s radial scans with 1024 A-scans each, 6 mm in length). Ophthalmic examinations, including BCVA measurement and OCT, were performed monthly. Digital simultaneous FA and ICGA using HRA-2 were also performed 3 months after the initial aflibercept injection.
In this study, we defined dry macula as the absence of both subretinal fluid (SRF) and intraretinal fluid (IRF) evaluated on all 12 radial scans on OCT. Complete resolution of polypoidal lesions 3 months after the initial intravitreal injection of aflibercept was defined as the detection of no polypoidal lesions on ICGA, while partial resolution was defined as a decrease in the number and/or size of polypoidal lesions. Each size of polypoidal lesions and branching vascular networks was determined by careful observation of ICGA results through the early to late phase, and measured as the greatest linear dimension using an electronic calliper within HRA-2. A greater than 20 % decrease or increase in the greatest linear dimension was defined as a decrease or increase respectively.
The differences in BCVA and foveal thickness between baseline and 3 months after the initial injection were analyzed using the paired Student’s t test. The logarithm of minimal angle of resolution (logMAR) unit calculated from decimal visual acuity was used to analyze the BCVA. Statistical analysis was performed using SPSS 11.5.1 for Windows (SPSS Inc, Chicago, IL, USA). In all analyses, P < 0.05 was taken to indicate statistical significance.
Results
Thirty-three patients (17 men, 16 women, mean age 75 ± 8.7 years, range, 60–89 years) were included in the study. When compared with baseline, the mean BCVA 14 months after the initial injection improved significantly (0.40 ± 0.34 vs 0.22 ± 0.20 log MAR unit, P < 0.001). Eight eyes (24 %) showed improvement in BCVA of ≥ 0.3 logMAR unit, and no eyes (0 %) had a decrease in BCVA of ≥ 0.3 logMAR unit.The mean foveal thickness improved significantly (348 ± 184 μm at baseline vs 194 ± 32 μm 3 months after the initial injection, P < 0.001). At baseline, OCT showed SRF in 32 eyes (97 %) and IRF in nine eyes (27 %). At 3 months, 31 eyes (97 %) achieved dry macula. The numbers of initial consecutive injections to achieve dry macula was one injection in 14 eyes (44 %), two injections in ten eyes (31 %) and three injections in seven eyes (22 %). Dry macula was not achieved in only one eye (3 %), even following three monthly injections. At baseline, pigment epithelial detachment (PED) was detected in seven eyes (21 %) (serous PED; two eyes, fibrovascular PED; two eyes, hemorrhagic PED; three eyes) on OCT. Complete disappearance of serous PED was recognized in two eyes by 2 months after the initial injection. The size of fibrovascular PED of two eyes was unchanged, and the size of haemorrhagic PED of three eyes reduced gradually through the 3-month treatment period.
At baseline, ICGA detected polypoidal lesions in all 33 eyes; 16 eyes (48 %) had multiple polypoidal lesions, and 17 eyes (52 %) had one lesion. OCT showed a steep, protruding retinal pigment epithelium (RPE) corresponding to polypoidal lesions in all eyes. The mean number of polypoidal lesions of 15 eyes with multiple lesions was 3.79 ± 2.55 (range: 2–12), except in one eye (Fig. 1) that had so many polypoidal lesions that we could not clearly determine the demarcation of each polyp and could not accurately count the number of polypoidal lesions. Three months after the initial intravitreal injection of aflibercept, the complete resolution of polypoidal lesions and partial resolution were detected on ICGA in 16 eyes (48 %) (Fig. 2) and in nine eyes (27 %) (Fig. 1) respectively. The remaining eight eyes (24 %) showed no changes in size and number of polypoidal lesions (Fig. 3). At baseline, a branching vascular network was also detected in 27 eyes (82 %). In the remaining six eyes (18 %), retinal and subretinal hemorrhage obscured a possible branching vascular network. Three months after the initial intravitreal injection of aflibercept, a branching vascular network remained and showed no change in greatest linear dimension of the branching vascular network in all 27 eyes in which the network was detected at baseline (Figs. 1 and 3).
The left eye of a 70-year-old man with polypoidal choroidal vasculopathy (PCV). a At baseline, indocyanine green angiography (ICGA) showed many polypoidal lesions and a branching vascular network. b Horizontal optical coherence tomography (OCT) showed protrusions of the retinal pigment epithelium (RPE) corresponding to polypoidal lesions on ICGA, subretinal fluid (SRF) and intraretinal fluid (IRF). c One month after the third intravitreal injection of aflibercept, ICGA showed decreases in number and size of polypoidal lesions. The branching vascular network remained and showed no change in diameter. The baseline greatest linear dimension of abnormal network and that after treatment were 5 030 μm and 5 040 μm, respectively (yellow bidirectional arrows). d OCT showed complete resolution of SRF and IRF. The decimal visual acuity improved from 0.1 to 0.3
The right eye of a 67-year-old woman with polypoidal choroidal vasculopathy (PCV). a At baseline, indocyanine green angiography (ICGA) showed a polypoidal lesion. b Horizontal optical coherence tomography (OCT) showed protrusion of the retinal pigment epithelium (RPE) corresponding to a polypoidal lesion on ICGA and 22 subretinal fluid (SRF). c One month after the third intravitreal injection of aflibercept, ICGA showed complete resolution of the polypoidal lesion. d OCT showed complete resolution of protrusion of the RPE and SRF. The decimal visual acuity improved from 0.8 to 0.9
The left eye of a 75-year-old man with polypoidal choroidal vasculopathy (PCV). a At baseline, indocyanine green angiography (ICGA) showed two polypoidal lesions and a branching vascular network. b Horizontal optical coherence tomography (OCT) showed mild protrusion of the retinal pigment epithelium (RPE) corresponding to polypoidal lesions on ICGA and subretinal fluid (SRF). c One month after the third intravitreal injection of aflibercept, ICGA showed no changes in two polypoidal lesions and branching vascular network. The baseline greatest linear dimension of abnormal network and that after treatment were 2 060 μm and 2 050 μm, respectively (yellow bidirectional arrows). d OCT showed complete resolution of SRF. The decimal visual acuity improved from 0.2 to 0.6
None of the patients developed systemic complications, such as thromboembolic events or cerebral vascular accidents. Ocular complications, including intraocular inflammation, elevated intraocular pressure, increase in cataracts, and endophthalmitis were not observed.
Discussion
In the present study, we investigated the short-term efficacy of aflibercept monotherapy in patients with treatment-naïve PCV. In VIEW 1 and VIEW 2 studies in which the patients with treatment-naïve wet AMD were given three monthly injections of 2.0 mg of aflibercept as loading doses, the mean change in BCVA from baseline to 3 months after the initial injection was almost 7 letters using the Early Treatment of Diabetic Retinopathy Study charts (ETDRS) [18]. In this study, the mean change in BCVA from baseline to 3 months after the initial injection was 8.9 letters when the change of BCVA measured by decimal visual acuity was converted to the change in ETDRS score using the conversion table reported by Tano et al. [26]. Table 1 shows the outcomes in the present and previous studies 3 months after the initial anti-VEGF monotherapy in eyes with treatment-naïve PCV [8–11]. Improvements in BCVA of ≥ 6 0.3 logMAR unit were 38 % in this study for aflibercept monotherapy, 0 % in bevacizumab monotherapy [11], and 24–38 % in ranibizumab monotherapy [8, 9] reported previously. The mean change in BCVA evaluated with ETDRS score in this study was almost equivalent to the result of +8.5 letters of the group treated with ranibizumab monotherapy in the EVEREST study (efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy) [10].
In previous studies of treatment-naïve PCV, polypoidal lesions decreased on ICGA in 9 % with bevacizumab monotherapy [11] and in almost 30–80 % with ranibizumab monotherapy [8–10]. The complete disappearance of polypoidal lesions in these previous reports was detected on ICGA in 9 % of cases treated with bevacizumab monotherapy [11], and in almost 19–33 % of those treated with ranibizumab monotherapy [8–10]. In this study, polypoidal lesions decreased in 25 eyes (76 %) and disappeared completely in 16 eyes (48 %) treated with aflibercept. Therefore, aflibercept may not be inferior to ranibizumab in resolution of polypoidal lesions of eyes with treatment-naïve PCV, and may be superior to ranibizumab for complete disappearance of polypoidal lesions.
In previous reports, the branching vascular network persisted in 77–100 % of eyes treated with ranibizumab [8–10] or bevacizumab [11] monotherapy. In this study, the disappearance of the small diameter of the branching vascular network or reduction in the diameter of the branching vascular network was not observed in all eyes, the branching vascular network remained in all 27 eyes in which the network was detected at baseline. This result was consistent with those of previous studies. Residual branching vascular networks after laser photocoagulation were reported to be associated with disease recurrence [27] and recurrent or newly developed polypoidal lesions after PDT, all of which were connected to a residual branching vascular network [28]. As aflibercept monotherapy did not resolve the branching vascular network of PCV in the present study, there is a potential risk of recurrence of polypoidal lesions after the induction phase of aflibercept therapy.
The complete resolution of SRF on OCT at 3 months after initial treatment with anti-VEGF drugs was 97 % in this study with aflibercept, 20 % with bevacizumab [11] and almost 65–66 % with ranibizumab [8, 9]. Therefore, aflibercept may be superior to bevacizumab and ranibizumab in reduction of SRF derived from polypoidal lesions and branching vascular network. On the other hand, the resolution of PED in this study was observed on OCT in 67 % of eyes, which was consistent with the results reported previously for other anti-VEGF drugs [8, 9, 11].
The possible mechanism underlying the superiority of aflibercept to bevacizumab and ranibizumab in complete resolution of SRF on OCT and polypoidal lesions on ICGA was considered to involve the potentially important roles of VEGF-A and other members of the VEGF family, including placental growth factor (PlGF) [29] and VEGF-B [30], in angiogenesis and hyperpermeability. These substances may be related to the activities of PCV. Bevacizumab and ranibizumab could only bind VEGF-A. Aflibercept could bind not only VEGF-A, but also VEGF-B and PlGF [17]. Another potential mechanism is the higher binding affinity of aflibercept to VEGF compared to ranibizumab [17]. These pharmacologically superior characteristics of aflibercept to bevacizumab and ranibizumab may be related to the greater efficacy of aflibercept for suppression of PCV vascular lesions. However, we were unable to draw definitive conclusions regarding the superiority of aflibercept or ranibizumab for treatment-naïve PCV, because the populations in the present study with regard to aflibercept and previous studies with regard to ranibizumab [8–10] were inherently different. PCV has a variety of clinical manifestations with different natural courses [31, 32]. Further randomised controlled clinical trials should be performed to confirm the superiority of aflibercept or ranibizumab for treatment-naïve PCV.
Koh et al. [10] reported that both combination therapy of PDT with ranibizumab and PDT monotherapy for treatment-naïve PCV were superior to ranibizumab monotherapy in achieving complete polyp regression at month 6 in the EVEREST study (77.8% and 71.4 % vs 28.6 %; P ≤ 0.01), which is the only randomised controlled clinical trial published to date. The results with respect to polyp regression in this study seem inferior to the results obtained in the EVEREST study with PDT. But the improvement of BCVA in this study (±8.9 letters) seem comparable to the result of the group treated with combination therapy of PDT with ranibizumab and PDT monotherapy in the EVEREST study (±10.9 and ±7.5 letters respectively). Improvements in BCVA and central retinal thickness favoured combination therapy, but the study did not have sufficient power to demonstrate the statistical significance of differences. Therefore, improvement of BCVA may be influenced by the existence of improvement of exudative changes on OCT rather than the existence of polyp regression on ICGA in the short time period in patients with treatment-naïve PCV. Lai et al. [33] concluded that combination therapy and PDT monotherapy were more effective for achieving complete regression of the polypoidal lesions in ICGA compared with ranibizumab monotherapy at 3 months in which there were a few cases of previously treated PCV, but there were no significant differences in BCVA or visual change between eyes initially treated with ranibizumab monotherapy, combination therapy, or PDT monotherapy at 12 months. There have been no previous studies concerning combination therapy of PDT with aflibercept for AMD, including PCV. Further studies are required for comparison of treatment results among combination therapy of PDT with aflibercept, PDT monotherapy, and aflibercept monotherapy in treatment-naïve PCV.
The present study provided evidence that one intravitreal injection of aflibercept monthly for 3 months improves visual and anatomical findings in patients with treatment-naïve PCV. Although the limitation of this study was the short follow-up, aflibercept therapy is safe and well-tolerated in eyes with treatment-naïve PCV. Long-term follow-up is necessary to determine the efficacy of aflibercept therapy for PCV.
References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B (1990) Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 10:1–8
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA (1995) Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 15:100–110
Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA (1997) The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 115:478–485
Yannuzzi LA, Wong DW, Sforzolini BS, Goldbaum M, Tang KC, Spaide RF, Freund KB, Slakter JS, Guyer DR, Sorenson JA, Fisher Y, Maberley D, Orlock DA (1999) Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 117:1503–1510
Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA (2004) Polypoidal choroidal vasculopathy. Surv Ophthalmol 49:25–37
Laude A, Tan LE, Wilson CG, Lascaratos G, Elashry M, Aslam T, Patton N, Dhillon B (2010) Intravitreal therapy for neovascular age-related macular degeneration and inter-individual variations in vitreous pharmacokinetics. Prog Retin Eye Res 29:466–475
Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, Ikuno Y, Tano Y (2008) One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 115:141–146
Hikichi T, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, Kosaka S, Matsushita R, Takami K (2010) Improvement of angiographic findings of polypoidal choroidal vasculopathy after intravitreal injection of ranibizumab monthly for 3 months. Am J Ophthalmol 150:674–682
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S (2011) Predictive factors of resolved retinal fluid after intravitreal ranibizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 95:1555–1559
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH (2012) Everest study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 32:1453–1464
Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M, Tano Y (2008) Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 92:70–73
Lai TY, Chan WM, Liu DT, Luk FO, Lam DS (2008) Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol 92:661–666
Akaza E, Yuzawa M, Mori R (2011) Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 55:39–44
Cho M, Barbazetto IA, Freund KB (2009) Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 148:70–78
Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP (2010) Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol 150:666–673
Stewart MW, Rosenfeld PJ (2008) Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 92:667–668
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U; VIEW 1 and VIEW 2 Study Groups (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548
Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, Sorenson J, Freund KB (2013) Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina 33:1605–1612
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK (2013) Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 156:29–35
Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR, Mahajan VB (2013) Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 156:15–22
Ho VY, Yeh S, Olsen TW, Bergstrom CS, Yan J, Cribbs BE, Hubbard GB 3rd (2013) Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol 156:23–28
Chang AA, Li H, Broadhead GK, Hong T, Schlub TE, Wijeyakumar W, Zhu M (2014) Intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Ophthalmology 121(1):188–192. doi:10.1016/j.ophtha.2013.08.035
Miura M, Iwasaki T, Goto H (2013) Intravitreal aflibercept for polypoidal choroidal vasculopathy after developing ranibizumab tachyphylaxis. Clin Ophthalmol 7:1591–1595. doi:10.2147/OPTH.S50634
Japanese study group of polypoidal choroidal vasculopathy (2005) Criteria for diagnosis of polypoidal choroidal vasculopathy [in japanese]. Nippon Ganka Gakkai Zasshi 109:417–427
Tano Y, Ohji M, Ishibashi T, Shiraga F, Tokoro T, Yuzawa M, Yoshimura N (2009) Re-treatment guideline of ranibizumab (genetical recombination) in the maintenance phase. Nihon Ganka Gakkai Zasshi 113:1098–1103
Yuzawa M, Mori R, Haruyama M (2003) A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 47:379–384
Wakabayashi T, Gomi F, Sawa M, Tsujikawa M, Tano Y (2008) Marked vascular changes of polypoidal choroidal vasculopathy after photodynamic therapy. Br J Ophthalmol 92:936–940
Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C, Nguyen L, Foidart JM, Noël A, Munaut C (2003) Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 44:3186–3193
Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, Kumar A, Rissanen TT, Wang B, Nagai N, Fons P, Fariss R, Zhang Y, Wawrousek E, Tansey G, Raber J, Fong GH, Ding H, Greenberg DA, Becker KG, Herbert JM, Nash A, Yla-Herttuala S, Cao Y, Watts RJ, Li X (2009) VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A 106:6152–6157
Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K, Nagai Y, Takahashi K (1999) Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 117:1035–1042
Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, Takahashi K, Matsumura M (2002) Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 133:639–648
Lai TY, Chan WM, Liu DT, Lam DS (2009) Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 29:750–756
Acknowledgments
The authors have no proprietary interest in any aspect of this report, and they report no financial support or financial conflict of interest. Involvement in tasks as follows: conception and design of study (S.I.), analysis and interpretation (S.I., K.S.), writing of the article (S.I.), critical revision of the article (S.I., K.S.), final approval of the article (S.I., K.S.), and data collection (S.I.). The current research followed the tenets of the Declaration of Helsinki, and informed consent was obtained from all subjects after explanation of the study protocol. The institutional review board at the Kanazawa University Hospital approved the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ijiri, S., Sugiyama, K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naïve polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 253, 351–357 (2015). https://doi.org/10.1007/s00417-014-2707-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2707-2