Abstract
Purpose
To evaluate the 1-year treatment outcomes of intravitreal aflibercept injections (IVA) using a treat-and-extend regimen for polypoidal choroidal vasculopathy (PCV).
Methods
Thirty-seven eyes with treatment-naive PCV treated with IVA using a treat-and-extend regimen for 1 year were reviewed retrospectively. The main outcome measures were changes in the best-corrected visual acuity (BCVA) and central retinal thickness (CRT), and the treatment interval at 1 year. The predictive factors for patients who could not continue to extend the treatment interval because of poor response to IVA or recurrence were analyzed.
Results
The mean logarithm of the minimum angle of resolution BCVA improved from 0.37 at baseline to 0.21 at 1 year (P < 0.001). The mean CRT decreased from 342.3 μm at baseline to 196.6 μm at 1 year (P < 0.001). The mean treatment interval was 9.7 weeks at 1 year (4 weeks in 11 eyes [29.7%], 6 weeks in 1 eye [2.7%], 8 weeks in 2 eyes [5.4%], 10 weeks in 1 eye [2.7%], and 12 weeks in 22 eyes [59.5%]). A larger number of polypoidal lesions at baseline was predictive for patients who could not continue to extend the treatment interval.
Conclusions
IVA using a treat-and-extend regimen is effective for improving BCVA and CRT in eyes with PCV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypoidal choroidal vasculopathy (PCV) is an exudative maculopathy affecting vision, with a prevalence of 10–54% in Asian patients and 8–12% in white patients with presumed exudative age-related macular degeneration (AMD) [1, 2]. Clinically, PCV, which is characterized by polypoidal lesions and a branching vascular network seen on indocyanine green angiography (ICGA) [3, 4], causes recurrent retinal hemorrhages, fluid accumulation, and lipid exudation, eventually resulting in chorioretinal atrophy and permanent visual loss [5].
The therapeutic approaches to PCV include photodynamic therapy (PDT), intravitreal injections of antivascular endothelial growth factor (VEGF) pharmacologic agents, and a combination of both. Although previous studies have reported that PDT is more effective than anti-VEGF drugs, such as ranibizumab, for causing regression of the polypoidal lesions, the visual outcomes were reportedly better in patients who received anti-VEGF therapy than in those who received PDT [2, 6]. The limited visual improvement with PDT might result from subretinal hemorrhages, pigment epithelial tears, or atrophy of both the retinal pigment epithelium and the choriocapillaris in the area to which PDT is applied [7,8,9]. Therefore, because of their efficacy and safety, anti-VEGF drugs are considered an important therapeutic option. Of the various anti-VEGF drugs available, aflibercept (Eylea; Regeneron, Tarrytown, USA) reportedly has a higher binding affinity to VEGF, a longer intravitreal half-life relative to other anti-VEGF agents, and the capacity to antagonize growth factors other than VEGF [10,11,12,13]. Recently, we reported the efficacy of intravitreal aflibercept injections (IVA) for improving visual acuity (VA) and causing regression of polyps in eyes with PCV [14].
Regarding the treatment regimens of anti-VEGF therapy, treat-and-extend regimens have recently been used to treat AMD [15, 16]. A treat-and-extend regimen is an individualized regimen that aims to decrease the numbers of clinic visits and injections by determining the optimal treatment interval [17]. Specifically, the interval between injections is extended gradually after resolution of the retinal exudation in response to initial monthly injections. With recurrence, the interval is shortened. The efficacy of a treat-and-extend regimen in controlling disease activity and improving VA with fewer clinical visits and injections as compared with other regimens, such as fixed dosing and pro re nata (PRN) regimens, has been reported in patients with AMD [18,19,20,21,22,23]. However, to the best of our knowledge, no reports have evaluated a treat-and-extend regimen for treating PCV.
In the current study, we evaluated the 1-year outcomes of a treat-and-extend regimen with IVA used to treat previously untreated PCV to clarify the effectiveness of this approach. We also sought to identify the prognostic factors for patients who could not continue to extend the treatment intervals because of poor response to IVA or recurrence of PCV.
Patients and methods
We retrospectively reviewed the medical records of 37 eyes of 37 consecutive patients with treatment-naive PCV who had been treated with IVA using a treat-and-extend regimen for at least 1 year at Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences between June 2013 and August 2014. The institutional review board of this institution approved the study, which was conducted according to the tenets of the Declaration of Helsinki. PCV was diagnosed on the basis of the presence of abnormal branching vascular networks and characteristic polypoidal vascular lesions seen on ICGA images. Eyes with other retinal diseases, i.e., diabetic retinopathy, retinal vein occlusion, or myopic degeneration, were excluded from the study, as were eyes that had undergone vitrectomy.
All the patients underwent a comprehensive ophthalmologic examination at all visits, which included measurement of decimal best-corrected VA (BCVA) using a Landolt C acuity chart at 5 m and intraocular pressure, indirect ophthalmoscopy, slit-lamp biomicroscopy with a contact lens, and optical coherence tomography (OCT) (swept-source OCT, DRI OCT-1 Atlantis; Topcon, Tokyo, Japan, or spectral-domain OCT, Spectralis; Heidelberg Engineering, Heidelberg, Germany). Fluorescein angiography (FA) and ICGA were performed at baseline and at 3 months and 1 year (12 months up to 15 months) after the primary aflibercept injection. FA and ICGA images were obtained using the Heidelberg Retina Angiograph system (Heidelberg Engineering, Dossenheim, Germany) with a confocal scanning laser ophthalmoscope. The greatest linear dimension (GLD) of the lesions was measured by both FA and ICGA. The GLD on FA includes areas of dye leakage, pigment epithelial detachments, and subretinal hemorrhages. The GLD on ICGA includes areas of polypoidal lesions and abnormal branching vascular networks.
All the patients were initially treated with at least 3 monthly IVA (2 mg). The monthly injections continued until no retinal exudation (i.e., new subretinal hemorrhages or subretinal and/or intraretinal fluid) was observed on OCT or slit-lamp biomicroscopy. When the retinal exudation resolved, the interval to the next injection and follow-up period was extended by 2 weeks up to a maximum of 12 weeks, at which point the treatment was maintained at that interval. If the exudation recurred, the interval was shortened by 2 weeks to a minimum interval of 4 weeks. When the retinal exudation resolved after a reduced interval, the treatment was maintained at this frequency. Interval extensions were restarted if no recurrence was observed for 6 consecutive months.
The BCVA was converted to logarithm of the minimum angle of resolution (logMAR) units for statistical analysis. After the BCVA had been assessed, the eyes were categorized into the following 3 groups on the basis of the BCVA change at 1 year from baseline (change defined as ≥0.3 logMAR units): improved, stable, and deteriorated. Complete resolution of the polypoidal lesions was evaluated by ICGA at 3 months and at 1 year after the first injection. The number of injections and visits during the first year and the intervals between the injections at 1 year were also calculated. The changes in the BCVA and central retinal thickness (CRT) between baseline and 1 year after the first injection were compared using the paired t test. We used the Mann–Whitney test to compare the changes in the BCVA and CRT between eyes with no recurrence and eyes with poor response or recurrence. Poor response to IVA was defined as persistent retinal exudative changes, including subretinal hemorrhages and fluid accumulation, for 1 year despite IVA every 4 weeks. To identify the prognostic factors for patients who could not continue to extend treatment intervals because of poor response to IVA or recurrence of PCV, multivariate logistic regression analysis was performed. The factors included in this analysis were patient age, BCVA, CRT, GLD on FA, GLD on ICGA, the number of polyps and size of the largest polyp at baseline, and regression of the polypoidal lesions at 3 months after the first IVA. We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) for poor response or recurrence of PCV. All statistical analyses were performed with version 22.0 SPSS software (IBM, Armonk, USA). Probability values less than 0.05 were considered significant.
Results
The mean patient age was 73.3 years (range 55–89). Seven of the 37 patients were female. All 37 eyes had retinal exudative changes, including subretinal hemorrhages and fluid accumulation involving the fovea, on the baseline OCT images. During the follow-up period, no adverse events occurred and all the patients could follow the protocol.
At baseline, the mean GLD on FA was 3629.1 µm (range 1459–9482); the mean GLD on ICGA was 2427.3 µm (range 1252–6358); the mean number of polyps was 2.9 (range 1–8); and the mean diameter of the largest polyp was 275.8 µm (range 122–581). The polypoidal vascular lesions or abnormal branching vascular networks were at the subfovea in all eyes.
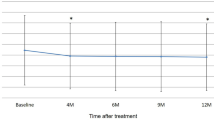
The mean logMAR BCVA of the 37 eyes improved significantly from 0.37 ± 0.37 (range −0.18 to 1.52) at baseline to 0.21 ± 0.29 (range −0.18 to 1.10) at 1 year (P < 0.001) (Fig. 1a). The BCVA improved in 11 eyes (29.7%), was stable in 25 eyes (67.6%), and deteriorated in 1 eye (2.7%). The mean ± standard deviation CRT decreased significantly from 342.3 ± 111.6 μm (range 130–677) at baseline to 196.6 ± 55.4 μm (range 112–323) at 1 year (P < 0.001) (Fig. 1b). One year after the first IVA, the OCT images showed that the retinal exudative changes had resolved in 27 eyes (73.0%) and remained in 10 eyes (27.0%).
a Mean changes in best-corrected visual acuity (BCVA) from baseline in 37 eyes treated with aflibercept using a treat-and-extend regimen. b Mean changes in central retinal thickness (CRT) from baseline in 37 eyes treated with aflibercept using a treat-and-extend regimen. Error bars standard error of the mean. *P < 0.001
ICGA images were available at baseline and at 3 months and 1 year after treatment. Because of allergy to ICGA, the ICGA images were unavailable at 1 year for one of the 37 patients. At baseline, all eyes had at least 1 polypoidal lesion on the ICGA images. Three months after the first injection, ICGA showed that the polypoidal lesions had resolved in 17 eyes (45.9%); at 1 year, the polypoidal lesions had resolved in 19 eyes (51.4%). Of the eyes in which the polypoidal lesions had resolved at 3 months, the lesions recurred in 1 eye at 1 year.The total number of injections during the first year ranged from 7 to 12 (mean 8.2; median 7). The number of visits was the same as the number of injections during the first year because all patients were examined and treated at the same time. The intervals between injections at 1 year ranged from 4 to 12 weeks (mean, 9.7; median, 12). The distributions of the intervals between injections at 1 year were 4 weeks in 11 eyes (29.7%), 6 weeks in 1 eye (2.7%), 8 weeks in 2 eyes (5.4%), 10 weeks in 1 eye (2.7%), and 12 weeks in 22 eyes (59.5%) (Fig. 2).
Twenty-two eyes (59.5%) had no recurrence after 3 monthly injections, whereas 15 eyes (40.5%) had a poor response or recurrence, i.e., a poor response to IVA in 3 eyes and at least 1 recurrence in 12 eyes. We compared the changes in the BCVA and CRT between the eyes with no recurrence and the eyes with a poor response or recurrence (Fig. 3). The mean BCVA improved in both groups until 6 months after the first injection. The eyes with no recurrence had improvements in the mean BCVA at 1 year. However, a significantly greater decrease in the mean BCVA occurred in the eyes with a poor response or recurrence than in the eyes with no recurrence (P < 0.05) (Fig. 3a). Of the 12 eyes with recurrence, although the retinal exudative changes had resolved in 5 eyes at 1 year, the BCVA did not improve significantly in these eyes at 1 year (P = 0.940). At 1 year, a significantly greater decrease in CRT occurred in the eyes with no recurrence than in the eyes with a poor response or recurrence (P = 0.001) (Fig. 3b). Figures 4 and 5 show representative cases.
a Mean changes in best-corrected visual acuity (BCVA) from baseline between polypoidal choroidal vasculopathy (PCV) eyes with no recurrence and those with a poor response to IVA or recurrence. b Mean changes in central retinal thickness (CRT) from baseline between PCV eyes with no recurrence and those with a poor response to IVA or recurrence. The circles indicate eyes with no recurrence; the squares indicate eyes with a poor response to IVA or recurrence. *P < 0.05
An 80-year-old woman with polypoidal choroidal vasculopathy in her right eye responded well to the treat-and-extend regimen and did not have recurrences during the 1-year follow-up. a At baseline, indocyanine green angiography (ICGA) showed a polypoidal lesion (arrow) and a branching network (arrowheads). b Optical coherence tomography (OCT) showed subretinal fluid (arrow) and protrusion of the retinal pigment epithelium (RPE) due to a polypoidal lesion (arrowhead). The best-corrected decimal visual acuity was 0.4. c Three months after the first aflibercept injection, ICGA showed complete regression of the polypoidal lesions. d OCT showed resolution of the subretinal fluid and RPE protrusion. The visual acuity was 0.6. e, f At 1 year, ICGA and OCT showed complete regression of the polypoidal lesions and exudative changes. The injection interval at the final visit was 12 weeks and the visual acuity was 0.9
An 81-year-old man with polypoidal choroidal vasculopathy in his right eye responded to intravitreal aflibercept injections but had a recurrence with extension of the interval between injections. a At baseline, indocyanine green angiography (ICGA) showed polypoidal lesions (arrows) and a branching network (arrowheads). b Optical coherence tomography (OCT) showed subretinal fluid (arrow) and protrusions of the retinal pigment epithelium due to polypoidal lesions (arrowheads). The best-corrected decimal visual acuity was 0.9. c, d Three months after the first aflibercept injection, ICGA and OCT showed complete regression of the subretinal fluid and polypoidal lesions. The visual acuity was 1.2. e At 9 months, when the interval between injections was extended to 10 weeks, OCT showed recurrence of the subretinal fluid (arrows) and protrusions of the retinal pigment epithelium (arrowheads). The visual acuity was 0.5. The interval was shortened by 2 weeks. f, g One year after the first aflibercept injection, when the interval between injections was 4 weeks, ICGA and OCT showed regression of the polypoidal lesions and subretinal fluid. However, the visual acuity was still 0.6
We evaluated factors predictive for patients who could not continue to extend the treatment intervals because of a poor response to IVA or recurrence of PCV. Table 1 shows the results of the multivariate logistic regression analysis. The number of polyps at baseline was associated with a poor response or recurrence of PCV (OR, 2.237; 95% CI, 1.034–4.843; P = 0.041). This result indicated that a large number of polyps at baseline might be associated with patients who cannot continue to extend treatment intervals during the first year of a treat-and-extend regimen.
Discussion
In the current study, we administered IVA for PCV patients according to a treat-and-extend regimen and found that this approach improved the BCVA and retinal exudative changes. Furthermore, 62.2% of the patients extended the intervals of both visits and injections to up to 10 weeks or longer. Yamamoto et al. treated PCV with bimonthly fixed dosing of IVA and reported improvement in the mean BCVA (logMAR) from 0.31 ± 0.32 to 0.17 ± 0.28, reduction of the mean CRT from 315 ± 146 to 204 ± 115 μm, and resolution of the retinal exudative changes in 71.1% of patients. The mean numbers of visits and injections at 1 year were 12 and 7.1, respectively [24]. In the current study, using a treat-and-extend regimen, we observed an improvement in the mean BCVA (logMAR) from 0.37 ± 0.37 to 0.21 ± 0.29, reduction in the mean CRT from 342.3 ± 111.6 to 196.6 ± 55.4 μm, and resolution of the retinal exudative changes in 73.0% of the patients. The mean numbers of visits and injections at 1 year were both 8.2. These results indicated that the degree of improvement in the BCVA, CRT, retinal exudative change, and number of injections with a treat-and-extend regimen is comparable to those with a bimonthly fixed dosing regimen. Regarding the number of visits, the treat-and-extend regimen resulted in a lower number of visits than did the bimonthly fixed dosing regimen. It should be noted that the mean treatment interval at 1 year was 9.7 weeks and the percentage of patients whose treatment interval was 10 weeks or longer was 62.2% and that of patients whose treatment interval was 12 weeks was 59.5%. Hikichi et al. treated 85 PCV patients with ranibizumab injection using a PRN regimen for 1 year and reported that 28% of the patients did not require any additional injections after the 3 initial monthly injections [25]. Since a treat-and-extend regimen requires injection of the eyes at each follow-up visit regardless of the disease activity, their result suggests that the treat-and-extend regimen may have led to overtreatment of approximately 30% of the patients in our study. Further study with a longer observation period is required to evaluate whether a treat-and-extend regimen is effective in extending the treatment interval more and in decreasing the patient and physician burdens.
The percentage of patients in whom the treatment interval increased to 12 weeks at 1 year (59.5%) was higher than that in previous studies of treat-and-extend regimens. In the Lucentis Compared to Avastin Study (LUCAS), the percentage of patients in whom the treatment interval increased to 12 weeks at 1 year was 25.1% with bevacizumab and 37.1% with ranibizumab [20]; in the Treat-and-Extend Protocol in Patients with Wet Age-Related Macular Degeneration (TREX-AMD), the percentage of patients was 26% with ranibizumab [21]. There are 2 possible explanations for the discrepancies among these 3 studies. The first is the differences in the pharmacologic action of anti-VEGF drugs used in these studies. Although bevacizumab and ranibizumab block the VEGF-A isoform only, aflibercept binds to both VEGF-A and B isoforms and to placental growth factor [26, 27]. The binding affinity of aflibercept to VEGF is reportedly substantially higher than that of ranibizumab, and aflibercept potentially has a longer intravitreal half-life relative to other anti-VEGF agents [10]. Indeed, in the VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD (VIEW) study, aflibercept was as effective as ranibizumab in improving the VA in patients with AMD, but required fewer injections to achieve the same effect [28]. The second explanation is the differences in the subtypes of the target diseases included in the 3 studies. Although LUCAS and TREX-AMD included patients with AMD without distinguishing among the subtypes, the current study included patients with PCV only. Differences in the therapeutic response of AMD subtypes to anti-VEGF therapy remain controversial. Although some studies have indicated that PCV is refractory to anti-VEGF therapy [29, 30], a recent study found that dry maculas were seen significantly less frequently in patients with typical AMD than in patients with PCV [31]. Further studies are required to clarify the role of the AMD subtypes in the therapeutic effect of anti-VEGF therapy using a treat-and-extend regimen.
The current study found that the degree of improvement in BCVA was smaller in patients with a poor response or recurrence, i.e., patients who could not continue to extend the treatment interval, than it was in patients without recurrence at 1 year (Fig. 3). This result corroborates the results of a previous study of a PRN regimen that showed that patients with at least 1 recurrence had significantly worse BCVA than did patients without a recurrence [32]. In the current study, 12 of the 37 eyes had at least 1 recurrence. Of these, the retinal exudation had resolved completely in 5 eyes at the final examination. Interestingly, despite complete resolution of the exudative changes, the BCVA did not improve significantly in any of these 5 eyes. This result is consistent with that of a previous study that found that intravitreal ranibizumab injections with a PRN regimen did not improve the BCVA significantly in AMD eyes with a recurrence, even in cases in which the exudation resolved completely [33]. These findings indicate the importance of avoiding a recurrence after initial resolution of the exudation with anti-VEGF drugs to improve vision. Therefore, proactive treatment regimens are considered more appropriate than reactive treatment regimens such as PRN regimens.
The multivariate analysis showed that the presence of a larger number of polyps at baseline predicted a poor response or recurrence of PCV. There was also a trend toward a greater percentage of a poor response or recurrence of PCV in those with remaining polypoidal lesions after the 3 initial monthly injections; however, this did not reach statistical significance. Recent studies have indicated that the features of polypoidal lesions are important predictors of the therapeutic efficacy of anti-VEGF drugs in PCV. Some studies have reported that polyps with smaller diameters are associated with successful regression of exudation and no recurrence, whereas other studies have reported that the regression of polypoidal lesions is related directly to fluid resolution [34,35,36]. Because the current results showed smaller BCVA improvements in patients with a poor response or recurrence than in those without a recurrence, special considerations that include reductions in the extended period after resolution of the retinal exudation might be required in patients with a large number of polyps.
The current study was limited by its small sample size, retrospective nature, and lack of a control group. The period of 1 year might be too short to accurately assess treatment effectiveness and perform comparisons with other treatment regimens. In addition, other treatment options for PCV such as PDT were not considered. Further prospective studies with larger sample sizes and longer follow-up periods should clarify the efficacy of IVA using a treat-and-extend regimen to treat PCV.
References
Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997;115:478–85.
Koh A, Lee WK, Chen L-J, Chen S-J, Hashad Y, Kim H, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K, Nagai Y, et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999;117:1035–42.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10.
Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002;133:639–48.
Oishi A, Miyamoto N, Mandai M, Honda S, Matsuoka T, Oh H, et al. LAPTOP Study: a 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology. 2014;121:1151–2.
Chan W-M, Lam DSC, Lai TYY, Liu DTL, Li KKW, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy. Ophthalmology. 2004;111:1576–84.
Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27:335–41.
Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115:141–6.
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85.
Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92:667–8.
Ohr M, Kaiser PK. Aflibercept in wet age-related macular degeneration: a perspective review. Ther Adv Chronic Dis. 2012;3:153–61.
Balaratnasingam C, Dhrami-Gavazi E, McCann JT, Ghadiali Q, Freund KB. Aflibercept: a review of its use in the treatment of choroidal neovascularization due to age-related macular degeneration. Clin Ophthalmol. 2015;9:2355–71.
Hosokawa M, Shiraga F, Yamashita A, Shiragami C, Ono A, Shirakata Y, et al. Six-month results of intravitreal aflibercept injections for patients with polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:1087–91.
Jeng KW, Wilgucki J, Halperin S, Feuer WJ, Fine HF, Roth D, et al. Retina specialists treating age-related macular degeneration recommend different approaches for patients than they would choose for themselves. Retina. 2014;34:1796–801.
Freund KB, Korobelnik J-F, Devenyi R, Framme C, Galic J, Herbert E, et al. Treat- and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–506.
Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143:679–80.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2010;117:2134–40.
Abedi F, Wickremasinghe S, Islam AFM, Inglis KM, Guymer RH. Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina. 2014;34:1531–8.
Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–52.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration. Ophthalmology. 2015;122:2514–22.
Rayess N, Houston SKS, Gupta OP, Ho AC, Regillo CD. Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol. 2014;159:3–8.
Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP, et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122:1212–9.
Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, Maruko I, et al. One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology. 2015;122:1866–72.
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol. 2012;154:117–24.
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–8.
Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96:1157–8.
Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;148:70–8.
Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol. 2010;150:666–73.
Oishi A, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Nakanishi H, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159:853–60.
Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;156:652–60.
Kuroda Y, Yamahsiro K, Miyake M, Yoshikawa M, Nakanishi H, Oishi A, et al. Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment. Ophthalmology. 2015;122:2303–10.
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Predictive factors of resolved retinal fluid after intravitreal ranibizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2011;95:1555–9.
Kang HM, Koh HJ, Lee SC. Baseline polyp size as a potential predictive factor for recurrence of polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1519–27.
Cho HJ, Han SY, Kim HS, Lee TG, Kim JW. Factors associated with polyp regression after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2014;59:29–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Hosokawa, None; Y. Morizane, None; M. Hirano, None; S. Kimura, None; F. Kumase, None; Y. Shiode, None; S. Doi, None; S. Toshima, None; M. Hosogi, None; A. Fujiwara, None; T. Mitsuhashi, None; F. Shiraga, Grants (Santen), Board membership (Santen), Consultant fees (Alcon, Byer, Novartis, Santen), Lecture fees (Alcon, Hoya, Novartis, Santen, Senju, Topcon).
About this article
Cite this article
Hosokawa, M., Morizane, Y., Hirano, M. et al. One-year outcomes of a treat-and-extend regimen of intravitreal aflibercept for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 61, 150–158 (2017). https://doi.org/10.1007/s10384-016-0492-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-016-0492-7