Abstract
Introduction
To compare the intraocular pressure (IOP)-lowering effects of 0.005% latanoprost to that of 0.004% travoprost in eyes with open-angle glaucoma (OAG).
Methods
Forty-two patients with OAG who received either latanoprost or travoprost every evening for 12 weeks, and then switched to the other medication for another 12 weeks. The IOP measurements were made with a Goldmann applanation tonometer (GAT) at the baseline, and at 1, 3, 4, and 6 months after the treatment. The IOP at the untreated baseline and at the end of each treatment period was measured at 10:00, 12:00, and 16:00 hours. The central corneal thickness (CCT) was measured at each visit using an ultrasonic pachymeter.
Results
The mean baseline IOP was 13.9 ± 2.5 mmHg, and the CCT was 536.7 ± 30.5 μm. Latanoprost reduced the IOP by 2.5 ± 1.7 mmHg and travoprost by 2.6 ± 1.5 mmHg from the baseline (p = 0.6807). The CCT decreased significantly to 531.9 ± 30.3 at 3 months (p = 0.0160) and to 529.4 ± 30.5 μm at 6 months (p = 0.0002) after the therapy. The decrease was significantly greater in eyes after travoprost (p = 0.0049).
Conclusions
Travoprost has similar effect as latanoprost in reducing the IOP in glaucoma patients with relatively low IOPs. The use of prostaglandin analogs can decrease the CCT, and this change should be considered when the IOPs obtained by GAT are analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Open-angle glaucoma (OAG) with low intraocular pressures (IOPs) of ≤21 mmHg is referred to as normal-tension glaucoma (NTG). Its prevalence in Japanese over 40 years of age is 3.6%, and eyes with NTG make up 92.3% of all eyes with OAG in Japan [1]. Although the pathogenesis of NTG has not been fully determined, a 30% reduction of the baseline IOP has been recommended to prevent the progression of the glaucomatous visual field defects, even in eyes with NTG [2].
Prostaglandin analogs (PGAs) and/or prostamide have become the preferred first-line therapy for the management of glaucoma. At present, four of these drugs (latanoprost, travoprost, tafluprost and bimatoprost) are available in Japan. Of these, travoprost was originally developed with benzalkonium chloride (BAK) as a preservative; however, only 0.004% travoprost ophthalmic solution without BAK (Travatan Z, Alcon Laboratories Inc, Fort Worth, TX, USA) is now available in Japan. It has been reported that travoprost without BAK solution has a similar IOP-lowering effect and safety to that of travoprost with BAK [3], and its effectiveness is equal or greater than that of latanoprost [4–13]. Both drugs reduce the IOP by approximately 20 to 40% of the baseline IOP [4–6, 8–13]. However, there is little information on the IOP-lowering effect in eyes with comparatively low IOPs such as those with NTG.
The PGAs have also been shown to decrease the central corneal thickness (CCT) [6, 14–21], and because Goldmann applanation tonometry underestimates the IOP in eyes with thinner corneas [22–25], questions have arisen about the accuracy of the IOPs measured after the use of PGAs. Although there are several studies on the effect of different PGAs on the CCT [6, 18–21], which PGA would reduce the CCT the most has not been determined.
Thus, the purpose of this study was twofold: first, to compare the IOP-lowering effects and safety of latanoprost to that of travoprost in eyes with open-angle glaucoma with low IOPs; and second, to investigate whether the reduction of the CCT is different for these two PGAs.
Materials and methods
This was a prospective, open-labeled, randomized, and crossover comparison study. The study was approved by the Institutional Review Board of Gifu University Graduate School of Medicine. All patients were fully informed and gave their written consent before participation.
We studied 42 patients with OAG at the Gifu University Hospital between August 2008 and July 2009. The diagnosis of OAG was based on the following criteria: 1) both eyes had a gonioscopically open angle, 2) at least one of the eyes had visual field defects whose location corresponded to the glaucomatous disc excavation, and 3) neuroradiological, rhinological, and general medical examinations did not disclose any pathology responsible for the optic nerve damage other than glaucoma. A glaucomatous visual field defect was defined as one in which three or more contiguous points in the visual field were reduced by >5 decibels with one point reduced by >10 decibels below the age-specific threshold on static automated perimetry (Humphrey 30–2, Humphrey Field Analyzer, Humphrey Instruments, San Leandro, CA, USA). A glaucomatous optic nerve appearance was defined as the presence of a focal or a diffuse defect of the optic disc rim to less than 10% of the disc diameter. Patients with any secondary factors that might induce glaucoma, such as uveitis and lens exfoliation, in even one eye were excluded. In addition, patients were excluded if they had any intraocular surgery including laser therapy, or had any corneal condition, e.g., pterygium, that prevented reliable Goldmann applanation tonometry (GAT). If the patients were taking any ocular hypotensive agents, they had a washout period of at least 4 weeks before beginning the experiment.
At the baseline, all patients underwent ocular examinations including the visual acuity, slit-lamp biomicroscopy, measurements of the central corneal thickness (CCT), direct ophthalmoscopy, and visual field examinations. The IOPs were measured by a single examiner (AS) throughout the examination period with a GAT at 10:00, 12:00, and 16:00 hours in a sitting position.
The CCT was measured with an ultrasonic pachymetry (SP-100 Handy Pachymeter; Tomey, Japan) once on two separate days, and the average was used as the baseline value. The CCT was always measured around 9:30 hours because of its diurnal variation.
After the initial examinations, one-half of the patients were randomly assigned to receive 0.005% latanoprost (Xalatan, Pfizer, Hellas, Athens, Greece) and the other half to receive 0.004% travoprost (Travatan Z, Alcon Laboratories Inc, Fort Worth, TX, USA). Each drug was applied each every evening at 21:00 into both eyes for 12 weeks. At the end of the 12 weeks, the patients were crossed over to the other drug (Fig. 1). No washout period separated the treatment periods.
The IOPs were measured before (baseline), and at 12 and 24 weeks. The measurements were made at 10:00, 12:00, and 16:00 hours. At 4 weeks and 16 weeks, the IOPs were measured only at 10:00 hours. The CCT was measured at every visit before the IOP measurements, and the average of five consecutive readings was used for the statistical analyses. The development of any complications based on the patients’ solicited complaints or the ocular findings obtained by one investigator were recorded at each examination. The complications included bulbar conjunctival hyperemia, itching, hypertrichosis, periocular hyperpigmentation, and a deepening of the superior sulcus.
One eye was randomly chosen for the statistical analyses. The equation used to correct for the CCT was that proposed by Doughty and Zaman [26], and it was used to determine the effect of the CCT on the GAT value. The formula used was: corrected GAT = measured GAT minus [(CCT − 535) × (2.5/50)]. Statistical analyses comparing the diurnal IOP curve at baseline and between treatments were performed by repeated ANOVA. The mean, maximum and minimum IOPs and the CCTs were analyzed by Wilcoxon signed-rank test or the Mann–Whitney U test. Differences in the CCT between treatments were analyzed using a mixed model analysis (Grizzles model) considering the carry-over effect due to the crossover design. Also, the adverse effects were evaluated by the chi-square test. The planned sample number of 21 patients/group was based on an expected 95% confidence interval estimated for the mean changes from baseline and effect of the numbers. A standard deviation of 3.5 mmHg was used in determining the sample size. This study had an 80% power to detect a 1.55 mmHg difference in the measured IOPs. The level of significance for each contrast was set at p < 0.05. All statistical analyses were performed with the STATA software version 11.1 (StataCorp, College Station, TX, USA.).
Results
Patients
All 42 of the patients completed the protocol, and their demographic data are shown in Table 1. The mean±standard deviation (SD) age was 53.2 ± 11.8 years, and 23 of the patients were men. The differences in the age, gender, baseline IOPs, CCT, and visual field indices were not significant between the two groups (Table 2).
Intraocular pressures
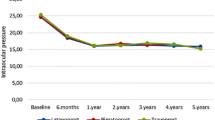
The mean baseline IOP was 13.9 ± 2.5 mmHg for all eyes. The baseline IOP was 14.4 ± 2.6 mmHg at 10:00 hours, 14.1 ± 2.7 mmHg at 12:00 hours, and 13.3 ± 2.5 mmHg at 16:00 hours. There were significant decreasing trends of the diurnal IOP curves at the baseline after the latanoprost and travoprost treatments (p < 0.001; repeated ANOVA). We also found a significant difference in diurnal IOP curves between the baseline and after treatment with latanoprost or travoprost (p < 0.001; repeated ANOVA). However, the differences in the IOPs for the individual times between the two treatments were not significant (10:00, p = 1.000; 12:00, p = 1.000; 16:00, p = 1.000: with Bonferroni correction). Both treatments significantly reduced the IOP from the baseline at each test time (all p < 0.001; Wilcoxon signed-rank test; Fig. 2). The mean diurnal IOP for patients treated with latanoprost was 11.4 ± 2.2 mmHg, and 11.4 ± 1.9 mmHg after travoprost (p = 0.9158; Wilcoxon signed-rank test). The values obtained at the different times were not significantly different between the two groups (Fig. 2). As with the absolute level of IOP, the mean percent reduction from the baseline for patients with latanoprost was 17.3 ± 10.9 %, and 16.9 ± 10.2 % with travoprost. This difference was not significant (Table 3).
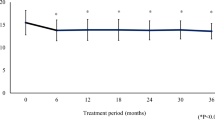
We then corrected the IOP values for the changes in the CCT. There was a significant difference in the corrected IOP at 10:00 hours (Fig. 3 and Tables 4).
Comparisons of corrected intraocular pressures (IOPs) at untreated baseline (open squares) versus latanoprost (open circles) and travoprost (closed circles). There was a significant difference in the IOP at 10:00 hours between drugs. The formula used was: corrected Goldmann applanation tonometry (GAT) = measured GAT minus [(CCT − 535) × (2.5/50)] proposed by Doughty and Zaman [26]. Errors bars indicate standard deviations
Central corneal thickness (CCT)
The mean baseline CCT was 536.7 ± 30.5 μm for all of the eyes. In eyes initially given travoprost, the CCT decreased significantly to 528.3 ± 31.3 μm at 3 months, to 530.2 ± 31.8 μm at 4 months, and to 528.4 ± 30.2 μm at 6 months (p = 0.0041, 0.0048, and 0.0011 respectively; Wilcoxon signed-rank tests; Table 5). There was a significant difference in CCT at 6 months in eyes initially treated with latanoprost compared to baseline CCT (p = 0.0473; Wilcoxon signed-rank test; Table 5). Additionally, a significant difference between the CCT at 3 months and 6 months was found in eyes initially started with latanoprost (p = 0.0305; Wilcoxon signed-rank test; Table 5). Mixed model analyses showed a significant difference in the CCT of eyes treated with travoprost and latanoprost (p = 0.049) even after considering the carry-over effects (p = 0.625).
Adverse effects
Mild bulbar conjunctival hyperemia was the most frequently adverse event, and was seen in 11 patients (26.2%) treated with latanoprost and 20 (47.6%) treated with travoprost (p = 0.0705; chi-square test). Hypertrichosis was observed in one patient treated with travoprost. Deepening of the upper lid sulcus was found in two female patients; one patient with travoprost, and the other patient with both drugs. None of the complications was severe enough to discontinue either drug.
Discussion
Earlier studies evaluated the effectiveness of travoprost and latanoprost in lowering the IOP in eyes with primary open-angle glaucoma or with ocular hypertension [6, 8–13, 18, 20, 21]. However, most of these studies examined patients with IOPs >21 mmHg. Our results demonstrated that the IOP-lowering ability of travoprost was not significantly different from that of latanoprost, even in eyes with OAG with comparatively low IOPs. We found a mean IOP reduction of 17.3% from the baseline with travoprost, and 16.9 % reduction with latanoprost. In addition, the CCT was found to decrease significantly more in eyes treated with travoprost than with latanoprost.
In earlier clinical studies comparing latanoprost to travoprost, some investigators concluded that the effectiveness of both agents to lower the IOP was comparable [8, 9, 11, 13], while others concluded that travoprost was more effective than latanoprost [4–6, 10, 12]. However, there were differences in the study design, baseline IOPs, types of glaucoma, endpoints chosen for analyses, and the statistical methods [7]. In the studies that reported that the reduction of the IOP by travoprost was not significantly different from that of latanoprost, the IOP was reduced by 22.7% to 44.0% of the untreated baseline [8–13]. These percentages are much higher than that observed in our study; however, our patients had relatively low baseline IOPs. In fact, it has also been reported that travoprost reduced the IOP in eyes with NTG by 16.1% to 20.2% of the baseline IOP [27–29], which is quite comparable to our findings. There is evidence that the CCT can affect the values of the IOP measured by an applanation tonometer, and formulas have been presented which can convert the measured IOP to the real IOP taking into account the CCT [26, 30, 31]. However, at present none of these formulas has been universally accepted [25], and some authors even question the clinical relevance of the corrections in the management of glaucoma [32, 33]. We found that the percentage reduction of the IOP at 10:00 hours was significantly greater after latanoprost than after travoprost when the CCT was considered. This tendency was just the opposite of one report which reported a better effectiveness of lowering the IOP in the early morning (8:00 and 10:00 hours) by travoprost than latanoprost when receiving each drug once every evening at 21:00 hours [13]. Additionally, some authors have pointed out the unique IOP-lowering characteristics of travoprost including an earlier time of reaching peak activity [5, 9], and a longer persistent effect over the 24-hour dosing schedule even with only one instillation [10, 11, 34]. This latter phenomenon is supported by a laboratory result [35] that showed that travoprost binds more strongly to the prostaglandin F2αreceptor than latanoprost.
Recently, the effect of prostaglandin analogues (PGAs) on CCT has been emphasized in the large-scale multi-center trials because it can alter the IOP values [16, 17]. Stefan and coworkers reported that the decrease in CCT at 3 months was not significantly different in patients using either travoprost (6.23 μm) or latanoprost (4.20 μm) [18]. Sen et al. demonstrated 6.7-μm and 7.7-μm CCT thinning in the groups treated respectively with latanoprost and bimatoprost at 24 months [19]. Hatanaka and colleague reported that groups treated with PGAs had a significant CCT decrease during an 8-week period (4.69 μm, 4.06 μm, and 6.22 μm decrease for latanoprost, bimatoprost and travoprost respectively) [20]. However, no significant differences were found among the three groups [20]. Arcieri et. al. reported after a 4-week trial that CCT was reduced by 1.15-μm, 3.15-μm, and 0.88-μm for latanoprost, bimatoprost, and travoprost respectively, and they concluded that only topical bimatoprost induced a statistically significant decrease in CCT [6]. Although all authors agree that the PGAs and prostamide can lead to a decrease in CCT; which one reduced CCT the most has not been agreed on. Our results showed a significantly greater decrease in CCT in eyes using travoprost after 3 months (6.74 μm) than using latanoprost (0.57 μm), which differs from Arcieri’s report [6]. Even when the same examiner measures CCT, significant variations of approximately 15 μm have been reported [36]. Additionally, the CCT readings with the ultrasound pachymeter on separate occasions (over the 3-month period) have significant fluctuations, with a mean difference of 9.6 μm in the right eye and 19.0 μm in the left eye [37]. For these reasons, the differences between drugs in the CCT alterations observed in this study might be tiny, within the measurement errors. However, we believe that our crossover design and the CCT measurements at the different times should cancel such variations in the CCT measurements.
In addition to PGAs and prostamides, it has been reported that brimonidine also reduces CCT [21], and that carbonic anhydrase inhibitors increase CCT [14, 38]. The exact mechanism how PGAs reduce CCT remains to be determined. One explanation is that lowering the IOP itself would cause a CCT decrease followed by a corneal hydration. However, this seems unlikely because in our study both drugs had a similar IOP-lowering effect. Matrix metalloproteinases (MMPs) are present in the cornea, and it has been suggested that an over-activity of certain gelotinoproteases (MMP-2) is involved in pathological corneal conditions such as keratoconus [39]. Thus, it is possible that PGAs or prostamide induce alterations of the corneal structure through direct activation of MMPs. In an experimental model, the MMP family, which includes approximately 20 types of enzymes, was found to activate cultured human muscle cells from the ciliary body [40], and rat conjunctiva [41] after exposure to latanoprost. Actually, latanoprost similarly activated the MMPs to increase the extracellular space between the ciliary muscle fibers in the uveoscleral outflow route for aqueous humor drainage [42].
None of our 42 patients dropped out from the study. Bulbar conjunctival hyperemia was the most frequent adverse effect that was found immediately after starting the drugs as has been reported [43, 44]. Because we had explained this complication before the beginning of the drugs, most patients were not surprised or upset with the mild hyperemia. Although two female participants reported a deepening of their upper lid sulcus, the examiner could not detect a change in the sulcus. There have been three reports describing this adverse event after the use of travoprost or bimatoprost [45–47], but no report was found that reported this change after latanoprost. Our patient who noticed this phenomenon after the administration of both drugs had initially used travoprost and subsequent latanoprost. Therefore, it is possible that this is not a complication induced by latanoprost, because it was pointed out that this change did not restore up to 6 months after a discontinuation of the drug [47].
The limitations of our study are the small number of patients, and the measurement of the IOP only three times/day during office hours. In addition, it might be better to show the longitudinal alterations of CCT. Based on our CCT results, it is difficult to predict whether changes in the CCT would continue if the PGAs were continued or be a short-term phenomenon.
In conclusions, our results demonstrated an equal IOP-lowering effect of travoprost and latanoprost. However in some glaucoma patients with low pressures, additional therapies should be considered for a greater IOP reduction of more than 30%. We also found a greater CCT decrease with travoprost than with latanoprost. Because of the reduction in the CCT, care should be taken in interpreting the IOP-lowering effect of PGAs and the prostamides.
References
Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G, Inoue Y, Kitazawa Y, Tajimi Study Group, Japan Glaucoma Society (2004) The prevalence of primary open-angle glaucoma in Japanese. The Tajimi Study. Ophthalmology 111:1641–1648
Collaborative Normal-Tension Glaucoma Study Group (1998) Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126:487–497
Gross RL, Peace JH, Smith SE, Walters TR, Dubiner HB, Weiss MJ, Ochsner KI (2008) Duration of IOP reduction with travoprost BAK-free solution. J Glaucoma 17:217–222
Chew PT, RojanaPongpun P, Euswas A, Lu D, Chua J, Hui S, Rait J, Goldberg I, Li B, Travatan CACG Study Group (2006) Intraocular pressure-lowering effect and safety of travoprost 0.04% and latanoprost 0.005% for the treatment of chronic angle closure glaucoma. Asian J Ophthalmol 8:13–19
Netland PA, Landry T, Sullivan EK, Andrew R, Silver L, Weiner A, Mallick S, Dickerson J, Bergamini MV, Robertson SM, Davis AA, Travoprost Study Group (2001) Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol 132:472–484
Arcieri ES, Pierre Filho PT, Wakamatsu TH, Costa VP (2008) The effects of prostaglandin analogues on the blood aqueous barrier and corneal thickness of phakic patients with primary open-angle glaucoma and ocular hypertension. Eye 22:179–183
Bean GW, Camras CB (2008) Commercially available prostaglandin analogs for the reduction of intraocular pressure: similarities and differences. Surv Ophthalmol 53(suppl 1):S69–S84
Maul E, Carrasco FG, Costa VP, Casiraghi JF, Vargas E, Sarmina JS, Mayol R (2007) A 6-week, multicenter, randomized, double-masked, parallel-group study comparing travoprost 0.004% to latanoprost 0.005% followed by 6-week, open-label treatment with travoprost 0.004%. Clin Ther 29:1915–1923
Hepsen IF, Ozkaya E (2007) 24-h IOP control with latanoprost, travoprost, and bimatoprost in subjects with exfoliation syndrome and ocular hypertension. Eye 21:453–458
García-Feijoo J, Martínez-de-la-Casa JM, Castillo A, Méndez C, Fernández-Vidal A, García-Sánchez J (2006) Circadian IOP-lowering efficacy of travoprost 0.004% ophthalmic solution compared to latanoprost 0.005%. Curr Med Res Opin 22:1689–1697
Dubiner HB, Sircy MD, Landry T, Bergamini MV, Silver LH, Darell Turner F, Robertson S, Andrew RM, Weiner A, Przydryga J (2004) Comparison of the diurnal ocular hypotensive efficacy of travoprost and latanoprost. Clin Ther 26:84–91
Yan DB, Battista RA, Haidich AB, Konstas AG (2008) Comparison of morning versus evening dosing and 24-h post-dose efficacy of travoprost compared with latanoprost in patients with open-angle glaucoma. Curr Med Res Opin 24:3023–3027
Yildirim N, Sahin A, Gultekin S (2008) The effect of latanoprost, bimatoprost, and travoprost on circadian variation of intraocular pressure in patients with open-angle glaucoma. J Glaucoma 17:36–39
Viestenz A, Martus P, Schlötzer-Schrehardt U, Langenbucher A, Mardin CY (2004) Impact of prostaglandin-F(2alpha)-analogues and carbonic anhydrase inhibitors on central corneal thickness – a cross-sectional study on 403 eyes. Klin Monatsbl Augenheilkd 221:753–756
Lass JH, Eriksson GL, Osterling L, Simpson CV, Latanoprost Corneal Effect Study Group (2001) Comparison of the corneal effects of latanoprost, fixed combination latanoprost-timolol, and timolol: a double-masked, randomized, one-year study. Ophthalmology 108:264–271
Brandt JD, Gordon MO, Beiser JA, Lin SC, Alexander MY, Kass MA (2008) Changes in central corneal thickness over time: the ocular hypertension treatment study. Ophthalmology 115:1550–1556
Harasymowycz PJ, Papamatheakis DG, Ennis M, Brady M, Gordon KD (2007) Relationship between travoprost and central corneal thickness in ocular hypertension and open-angle glaucoma. Cornea 26:34–41
Stefan C, Dumitrica DM, Tebeanu E, Nae I, Sapundgieva A, Dragomir L (2007) Prostaglandin analogues and central corneal thickness. Oftalmologia 51:95–99
Sen E, Nalcacioglu P, Yazici A, Aksakal FN, Altinok A, Tuna T, Koklu G (2008) Comparison of the effects of latanoprost and bimatoprost on central corneal thickness. J Glaucoma 17:398–402
Hatanaka M, Vessani RM, Elias IR, Morita C, Susanna R Jr (2009) The effect of prostaglandin analogs and prostamide on central corneal thickness. J Ocul Pharmacol Ther 25:51–53
Johnson TV, Toris CB, Fan S, Camras CB (2008) Effects of central corneal thickness on the efficacy of topical ocular hypotensive medications. J Glaucoma 17:89–99
Johnson M, Kass MA, Moses RA, Crodzki WJ (1978) Increased corneal thickness simulating elevated intraocular pressure. Arch Ophthalmol 96:664–665
Whitacre MM, Stein R (1993) Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol 38:1–30
Whitracre MM, Stein RA, Hassanein K (1993) The effect of corneal thickness on applanation tonometry. Am J Ophthalmol 115:592–596
Brandt JD (2004) Corneal thickness in glaucoma screening, diagnosis, and management. Curr Opin Ophthalmol 15:85–88
Doughty MJ, Zaman ML (2000) Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol 44:367–408
Suh MH, Park KH, Kim DM (2009) Effect of travoprost on intraocular pressure during 12 months of treatment for normal-tension glaucoma. Jpn J Ophthalmol 53:18–23
Nomura Y, Nakamura S, Moriwaki M, Takahashi Y, Shiraki K (2010) Effect of travoprost on 24-hour intraocular pressure in normal tension glaucoma. Clin Ophthalmol 4:643–647
Ang GS, Kersey JP, Shepstone L, Broadway DC (2008) The effect of travoprost on daytime intraocular pressure in normal tension glaucoma: a randomized controlled trial. Br J Ophthalmol 92:1129–1133
Ehlers N, Bramsen T, Sperling S (1975) Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 53:34–43
Whitacre MM, Stein RA, Hassanein K (1993) The effect of corneal thickness on applanation tonometry. Am J Ophthalmol 115:592–596
Brandt JD, Beiser JA, Kass MA, Gordon MO (2001) Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 108:1779–1788
Iester M, Mete M, Figus M, Frezzotti P (2009) Incorporating corneal pachymetry into the management of glaucoma. J Cataract Refract Surg 35:1623–1628
Sit AJ, Weinreb RN, Crowston JG, Kripke DF, Liu JH (2006) Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am J Ophthalmol 141:1131–1133
Sharif NA, Kelly CR, Crider JY, Williams GW, Xu SX (2003) Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther 19:501–515
Miglior S, Albe E, Guareschi M, Mandelli G, Gomarasca S, Orzalesi N (2004) Intraobserver and interobserver reproducibility in the evaluation of ultrasonic pachymetry measurements of central corneal thickness. Br J Ophthalmol 88:174–177
Wickham L, Edmunds B, Murdoch IE (2005) Central corneal thickness: will one measurement suffice? Ophthalmology 112:225–228
Ornek K, Gullu R, Ogurel T, Ergin A (2008) Short-term effect of topical brinzolamide on human central corneal thickness. Eur J Ophthalmol 18:338–340
Smith VA, Hoh HB, Littleton M, Easty DL (1995) Over-expression of a gelatinase A activity in keratoconus. Eye 9:429–433
Weinreb RN, Lindsey JD (2002) Metalloproteinase gene transcription in human ciliary muscle cells with latanoprost. Invest Ophthalmol Vis Sci 43:716–722
Mietz H, Schlötzer-Schrehardt U, Strassfeld C, Krieglstein GK (2001) Effect of latanoprost and timolol on the histopathology of the rabbit conjunctiva. Invest Ophthalmol Vis Sci 42:679–687
Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN (1999) Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol 117:794–801
Fellman RL, Sullivan EK, Ratliff M, Silver LH, Whitson JT, Turner FD, Weiner AL, Davis AA, Group TS (2002) Comparison of travoprost 0.0015% and 0.004% with timolol 0.5% in patients with elevated intraocular pressure: a 6-month, masked, multicenter trial. Ophthalmology 109:998–1008
Camras CB (1996) Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology 103:138–147
Peplinski LS, Albiani Smith K (2004) Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci 81:574–577
Yang HK, Park KH, Kim TW, Kim DM (2009) Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol 53:176–179
Yam JC, Yuen NS, Chan CW (2009) Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol Ther 25:471–472
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary or financial interest in any products used in this study.
Rights and permissions
About this article
Cite this article
Sawada, A., Yamamoto, T. & Takatsuka, N. Randomized crossover study of latanoprost and travoprost in eyes with open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 250, 123–129 (2012). https://doi.org/10.1007/s00417-011-1762-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1762-1