Abstract
Purpose
To retrospectively evaluate the 3-year efficacy and safety of single-agent omidenepag isopropyl in patients with normal tension glaucoma (NTG).
Study design
Retrospective.
Methods
One hundred patients (100 eyes) who had newly been administered omidenepag isopropyl were enrolled in this study. Intraocular pressure (IOP) was compared at baseline and 6, 9, 12, 18, 24, 30, and 36 months after administration. The mean deviation values at baseline and 12, 24, and 36 months measured using the Humphrey visual field test (30-2 Swedish Interactive Threshold Algorithm standard) were compared. Adverse reactions and dropouts were assessed.
Results
IOP significantly decreased from 15.5±2.7 mmHg at baseline to 13.8 ±2.3 mmHg after 6 months, 13.9± 2.3 mmHg after 12 months, 13.9±2.3 mmHg after 18 months, 13.8±2.1 mmHg after 24 months, 13.9±2.0 mmHg after 30 months, and 13.6±1.7 mmHg after 36 months (P < 0.0001). There was no significant difference in the mean deviation values at baseline (-3.66±3.49 dB), 12 months (-3.41±3.80 dB), 24 months (-3.13±3.81 dB), and 36 months (-3.06±3.30 dB). Adverse reactions occurred in 11 patients (11.0%), including conjunctival hyperemia in 6 patients. Fifty-two patients (52.0%) were excluded from the analysis because they discontinued treatment either due to IOP measurement by NCT or the use of additional drugs.
Conclusion
After the administration of omidenepag isopropyl, IOP in patients with NTG decreased within 3 years, visual fields were maintained, and safety was satisfactory. Thus, omidenepag isopropyl can be used as the first-line treatment for patients with NTG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma treatment begins with a single dose of eye drops [1]. Prostaglandin F (FP)-receptor eye drops are the first-line medication because of their strong efficacy in intraocular pressure (IOP)-lowering, low incidence of systemic side effects, and convenience of once-daily ophthalmic administration. However, they can cause topical periocular adverse reactions, including eyelid pigmentation, iris pigmentation, eyelash growth, and deepening of the upper eyelid sulcus [2, 3]. Omidenepag isopropyl ophthalmic solution (omidenepag) [4,5,6,7,8,9] was developed as an ophthalmic solution with an IOP-lowering efficacy comparable to that of FP-receptor ophthalmic solutions while presenting fewer localized ocular side effects.
There are numerous reports on the 1-year efficacy and safety of omidenepag eye drops for glaucoma and ocular hypertension [10,11,12,13,14,15]. However, there are few reports on the efficacy and safety of omidenepag in cases of normal tension glaucoma (NTG) [10, 11], which is common in the Japanese population. Long-term efficacy and safety studies are imperative as glaucoma treatment is inherently a long-term process. We previously reported the efficacy and safety of omidenepag eye drops in patients with NTG over a 1-year period [10]. In the present study, we extended the follow-up period of 100 cases from this cohort to 3 years and reported the outcomes and safety profile.
Subjects and methods
Omidenepag isopropyl (EYBELIS ophthalmic solution 0.002%, Santen Pharmaceutical Co., Ltd.) was administered to 100 patients (100 eyes) with NTG treated at the Inouye Eye Hospital Group (Inouye Eye Hospital, Nishikasai Inouye Eye Hospital, Omiya Inouye Eye Clinic, and Sapporo Inouye Eye Clinic) between December 2018 and September 2020. The definition of NTG was revised as per the Japanese Glaucoma Society Guidelines for Glaucoma 5th edition, published in May 2021 [16]. It is described as: “Primary open-angle glaucoma (broad)”, characterized by chronic progressive optic neuropathy with morphologic features such as an enlarged disc cup, thinning of the rim, and RNFL defect, all without the presence of any other diseases or congenital abnormalities. Although gonioscopy shows a normal open angle, this does not exclude the possibility of functional abnormalities in the chamber angle. NTG represents a subtype of “primary open-angle glaucoma (broad)” in which the IOP consistently falls within the statistically determined normal range during the development of glaucomatous optic neuropathy. This study was conducted retrospectively using patients’ medical records. Omidenepag isopropyl was administered to the patients once daily, specifically in the morning. Administered at night, it would be difficult to notice any adverse effects since the patient could be asleep. IOP was assessed at baseline and at 6, 12, 18, 24, 30, and 36 months after initial administration, using a Goldmann tonometer. IOP values at these time points were compared, and the ranges and rates of IOP reduction from baseline to each follow-up visit were calculated and compared. The mean deviation (MD) values were measured using the Humphrey visual field test program (30-2 Swedish Interactive Threshold Algorithm Standard) and compared at baseline and at 12, 24, and 36 months after initial administration. Adverse reactions and dropout rates were assessed after admission.
For patients with both eyes satisfying the inclusion criteria, the eye with the higher IOP at baseline was enrolled; however, if the IOP was the same OU, the right eye was enrolled. Changes in IOP were analyzed using one-way ANOVA and Bonferroni/Dunn tests. The IOP reduction range, rate, and MD were compared and analyzed using Friedman’s test. Statistical significance was set at P < 0.05. The study protocol adhered to the tenets of the Declaration of Helsinki and approved by the Ethics Committee of Inouye Eye Hospital. Study information was provided at the hospital, and the participants had the opportunity to refuse inclusion.
Results
The participants comprised 36 men and 64 women. The mean age was 55.3±12.8 years (mean±standard deviation; range, 22-83 years). IOP measurements prior to treatment were 15.5±2.7 mmHg, with a range of 10-21 mmHg. MD values (100 eyes) for the Humphrey visual field testing program center 30-2 SITA-Standard were -3.66 ± 3.49 dB (range, -16.10 to 2.28 dB).
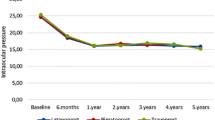
The results of the IOP measurements are shown in Fig. 1. IOP significantly decreased from 15.5±2.7 mmHg (range: 10 to 21 mmHg) at baseline (100 eyes) to 13.8±2.3 mmHg (range: 10 to 21 mmHg) after 6 months (92 eyes), 13.9±2.3 mmHg (range: 8 to19.5 mmHg) after 12 months (81 eyes), 13.9±2.3 mmHg (range: 9 to 19 mmHg) after 18 months (70 eyes), 13.8±2.1 mmHg (range: 8 to 18.5 mmHg) after 24 months (67 eyes), 13.9±2.0 mmHg (range: 9 to 20 mmHg) after 30 months (57 eyes), and 13.6±1.7 mmHg (range: 10 to 18 mmHg) after 36 months (48 eyes) (P< 0.0001). There was no significant difference in the IOP reduction range, which was 1.5±1.9 mmHg (range: -4 to 5 mmHg) after 6 months, 1.5±2.2 mmHg (range: -4 to 8 mmHg) after 12 months, 1.4±2.5 mmHg (range: -4 to 7 mmHg) after 18 months, 1.3±2.2 mmHg (range: -4 to 6 mmHg) after 24 months, 1.2±2.1 mmHg (range: -3 to 7 mmHg) after 30 months, and 1.3±2.3 mmHg (range: -3 to 7 mmHg) after 36 months (P= 0.2469). There was also no significant difference in IOP reduction rate and range, which were 9.1±12.9% (range: -40.0 to 31.3%) after 6 months, 8.4±13.0% (range: -26.7 to 50.0%) after 12 months, 7.5±15.8% (range: -31.8 to 37.5%) after 18 months, 7.0±14.7% (range: -30.0 to 33.3%) after 24 months, 6.6±13.6% (range: -30.0 to 43.8%) after 30 months, and 7.0±14.8% (range: -25.0 to 37.5%) after 36 months (P= 0.2469). There was no significant difference in the MD values as assessed by the Humphrey visual field test program, which was -3.66±3.49 dB (100 eyes) before treatment and -3.41±3.80 dB (79 eyes) after 12 months, -3.13±3.81 dB (65 eyes) after 24 months, and -3.06±3.30 dB (42 eyes) after 36 months (P=0.060).
Adverse reactions occurred in 11 patients (11.0%) (Table 1): conjunctival hyperemia in 6 (6.0%) patients, 1 after 1 week, 1 after 2 weeks, and 4 after 1 month, eye pain in 1 (1.0%) patient after 1 month, iritis in 1 (1.0%) and blepharitis in 1 (1.0%) patient after 7 months, eyelid swelling in 1 (1.0%) patient after 21 months, and corneal epithelial disorder in 1 (1.0%) patient after 33 months. Fifty-two patients (52.0%) discontinued administration (Table 2) due to interruption of visits in 15 (15.0%) patients, added medication in 8 (8.0%), visual field disorder progression in 7 (7.0%), adverse reactions in 6 (6.0%) (conjunctival hyperemia in 2, eye pain in 1, iritis in 1, blepharitis in 1, and eyelid swelling in 1), hospital transfer in 6 (6.0%), IOP measurement with a non-contact tonometer in 6 (6.0%), change of medications in 2 (2.0%), and cataract surgery in 2 (2.0%) patients.
Discussion
In this study, we investigated the long-term efficacy and safety of omidenepag eye drops over a 3-year period in patients with NTG. IOP was significantly lower after than before treatment over a 36-month period. A possible reason for the high number of discontinuations could be the coronavirus disease 2019 (COVID-19) pandemic.
In the Phase 3 RANGE study in Japan [11], omidenepag eye drops were administered for 52 weeks to treat primary open-angle glaucoma (POAG) and ocular hypertension (OH). Patients were categorized into two groups based on baseline IOP: 16-22 mmHg (group 1) and 22-34 mmHg (group 2). The baseline IOP was 18.71±1.68 mmHg in group 1 and 24.06±2.36 mmHg in group 2. The IOP reduction range after 52 weeks was 3.7±0.3 mmHg (group 1) and 5.6±0.5 mmHg (group 2). NTG was considered equivalent to Group 1. Nakazawa et al. treated patients with glaucoma and OH with omidenepag eye drops for 1 year [12]. IOP was 17.0 mmHg before treatment and significantly decreased to 13.8 mmHg after 12 months of treatment; the IOP reduction range was 3.2 mmHg. Ozaki et al. treated patients with POAG using omidenepag eye drops for 1 year [13]. IOP was 14.1±3.9 mmHg before treatment and significantly decreased to 11.8±3.1 mmHg 12 months after treatment, demonstrating an IOP reduction range of 2.3 mmHg. Rikiishi et al. treated patients with glaucoma and OH with omidenepag eye drops for 1 year [14]. IOP was 17.4±3.5 mmHg before treatment and significantly decreased to14.1±2.9 mmHg 12 months after treatment, highlighting an IOP reduction range of 3.3 mmHg. Kozaki et al. treated patients with POAG and OH using omidenepag eye drops for 1 year [15]. IOP significantly decreased 12 months after treatment with an IOP reduction range of 3.7±2.8 mmHg. Ozaki et al. [13] had a lower pre-dose IOP than in the present study; however, the IOP reduction was greater. Other reports [11, 12, 14] reported a higher pre-dose IOP than in the present case, which may have contributed to the slightly poorer IOP reduction observed in this study.
The 3-year efficacy and safety of PG-related drug eye drops for NTG are reported [17,18,19,20].
The IOP reduction rate for eye drops over 3 years was as follows: bimatoprost, 18.6 to 24.9% [17]; travoprost, 16.7 to 19.6% [18]; tafluprost, 11.4 to 15.6% [19]; and latanoprost, 14% [20]. The IOP reduction rate of omidenepag isopropyl in this study ranged from 6.6 to 9.1%, suggesting that omidenepag isopropyl may have a lower IOP reduction effect than other PG-related drugs. However, the baseline IOP was 16.7 ± 2.2 mmHg for bimatoprost and 16.8 ± 2.6 mmHg for Travoprost, which is higher than the 15.5 ± 2.7 mmHg observed for omidenepag isopropyl in the present study. This difference in baseline IOP may have influenced the results. In the Phase 3 RANGE Study [11], ocular local adverse reactions occurred in 47.9% of patients in group 1, including conjunctival hyperemia in 16.7%, macular edema in 10.4%, and cystoid macular edema in 6.3% with other reactions. Nakazawa et al. [12] report adverse reactions in 23.2% of patients, including conjunctival hyperemia in 3.5%, refractive disorder in 2.8%, and myopia in 1.3% with other reactions. Ozaki et al. [13] report adverse reactions in 4.9% of the patients, all of whom had conjunctival hyperemia. Rikiishi et al. [14] report adverse reactions in the study group, including conjunctival hyperemia in 3.2% and photophobia in .8%. In this study, adverse reactions occurred in 11 patients (11.0%), including conjunctival hyperemia in 6 (6.0%), eye pain in 1 (1.0%), iritis in 1 (1.0%), blepharitis in 1 (1.0%), eyelid swelling in 1 (1.0%), and corneal epithelial disorders in 1 (1.0%). The number of cases in this study was larger than those in previous reports [11, 13, 14] except for the report by Nakazawa et al. [12], which resulted in a lower rate of adverse drug reactions. In previous reports [11,12,13,14], conjunctival hyperemia was common; however, in our case, patient reports of conjunctival hyperemia were fewer because the patients had been informed before administration that conjunctival hyperemia could occur and because considerable time had elapsed since the administration of eye drops.
In this study, 9 of 11 (81.8%) adverse reactions occurred within 1 year of treatment, with eyelid swelling (after 21 months of treatment) and corneal epithelial disorders (after 33 months of treatment) occurring after 1 year. Particular attention should be paid to the appearance of side effects for up to 1 year after administration.
In this study, 6.0% of patients discontinued the drug owing to adverse reactions. In the Phase 3 RANGE Study [11], 11 patients (22.9%) in Group 1 discontinued treatment. Among these, 8 patients had adverse reactions, and two patients had inadequate IOP reduction. Nakazawa et al. [12] report that, in all treatment groups, 17.6% of discontinuations were due to side effects or inadequate IOP reduction. The most common cases of discontinuation in this study included 8 cases of medication issues (inadequate IOP-lowering effect) and 7 cases of progression of visual field disorder. In addition, 15 cases were of interrupted visits, 6 cases of transfers, and 6 cases of IOP measurement with a non-contact tonometer, which together accounted for 27% of the cases; however, these were cases of discontinuation, regardless of the efficacy or safety of the eye drops. The widespread disruption that COVID-19 caused may have also contributed. In Japan, a state of emergency had been declared four times since April 2020 (for 17 months); however, at present people are no longer required to refrain from going out or traveling. In some cases, IOP was measured using a non-contact tonometer rather than a contact Goldmann applanation tonometer owing to the fear of COVID-19 infection. Excluding these discontinued cases, 25 (25.0%) other patients discontinued treatment, which is almost the same as that reported previously.
The MD values of the Humphrey visual field test were compared year-to-year in this study and were maintained over a 3-year period. However, in 7 cases, additional treatment was required owing to the progression of visual field defects, and the medication was discontinued. The MD slope for all cases was calculated to be 0.00±1.15 dB/year, ranging from -1.71 to 1.28 dB/year. Some cases exhibited a rapid progression of visual field impairment and required careful follow-up.
A limitation of this study is that the interruption of visits and transfers owing to the COVID-19 outbreak prevented an accurate evaluation of omidenepag ophthalmic solutions. Omidenepag eye drops are contraindicated in patients with pseudophakia or aphakic eyes; therefore, they are not administered to patients who had undergone cataract surgery.
Since omidenepag eye drops have become available, their long-term efficacy and safety over a 3-year period in patients with NTG have been investigated. In these investigations, IOP proved significantly lower after treatment over a 36-month period. Visual field impairment remained unchanged until 36 months after the treatment. Adverse reactions occurred in 11 (11.0%) patients, including conjunctival hyperemia, eye pain, iritis, blepharitis, eyelid swelling, and corneal epithelial disorders. The long-term efficacy and safety of omidenepag isopropyl in patients with NTG were thus satisfactory. In conclusion, it is possible to use omidenepag isopropyl as the first-line treatment for patients with NTG.
References
Kiuchi Y, Inoue T, Shoji N, Nakamura M, Tanito M, Glaucoma Guideline Preparation Committee, Japan Glaucoma Society. The Japan Glaucoma Society guidelines for glaucoma, 5th edition. Jpn J Ophthalmol. 2023;67:189-254.
Inoue K, Shiokawa M, Higa R, Sugahara M, Soga T, Wakakura, et al. Adverse periocular reactions to five types of prostaglandin analogs. Eye (Lond). 2012;26:1465–72. https://doi.org/10.1038/eye.2012.195.
Inoue K, Shiokawa M, Wakakura M, Tomita G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22:626–31.
Aihara M, Fenghe Lu, Kawata H, Iwata A, Liu K, Odani-Kawabata N, et al. Phase 2, randomized, dose-finding studies of Omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28:375–85. https://doi.org/10.1097/IJG.0000000000001221.
Aihara M, Lu F, Kamata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: The Phase 3 AYAME Study. Am J Ophthalmol. 2020;220:53–62.
Olander KW, Sato MA, Abrams MA, Jerkins GW, Lu F, Dinh P, et al. A randomized phase 2 trial comparing Omidenepag isopropyl 0.002% once and twice daily in subjects with primary open-angle glaucoma or ocular hypertension (SPECTRUM-6). J Glaucoma. 2021;30:473–80.
Aihara M, Ropo A, Lu F, Kawata H, Iwata A, Odani-Kawabata N, et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64:398–406.
Inoue K, Shiokawa M, Katakura S, Shimizu K, Ishida K, Tomita G. Periocular adverse reactions to omidenepag isopropyl. Am J Ophthalmol. 2022;237:114–21.
Sakata R, Fujishiro T, Saito H, Nakamura N, Honjo M, Shirato S, et al. Recovery of deepening of the upper eyelid sulcus after switching from prostaglandin FP receptor agonists to EP2 receptor agonist: a 3-month prospective analysis. Jpn J Ophthalmol. 2021;65:591–7.
Inoue K, Shiokawa M, Kunimatsu-Sanuki S, Nozaki N, Shimizu K, Ishida K, et al. One-year efficacy and safety of omidenepag isopropyl in patients with normal-tension glaucoma. J Ocul Pharmacol Ther. 2022;38:354–8.
Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N. Twelve-month efficacy of omidenepag isopropyl, a selective ER2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study. Jpn J Ophthalmol. 2021;65:810–9.
Nakazawa T, Takahashi K, Kuwayama Y, Nomura A, Shimada F. Interim results of post-marketing observational study of omidenepag isopropyl for glaucoma and ocular hypertension in Japan. Adv Ther. 2022;39:1359–74.
Ozaki H, Kobayashi S, Yokoo Y, Nozaki N, Shimizu K, Ishida K. One-year outcome of omidenepag isopropyl in primary open angle glaucoma and preperimetric glaucoma. Rinsho Ganka. 2022;76:1380–6 (in Japanese).
Chikaraishi Y, Arakaki Y, Koizumi H. Evaluation of the long-term safety and efficacy of omidenepag isopropyl ophthalmic solution. Atarashii Ganka. 2022;39:1530–3 (in Japanese).
Kozaki J, Maeda N, Kosaki R. Efficacy and safety of selective prostaglandin EP2 agonist in primary open angle glaucoma and ocular hypertension. Rinsho Ganka. 2022;76:85–92 (in Japanese).
Kikuchi Y, Inoue T, Shoji N, Nakamura M, Tanito M. The Japan Glaucoma Society guidelines for glaucoma 5th edition. Jpn J Ophthalmol. 2023;67:189–254.
Inoue K, Shiokawa M, Fujimoto T, Tomita G. Effects of treatment with bimatoprost 0.03% for 3 years in patients with normal-tension glaucoma. Clin Ophthalmol. 2014;8:1179–83.
Inoue K, Tanaka A, Tomita G. Effects of tafluprost treatment for 3 years in patients with normal-tension glaucoma. Clin Ophthalmol. 2013;7:1411–6.
Inoue K, Iwasa M, Wakakura M, Tomita G. Effects of BAK-free travoprost treatment for 3 years in patients with normal tension glaucoma. Clin Ophthalmol. 2012;6:1315–9.
Takemoto D, Higashide T, Saito Y, Ohkubo S, Udagawa S, Takeda H, et al. Intraocular pressure and visual field changes in normal-tension glaucoma patients treated using either unoprostone or latanoprost: a prospective comparative study. Clin Ophthalmol. 2017;11:1617–24.
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K. Inoue, Grants or contracts (Otsuka, Wakamoto), Lecture fee (Senju, Otsuka, Kowa, Santen, Allergan, Novartis, HOYA, Pfizer, Viatris, Rohto Nitten); M. Shiokawa, Lecture fee (Otsuka); S. K-Sanuki, Lecture fee (Santen, Otsuka, Kowa, Nitto Medic, Novartis); J. Kang, None; T. Uraki, None; G. Tomita, Grants or contracts (Pfizer, Santen, Senju, Eisai, Handaya, Kowa, Otsuka), Lecture fee (Santen, Senju, Allergan, TOPCON); K. Ishida, Lecture fee (Alcon, Pfizer, Santen, Senju, Otsuka, Kowa, Sucampo, GSK, JFC Sales Plan, Novartis, Wakamoto, Allergan, Bayer, Nitto Medic).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Kenji Inoue.
About this article
Cite this article
Inoue, K., Shiokawa, M., Kunimatsu-Sanuki, S. et al. Three-year efficacy and safety of omidenepag isopropyl in patients with normal tension glaucoma. Jpn J Ophthalmol 68, 206–210 (2024). https://doi.org/10.1007/s10384-024-01052-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-024-01052-8