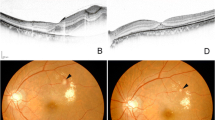
Abstract
Purpose
To assess the efficacy and complications of intravitreal injection of sulfur hexafluoride (SF6) gas with/without tissue plasminogen activator (tPA) for displacing submacular hemorrhage.
Methods
The medical records of 53 eyes that underwent pneumatic displacement for submacular hemorrhage were reviewed retrospectively. Submacular hemorrhage was related to exudative age-related macular degeneration (AMD) in 39 eyes and ruptured retinal arterial macroaneurysms in 14 eyes, and treated with intravitreal injection of SF6 gas with or without tPA.
Results
Compared with preoperatively (mean follow-up, 18.4 months), the final visual acuity (VA) improved by 0.3 or more logMAR unit in 34 eyes (64.2%), stabilized within 0.3 logMAR in 15 eyes (28.3%), and deteriorated in four eyes (7.5%). In eyes with AMD, hemorrhage including vitreous hemorrhage recurred in eight (22.2%) of 36 eyes treated with tPA and one (33.3%) of three eyes not treated with tPA. In eyes with macroaneurysms, hemorrhage recurred in four (100%) of four eyes treated with tPA and in one (10.0%) of ten eyes without tPA (p < 0.005). Eight eyes underwent vitrectomy for recurrent hemorrhage. During follow-up, photodynamic therapy or intravitreal ranibizumab or pegaptanib was administered in 16 (41.0%) of 39 eyes with AMD. Postoperative ocular hypertension persisting over 3 days was not observed.
Conclusions
Intravitreal SF6 gas plus tPA may be well-accepted, with good visual outcomes and no remarkable complications for treating submacular hemorrhage secondary to AMD. tPA is not recommended for ruptured retinal arterial macroaneurysms, because of a higher incidence of subsequent vitreous hemorrhage. Pneumatic displacement of submacular hemorrhage without tPA may provide good visual outcomes with less re-bleeding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Submacular hemorrhage is an important cause of sudden visual loss in eyes with age-related macular degeneration (AMD) and retinal arterial macroaneurysms [1–5]. A variety of therapeutic approaches has been developed with the common goal of clearing subfoveal hemorrhages to minimize permanent damage to the photoreceptors and retinal pigment epithelium [1].
Heriot first described managing submacular hemorrhage with injection of intravitreal tissue plasminogen activator (tPA) injection and pneumatic displacement of subfoveal hemorrhages [6]. This simple procedure provides a high anatomic success rate with few complications. However, Kokame reported cases of dense vitreous hemorrhage after intravitreal tPA, and pneumatic displacement of submacular hemorrhage associated with retinal arterial macroaneurysms [7]. Other investigators had successfully displaced submacular hemorrhage using an intravitreal gas injection alone [8, 9]. Thus, the need for tPA is controversial.
In our hospital, we have had four consecutive cases of submacular hemorrhage associated with retinal arterial macroaneurysms with severe vitreous hemorrhage after intravitreal injection of sulfur hexafluoride (SF6) gas and tPA. Thereafter, we treated eyes with submacular hemorrhage secondary to macroaneurysms with intravitreal injections of SF6 gas only, and we treated AMD-related submacular hemorrhage with SF6 gas with tPA in most cases.
The objective of the current study was to evaluate the efficacy and complications of pneumatic displacement with or without tPA for treating submacular hemorrhage.
Materials and methods
We retrospectively reviewed the medical records of 53 consecutive patients in our institution who had undergone intravitreal injection of SF6 gas with or without tPA for fibrinolysis and displacement of submacular hemorrhage. Submacular hemorrhage was associated with exudative AMD in 39 eyes, and with retinal arterial macroaneurysms in 14 eyes. The procedure was performed after topical instillation of 4% xylocaine under sterile conditions. Paracentesis was followed by transconjunctival intravitreal injection of tPA (40 kIU) in some cases and pure SF6 gas (0.3–0.6 ml) 3.5 to 4.0 mm posterior to the limbus using a 30-gauge needle. The patients were required to maintain the facedown position for the following 3 days or longer. The best-corrected visual acuity (BCVA) and intraocular pressure were measured, and funduscopy carried out preoperatively and postoperatively. Complications and additional treatments were assessed. For statistical analysis, counting fingers was categorized as a VA of 0.004, hand motions as 0.002, and light perception as 0.001. Improvement and deterioration of BCVA were defined as a change of 0.3 or more in logarithm of minimal angle of resolution (logMAR) VA compared with the preoperative value. Fisher’s exact test was used to compare the incidence rates of recurrent hemorrhage.
Results
Of 39 eyes with AMD, 36 eyes were treated with intravitreal injection of SF6 gas with tPA; three eyes were not treated with tPA. Of 14 eyes with ruptured retinal arterial macroaneurysms, four consecutive patients were treated with intravitreal injection of SF6 gas and tPA, and the next ten cases underwent intravitreal injection of SF6 alone. The mean follow-up period was 18.4 ± 16.6 months (range, 3–61 months). The mean patient age was 72.6 ± 10.2 years (range, 50–90 years). The mean baseline decimal BCVA, the mean maximal BCVA, and the mean final BCVA were 0.09, 0.35, and 0.24 respectively (Fig. 1). At the last visit, 13 (33.3%) eyes with AMD and six (42.9%) eyes with macroaneurysms had a decimal BCVA of 0.5 or better. Comparison of the baseline BCVA and the best BCVA showed that the BCVA improved in 30 eyes (76.9%), was unchanged in nine eyes (23.1%), and deteriorated in no eyes (0.0%) in patients with AMD. At the last visit, however, the improved BCVA was sustained in 21 eyes (53.8%). In four eyes (10.3%), the last BCVA deteriorated. In contrast, the BCVA improved in 13 eyes (92.9%), and did not decrease in any eyes throughout the observation period in patients with macroaneurysms (Fig. 2). The major complication was recurrent hemorrhages, including vitreous hemorrhages, in 14 of 53 eyes. Recurrent hemorrhages were caused by fresh re-bleeding in 11 eyes, while the other three eyes might possibly have suffered translocation of preoperatively existing hemorrhages to vitreous cavity. Of the 14 eyes, eight eyes had AMD treated with tPA, one eye AMD without tPA, four eyes macroaneurysms treated with tPA, and one eye macroaneurysm without tPA. The incidence rates of re-bleeding were 22.2% and 33.3% in eyes with AMD treated with or without tPA and 100.0% and 10% in eyes with macroaneurysms with or without tPA respectively (Fig. 3). Macroaneurysms had a significantly higher risk of re-bleeding when tPA was used, compared with macroaneurysms treated without tPA and AMD treated with tPA (p < 0.01). These complicated cases were treated with pars plana vitrectomy, with no adverse events that decreased the vision. Postoperative transient ocular hypertension was observed in five eyes that normalized within 2 days after administration of anti-glaucoma eye drops. During the observation period, additional therapies were administered in 16 patients with AMD (41.0%), which included photodynamic therapy (PDT) in 11 eyes (28.2%) and antivascular endothelial growth factor (VEGF) therapies in eight eyes (20.5%) (Fig. 4). Six eyes were treated with intravitreal ranibizumab (Lucentis®, Novartis Pharma K.K., Tokyo, Japan) and two eyes with pegaptanib (Macugen®, Pfizer Japan Inc., Tokyo, Japan).
Discussion
The natural course of submacular hemorrhage often leads to irreversible visual loss [1–5]. Several mechanisms, including the toxic effects of hemoglobin-derived iron, shearing damage to the photoreceptors by fibrin clots and subsequent fibrinolytic response, and a longstanding mechanical barrier between the retina and choriocapillaris, have been postulated as explanations for retinal damage caused by thick subretinal blood [1, 4, 10, 11]. The natural history and experimental data underscore the need for safe and effective treatments to remove thick subfoveal blood and prevent irreversible blood-induced damage to the outer retina.
In 1996, Heriot introduced a new procedure to lyse and displace submacular blood without intraocular surgery by injecting intravitreal tPA and a bubble of long-acting expansile gas into the vitreous cavity [6]. Intravitreal tPA as an adjuvant has been used, along with pneumatic displacement of submacular hemorrhage. Hassan et al., who used intravitreal tPA and expansible gas, described displacement of submacular hemorrhage from the center of the fovea in 15 of 15 eyes, and reported that the VA improved by two or more lines in 14 of 15 eyes, with a low rate of serious complications [12]. The investigators concluded that intravitreal injection of tPA and gas followed by brief prone positioning effectively displaces thick submacular blood, and facilitates visual improvement in most patients.
In the current study, the postoperative BCVA improved in 30 eyes (76.9%) or was unchanged in nine eyes (23.1%), while no eyes with a submacular hemorrhage associated with AMD had decreased VA. Our visual results were comparable to those in eyes treated with surgical evacuation of hemorrhage or those observed over the natural course [13–18]. There was no significant (p = 0.578) difference in the incidence of recurrent hemorrhage between the eyes treated with and without tPA, although the number of patients with AMD treated without tPA was too small for statistical analysis.
During the current follow-up period, 16 (41.0%) of 39 patients with AMD also were treated with PDT or anti-VEGF therapies such as intravitreal ranibizumab and pegaptanib. In our previous study, 11 (28.2%) of 39 eyes with exudative AMD, which were treated primarily with a sub-Tenon injection of triamcinolone acetonide and PDT, required additional PDT during the mean follow-up period of 12.7 months. Most cases that required no additional therapy had polypoidal choroidal vasculopathy, which when ruptured may form a dense subretinal hemorrhage, but frequently result in spontaneous closure of polyps. Taken together, the current results suggested that tPA might be well-tolerated and achieve good visual outcomes, without an increased risk of postoperative hemorrhage for pneumatic displacement of submacular hemorrhages.
However, we observed severe vitreous hemorrhages in four consecutive cases of submacular hemorrhages associated with retinal arterial macroaneurysms after intravitreal injection of SF6 gas with tPA. Kokame also reported two cases of severe vitreous hemorrhage 1 day after intravitreal injection of SF6 gas with tPA in eyes with submacular hemorrhage associated with retinal arterial macroaneurysms [7]. It is easy to speculate that severe postoperative vitreous hemorrhage may be an adverse effect of tPA in eyes with ruptured retinal arterial macroaneurysms. Thereafter, we had ten additional consecutive cases of submacular hemorrhage associated with macroaneurysms treated with intravitreal injection of SF6 gas without tPA. As expected, vitreous hemorrhage occurred in only one eye (10.0%). Moreover, the postoperative BCVA improved in all cases. There was a significant difference in the incidence of recurrence of hemorrhages between the groups treated with and without tPA (p = 0.005). Our results indicated that a higher incidence of subsequent vitreous hemorrhage associated with tPA should be considered in eyes with macroaneurysms. Currently, gas injection without tPA can be recommended in patients with ruptured retinal arterial macroaneurysms.
A limitation of our study was the small sample size of eyes in the group with AMD-associated submacular hemorrhages treated with intravitreal injection of SF6 gas alone. Since potent retinal toxicity of tPA has been reported [19, 20], the necessity for tPA as an adjuvant for pneumatic displacement of submacular hemorrhage associated with AMD should be evaluated further. Ron et al. reported that use of only gas injection to displace a submacular hemorrhage can significantly improve the VA [21]. Ohji et al. reported that when a subretinal hemorrhage is relatively fresh, only injection of gas can remove it from the subfoveal space [8]. However, Gopalakrishan et al. reported that pneumatic displacement without tPA may not be worthwhile in eyes with a submacular hemorrhage, due to wet AMD [22]. More recently, the addition of intravitreal bevacizumab to the injection of tPA and gas is reported to be safe, and superior to the displacement of submacular hemorrhage alone with tPA and gas [23, 24]. Further investigations are needed to address these issues.
In conclusion, the results of our retrospective study suggested that intravitreal injection of gas and tPA in patients with submacular hemorrhage secondary to AMD provide good visual outcomes without the possible adverse effects of tPA such as recurrent hemorrhages. In contrast, a higher incidence of subsequent vitreous hemorrhage associated with tPA should be considered in eyes with retinal arterial macroaneurysms. Alternatively, gas injection without tPA is recommended with good visual outcomes.
References
Bennett SR, Folk JC, Blodi CF, Klugman M (1990) Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol 109:33–37
Avery RL, Fekrat S, Hawkins BS, Bressler NM (1996) Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 16:183–189
Saito K, Iijima H (1997) Visual prognosis and macular pathology in eyes with retinal macroaneurysms. Nippon Ganka Gakkai Zasshi 101:148–151
Berrocal MH, Lewis ML, Flynn HW Jr (1996) Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol 122:486–493
Hochman MA, Seery CM, Zarbin MA (1997) Pathophysiology and management of subretinal hemorrhage. Surv Ophthalmol 42:195–213
Heriot WJ (1997) Further experience in management of submacular hemorrhage with intravitreal tPA. In: Proceedings of the Update on Macular Surgery. American Academy of Ophthalmology, San Francisco, pp 82–84
Kokame GT (2000) Vitreous hemorrhage after intravitreal tissue plasminogen activator (tPA) and pneumatic displacement of submacular hemorrhage. Am J Ophthalmol 129:456–457
Ohji M, Saito Y, Hayashi A, Lewis JM, Tano Y (1998) Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch Ophthalmol 116:1326–1332
Daneshvar H, Kertes PJ, Leonard BC, Peyman GA (1999) Management of submacular hemorrhage with intravitreal sulfur hexafluoride: a pilot study. Can J Ophthalmol 34:385–388
Avery RL, Fekkrat S, Hawkins BS, Bressler NM (1996) Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 16:183–189
Scupola A, Coscas G, Soubrane G, Balestrazzi E (1999) Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica 213:97–102
Hassan AS, Johnson MW, Schneiderman TE, Regillo CD, Tornambe PE, Poliner LS, Blodi BA, Elner SG (1999) Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology 106:1900–1906
Kamei M, Tano Y, Maeno T, Ikuno Y, Mitsuda H, Yuasa T (1996) Surgical removal of submacular hemorrhage using tissue plasminogen activator and perfluorocarbon liquid. Am J Ophthalmol 121:267–275
Ibanez HE, Williams DF, Thomas MA, Ruby AJ, Meredith TA, Boniuk I, Grand MG (1995) Surgical management of submacular hemorrhage: a series of 47 consecutive cases. Arch Ophthalmol 113:62–69
Lewis H (1994) Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol 118:559–568
Vander JF, Federman JL, Greven C, Slucher MM, Gabel VP (1991) Surgical removal of massive subretinal hemorrhage associated with age-related macular degeneration. Ophthalmology 98:23–27
de Juan E, Jr MR (1988) Vitreous surgery for hemorrhagic and fibrous complications of age-related macular degeneration. Am J Ophthalmol 105:25–29
Lim JI, Drews-Botsch C, Sternberg P Jr, Capone A Jr, Aaberg TM Sr (1995) Submacular hemorrhage removal. Ophthalmology 102:1393–1399
Irvine WD, Johnson MW, Hernandez E, Olsen KR (1991) Retinal toxicity of human tissue plasminogen activator in vitrectomized rabbit eyes. Arch Ophthalmol 109:718–722
Johnson MW, Olsen KR, Hernandez E, Irvine WD, Johnson RN (1990) Retinal toxicity of recombinant tissue plasminogen activator in the rabbit. Arch Ophthalmol 108:259–263
Ron Y, Ehrlich R, Axer-Siegel R, Rosenblatt I, Weinberger D (2007) Pneumatic displacement of submacular hemorrhage due to age-related macular degeneration. Ophthalmologica 221:57–61
Gopalakrishan M, Giridhar A, Bhat S, Saikumar SJ, Elias A, Sandhya N (2007) Pneumatic displacement of submacular hemorrhage. Retina 27:329–334
Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG (2008) Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol 86:490–494
Guthoff R, Guthoff T, Meigen T, Goebel W (2011) Intravitreous injection of bevacizumab, tissue plasminogen activator, and gas in the treatment of submacular hemorrhage in age-related macular degeneration. Retina 31:36–40
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interest in any aspect of this report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 31 kb)
Rights and permissions
About this article
Cite this article
Mizutani, T., Yasukawa, T., Ito, Y. et al. Pneumatic displacement of submacular hemorrhage with or without tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol 249, 1153–1157 (2011). https://doi.org/10.1007/s00417-011-1649-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1649-1