Abstract
Purpose
Contradictory evidence exists over the best approach for the management of submacular hemorrhage (SMH). In this study, we compared the outcomes of subretinal versus intravitreal injection of recombinant tissue plasminogen activator (r-tPA) and gas in cases of SMH secondary to age-related macular degeneration (AMD).
Methods
Twenty five eyes with SMH were retrospectively divided in 2 groups. Group A underwent vitrectomy, subretinal r-tPA and gas (Vitrectomy group, n = 14), and group B received intravitreal r-tPA and gas (Pneumatic group, n = 11). SMH displacement and change in subfoveal hemorrhage thickness (SFHT) at 1 month post-op were assessed. Additionally, best corrected visual acuity (BCVA) and central retinal thickness (CRT) at the end of the 12 month follow-up (FU) were analyzed. Clinical and epidemiological prognostic factors were tested.
Results
Mean duration of SMH prior intervention was 8.2(± 7.3) days. Baseline BCVA was 1.53 ± 0.73 LogMAR, mean extension of SMH was 4.604 ± 2079 μm and mean CRT pre-treatment was 795 ± 365 μm. SMH displacement at 1 month post-treatment was total in 9/14 versus 6/11 and partial in 4/14 versus 2/11 in Group A and Group B, respectively (Fisher's exact test p = 0.38). SFHT reduced by 404 ± 312 μm in Group A versus 376 ± 405 μm in group B (p = 0.86). BCVA improvement and reduction of CRT were highly significant at the end of FU (p = 0.002 and p < 0.001 respectively) but did not differ between the 2 groups. Only baseline BCVA and preoperative CRT proved to be significant prognostic factors for the final functional outcome (p = 0.013 and p = 0.047 respectively).
Conclusion
Both treatment options proved equal efficacy in displacing SMH in AMD. A multicenter trial may delineate a desirable algorithm of treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced age-related macular degeneration (AMD) is a leading cause of severe visual impairment in the elderly [1]. Submacular hemorrhage (SMH) is a serious complication of wet AMD, with reported annual incidence of 5.4 [2] to 25 per million per year [3]. It causes acute, extensive loss of visual acuity and may irreversibly damage the retina, if left untreated [4].
Many studies concerning the treatment options for SMH evaluated the use of intravitreal gas for pneumatic hemorrhage displacement and the use of recombinant tissue plasminogen activator (r-tPA), following pars plana vitrectomy (PPV) [5,6,7,8,9,10]. Head posturing for a variable number of days is required to facilitate the action of gas buoyancy.
Heriot was the first to describe pneumatic displacement of submacular hemorrhage in 1996 [11]. Both pneumatic displacement and PPV with subretinal r-tPA were later combined with intravitreal anti-vascular endothelial growth factor (anti-VEGF) injection [12,13,14] which currently represents the mainstay of treatment for neovascular AMD.
Both treatment modalities are primarily performed for recent-onset—less than 14 days—submacular hemorrhage [15]. Despite visual improvement, patient satisfaction with plasminogen activator injection treatment is often low [16].
This study aims to demonstrate the effects of intravitreal and subretinal r-tPA injection with pneumatic displacement for subretinal hemorrhage and to compare the results of the two procedures.
Materials and methods
Medical records of patients with consecutive wet AMD (wAMD) presenting at the Ophthalmica Eye Institute (Thessaloniki, Greece) with sudden visual loss due to submacular hemorrhage, from January 2017 to December 2018, were analyzed. The study was approved by the institution’s ethics committee and the tenets of the Declaration of Helsinki were respected. Written consent for the use of data for research purposes was obtained from all patients.
In all selected eyes there was evidence of significant subretinal and/or retinal pigment epithelium (RPE) hemorrhage, with or without vitreous hemorrhage and with a history of less than 15 days duration. Individuals who declined treatment or with a history of visually significant comorbidities such as glaucoma, corneal opacification, uveitis or previous ocular surgery other than phacoemulsification were excluded from the analysis. Patients with missing data or incomplete follow-up were further excluded.
Patients were divided in 2 groups, based on the offered procedure. Individuals in Group Α (“vitrectomy group”) underwent PPV, subretinal injection of r-tPA (25μ/0.1 ml) and Bevacizumab and intravitreal gas in nonexpansile concentration. Patients in Group B (the “pneumatic displacement group”) received intravitreal expansile gas and r-tPA along with intravitreal injection of Bevacizumab should the interval from the previous anti-VEGF injection was greater than 1 month. These patients were included in the analysis both as a whole and as subgroups.
The diagnosis of subretinal and/or sub-RPE hemorrhage was initially based on clinical examination. The exact anatomical site of the hemorrhage was confirmed with Optical Coherence Tomography (OCT) scans, color fundus photographs and/or fluorescein/indocyanine green angiography at physician’s choice. All data were recorded at baseline and at 1, 3, 6 and 12 months after treatment and included: best-corrected visual acuity (BCVA), anatomical site of the hemorrhage (subretinal and/or sub-RPE), central retinal thickness (CRT), extension of submacular hemorrhage and subfoveal hemorrhage thickness (SFHT).
When the procedure of choice involved vitrectomy, a standard 25 G 3-port PPV was performed, followed by subretinal delivery of r-tPA and Bevacizumab through a 41 G vitreoretinal cannula. Fluid-air exchange was carried out using SF6 or C3F8 in nonexpansile concentration and the patient was instructed to keep face-down posturing for 5 days. In the pneumatic displacement group, an expansile concentration of gas (SF6 or C3F8) and r-tPA were injected intravitreally through a 30 G needle. Patients assumed a supine position for 5 h and subsequently, similarly to the vitrectomy group, face-down posturing for 5 days was instructed. During the 12 months FU, should development of intra- or subretinal fluid be noted, patients received anti-VEGF treatment.
The two main outcome measures were BCVA at final follow-up and hemorrhage displacement at 1 month post-intervention. BCVA was assessed by two optometrists and recorded in logMAR units (LogMAR ETDRS Number Chart). Hemorrhage displacement was documented based on the method described by Chang and co-workers [17]: complete displacement defined as no blood or scarce blood within 1 disc diameter of the foveal center; partial displacement defined as blood under the fovea obscuring RPE but not causing clinically visible elevation of the retina; no displacement defined as unchanged amount of blood under the fovea causing clinically evident elevation of the retina.
Secondary outcome measures included postoperative complications and changes in OCT parameters: mean CRT (μm), hemorrhage extent (μm) and SFHT (μm). Hemorrhage displacement was graded as described above. Mean CRT was automatically calculated from the box function of the OCT software. Hemorrhage extent was measured on the largest diameter using the OCT software. SFHT was measured vertically on the central OCT scan. All OCT scans were obtained with the Spectralis HRA + OCT (Heidelberg Engineering GmbH, Heidelberg, Germany). All OCT data were extracted by two different investigators (G.N.T. and S.K.) in order to identify potential mistakes.
Data analysis was performed using the R program (version 3.5.2, R Foundation for Statistical Computing). Continuous variables of pre- and postoperative data were analyzed with paired t tests. Continuous variables between the operation groups were examined with the Student’s t test or the Mann–Whitney U test. The associations of continuous variables with the three levels of displacement were investigated with the ANOVA or the Kruskal–Wallis test. Categorical values were analyzed with Fisher’s exact test; pairwise post-hoc analysis was employed when needed. Pearson or Spearman correlation coefficients were calculated to assess the potential correlation of BCVA with other continuous and categorical variables at each month of follow-up. Statistical significance was set at 0.05 for all analyses.
Results
Baseline data
Twenty five eyes of 25 patients (14 males and 11 females) were included in the analysis. Group A (vitrectomy group) consisted of 14 eyes and group B (pneumatic group) of 11 eyes. Baseline data of the two groups showed similar distribution (Table 1). Mean age of the subjects was 80 years (ranging from 60 to 96 years). All patients were followed-up for a minimum of 12 months and attended regularly the requested visits at 1 week and at 1, 3, 6 and 12 months post-treatment. Baseline logMAR BCVA was 1.53 ± 0.73 and mean duration of SMH prior to surgery was 8.2 (± 7.3) days. Regarding anatomical localization, hemorrhage was defined as subretinal in 14 eyes (56%), sub-RPE in 6 eyes (24%) and combined subretinal and sub-RPE in 5 eyes (20%). Mean CRT was 795 ± 365 μm and mean extension of SMH pre-treatment was 4.604 ± 2079 μm. Maximum SMH thickness was 559 ± 370 μm and intraoperative anti-VEGF injection was performed in 18 of the 25 patients. SF6 was the preferred choice in 12 and 7 patients of Group A and B, respectively, whereas C3F8 was used in the rest of the cases.
Primary outcome features display
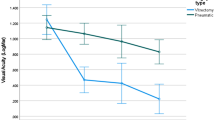
BCVA showed significant improvement at all time points, reaching a mean value of 0.91 ± 0.66 logMAR at 12 months (p = 0.002). Although BCVA improvement was greater in the vitrectomy group as opposed to pneumatic group, it failed to reach statistically significant levels (p = 0.68) (Fig. 1). Total displacement of SMH was observed in 16 eyes (64%), partial displacement in 4 eyes (16%) and no displacement in 5 eyes (20%) (Table 2). SMH displacement showed no statistical difference between the 2 groups (Fisher's exact test p = 0.38). Displacement was total in 9/14 versus 6/11 and partial in 4/14 versus 2/11 in Group A and Group B, respectively (Fig. 2).
Postoperative variation of BCVA (LogMAR) improvement during the 12 months follow-up. Comparison between the 2 groups failed to reach statistically significant levels at any time point of the study. X axis: time (months) of postoperative observation. Y axis: mean change in best corrected visual acuity (BCVA) (LogMAR)
Secondary outcome features display
Overall CRT changed significantly throughout follow-up, showing a mean reduction of 404 ± 292 μm at 1 month (p < 0.001) and 498 ± 366 μm at 12 months (p < 0.001). Similarly, overall extent of SMH and subfoveal hemorrhage thickness (SFHT) decreased significantly at all stages of follow-up with no statistically difference between the two groups (p = 0.6 and p = 0.9 respectively) (Table 2). Regarding postoperative complications, one rhegmatogenous retinal detachment was developed in the vitrectomy group 6 weeks after the initial procedure and 1 RPE tear in the pneumatic displacement group noticed 2 weeks post-treatment (OR: 0.6, p = 1). One patient developed rhegmatogenous retinal detachment 12 months following intervention in group B which was not considered to be related to the pneumatic approach and was not included in the analysis.
Several factors were considered in the evaluation of SMH displacement. Duration and type of hemorrhage (p = 0.25 and p = 0.16, respectively), gas type (p = 0.37) and intraoperative anti-VEGF (p = 0.38) showed no statistical influence on the magnitude of displacement. Similar results were revealed when the aforementioned parameters were analyzed in the Vitrectomy and Pneumatic group separately (Table 3). As expected, displacement of SMH hemorrhage was associated with greater final BCVA (p = 0.027) and reduced CRT at 12 months (p = 0.033). Functional outcome expressed by final BCVA and change of BCVA was associated with baseline BCVA (p = 0.013 and p = 0.033 respectively). Final BCVA was also related to preoperative CRT (p = 0.047). We failed to reveal other significant determinants for final BCVA including preoperative duration (p = 0.23), extent (p = 0.64) and location (p = 0.89) of hemorrhage, intraoperative anti-VEGF (p = 0.28) and gas type (p = 0.7), as none proved to impact functional outcome.
Discussion
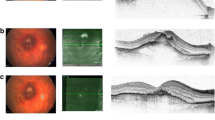
The onset of submacular hemorrhage in wAMD patients is a devastating complication, causing fast and irreversible visual loss in eyes that could otherwise maintain decreased but potentially useful vision for a longer period of time. Appropriate therapeutic approach for prompt and effective hemorrhage removal may prove life quality defining. In this study, we retrospectively compared vitrectomy with subretinal r-tPA and intravitreal gas (vitrectomy group) and intravitreal r-tPA and gas (pneumatic displacement group). Our results show that both techniques were effective in promoting complete or partial SMH displacement (81%) with significant functional benefit since BCVA improved in both groups throughout the 12-month follow-up. Interestingly, we did not notice any significant difference in outcome when comparing the 2 groups, although it is of note that the pneumatic group had 3 failures with no significant displacement as opposed to only one failure in the vitrectomy group. Moreover, of all preoperative findings tested, baseline BCVA and CRT were the only determinants to ultimately alter final functional outcome (Figs. 3, 4).
Example of submacular haemorrhage treated with vitrectomy-subretinal r-tPA and gas (vitrectomy group), pre-treatment and at 1, 3 and 6 months postoperative (clockwise). This case was classified as partial displacement because of the superior extrafoveal persistence of the haemorrhage. There is mild re-occurence of temporal and inferior extrafoveal haemorrhage at 3 months which further decreased at 6 months and never involved the subfoveal area
A considerable number of previous reports focused on presenting the outcomes of one single technique or comparing different techniques.
Vitrectomy with gas and subretinal r-tPA was first performed by Lewis in 1996 [18], using C3F8 tamponade in 24 consecutive recent-onset (< 14 days) SMH due to exudative AMD and reporting significant anatomical and functional improvement. More recently, Ritzau-Tondrow et al. [19] prospectively studied 34 eyes with wAMD-related SMH that underwent only core vitrectomy with subretinal r-tPA and SF6 tamponade. They concluded that the procedure achieved significant hemorrhage displacement and visual improvement. Martel and Mahmoud [20] described a technique similar to Lewis, injecting subretinally r-tPA with air via 41-gauge cannulas after vitrectomy. Later, Kadonosono et al.[21] presented a variation of this technique, performing 25-gauge vitrectomy with submacular r-tPA and 0.4 ml of air using a 47-μm microneedle (50-μm outer diameter) and reported 100% clot displacement with early visual improvement. Using a technique similar to ours, Juncal et al. [22] conducted a retrospective study on 99 eyes with SMH secondary to various causes who underwent vitrectomy with subretinal r-tPA and intravitreal gas. The authors reported significant blood displacement and visual improvement. In our study, we injected r-tPA subretinally using a 41-gauge cannula, without air. Air use was avoided in order to prevent macular hole formation, a potential complication that would increase operation time and complexity. The percentage of total SMH displacement (total and partial 80% overall) was comparable to the previously mentioned studies. Our results confirmed that SMH size does not correlate with final outcome, therefore cannot be used as predictive factor or biomarker for therapeutic decision-making. Similarly, duration of submacular hemorrhage does not seem to alter the outcome provided it does not exceed 2 weeks. In the vitrectomy group, final BCVA was significantly better than pre-op BCVA and overall better when compared to other reports [17, 22], although the difference to other studies may reflect our slightly better baseline visual acuity and the fact that we have included only wet AMD cases.
The technique of intravitreal r-tPA and gas for SMH displacement was introduced by Heriot as an outpatient procedure [11]. Since then, numerous reports have shown positive outcomes. In 2 prospective studies, Hattenbach et al. [23, 24] reported significant and accelerated visual improvement. Mozaffarieh et al. [16] conducted a prospective study with 101 cases of subretinal hemorrhage of at least one disc diameter and found significant visual and anatomical improvement at 12 months. In a retrospective study conducted by Schulze et al. [25], 67 patients with SMH due to exudative AMD received r-tPA and gas injection and it was reported that preoperative visual acuity was a prognostic factor for functional outcome. They also added that SMH diameter was the second prognostic factor, predicting a poor visual outcome when greater than 5 mm. In a retrospective study with 104 eyes treated for SMH with intravitreal injection of 30–100 mcg of tPA and expansile gas (SF6 or C3F8), conducted by Chen et al. [26], visual acuity improvement was significantly associated with preoperative VA, submacular blood displacement and the underlying cause of SMH. Complications included breakthrough vitreous hemorrhage in 8 eyes (8%) and retinal detachment in 3 eyes (3%). In the study by Tsymanava et al. [27], the best functional and anatomical results were reported for patients treated with 50 and 100 μg intravitreal r-tPA, with further BCVA improvement when additional gas was injected. In our study, intravitreal r-tPA with gas had a high success rate with complete or partial displacement (72.7%). One RPE tear (9%), needs to be taken into account. Compared to previous studies, our results are on the upper success limit, both for hemorrhage displacement and for visual acuity, and one possible explanation is the relatively short mean duration of submacular hemorrhage in the pneumatic group, less than 7 days.
Like in every therapeutic approach, direct comparison of surgical options is a basic branch of research appreciation. While Hillenkamp et al. [6] in their retrospective comparative study of 47 patients, found significant benefit of vitrectomy with subretinal t-PA over intravitreal t-PA (55% vs 22%) in terms of hemorrhage displacement, Shultz and Bakri [28], in their literature review of 2011, were not able to find clear advantage of one technique over the other. Similar conclusions were presented by Zeeburg and Van Muers in their 2013 review [15], though highlighting the lesser complications associated with intravitreal r-tPA without vitrectomy. A randomized prospective study by De Jong et al. [29] in 2016 involved 24 eyes with hemorrhage span up to 14 days, receiving either intravitreal r-tPA, gas and bevacizumab or vitrectomy, subretinal r-tPA, gas and bevacizumab. They found no difference in effectiveness for the 2 modalities and median relative volume reduction over 90% for both techniques [29]. Our study showed equal results for both techniques in terms of hemorrhage displacement, and even more markedly, final visual acuity. We could not prove one technique superior to the other, even when we tested the 2 groups for additional factors such as hemorrhage extent, duration and reduction rate over the 12-month follow-up. It is of note that, in contrast to other studies [22], we did not detect any recurrence of SMH.
This is one of few studies offering a head-to-head comparison of the 2 aforementioned techniques. Nevertheless, its retrospective design is a major limitation. As expected, some of the phakic patients in group A might have developed progressive nuclear sclerosis postoperatively. Since cataract progression was not documented with the “LOCS” classification system, it would be inappropriate to include lens data in the analysis. However, at no time point within the reported follow up, any of the eyes showed significant progression of cataract to require cataract surgery. We acknowledge the fact that final visual acuity in group A, although best corrected, might be slightly underestimated. Moreover, given the lack of predetermined guidelines concerning patient eligibility for either vitrectomy and subretinal r-tPA or pneumatic hemorrhage displacement, procedure choice was upon surgeon’s preference potentially introducing selection bias. We tried to partially counterbalance these limitations with a long follow-up and rigorous statistical analysis, obtaining a reasonably even distribution of baseline features between the 2 groups. Furthermore, our sample is similar to the majority of studies comparing the effectiveness of the two procedures. Finally, defining the types of displacement (total, partial and absent) in accordance to Chang et al. [17] and Kitagawa et al. [30], contributes to data validity.
In conclusion, this study indicates equal effectiveness of vitrectomy, subretinal r-tPA and gas versus intravitreal r-tPA and gas for the treatment of submacular hemorrhage due to neovascular AMD. Baseline BCVA and CRT appear to be significant prognosticators related to favorable prognosis.
References
Mehta S (2015) Age-related macular degeneration. Prim Care - Clin Off Pract 42(3):377–391. https://doi.org/10.1016/j.pop.2015.05.009
Al-Hity A, Steel DH, Yorston D et al (2019) Incidence of submacular haemorrhage (SMH) in Scotland: a Scottish Ophthalmic Surveillance Unit (SOSU) study. Eye 33(3):486–491. https://doi.org/10.1038/s41433-018-0239-4
McGowan F, Steel D, Yorston D (2014) AMD with submacular hemorrhage: new insights from a population-based study. Invest Ophthalmol Vis Sci 55(13):662
Stanescu-Segall D, Balta F, Jackson TL (2016) Submacular hemorrhage in neovascular age-related macular degeneration: A synthesis of the literature. Surv Ophthalmol 61(1):18–32. https://doi.org/10.1016/j.survophthal.2015.04.004
Haupert CL, McCuen BW 2nd, Jaffe GJ, et al (2001) Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol 131(2):208–215. https://doi.org/10.1016/S0002-9394(00)00734-0
Hillenkamp J, Surguch V, Framme C, Gabel VP, Sachs HG (2010) Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefe’s Arch Clin Exp Ophthalmol 248(1):5–11. https://doi.org/10.1007/s00417-009-1158-7
Rishi E, Gopal L, Rishi P, Sengupta S, Sharma T (2012) Submacular hemorrhage: A study amongst Indian eyes. Indian J Ophthalmol 60(6):521–525. https://doi.org/10.4103/0301-4738.103779
Olivier S, Chow DR, Packo KH, MacCumber MW, Awh CC (2004) Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in Age-Related macular degeneration. Ophthalmology 111(6):1201–1208. https://doi.org/10.1016/j.ophtha.2003.10.020
Singh RP, Patel C, Sears J (2006) Management of subretinal macular haemorrhage by direct administration of tissue plasminogen activator. Br J Ophthalmol 90(4):429–431. https://doi.org/10.1136/bjo.2005.085001
Thompson JT, Sjaarda RN, Mieler WF (2005) Vitrectomy for the treatment of submacular hemorrhages from macular degeneration: A comparison of submacular hemorrhage/membrane removal and submacular tissue plasminogen activator-assisted pneumatic displacement. Trans Am Ophthalmol Soc 103:98–107
WJ H, (1996) Intravitreal gas and TPA: an outpatient procedure for submacular hemorrhage. Published online, Am Acad Ophthalmol Annu Vitreoretin Updat, p 1996
Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG (2008) Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: A retrospective pilot study. Acta Ophthalmol 86(5):490–494. https://doi.org/10.1111/j.1600-0420.2007.01125.x
Sacu S, Stifter E, Vécsei-Marlovits PV et al (2009) Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye 23(6):1404–1410. https://doi.org/10.1038/eye.2008.267
Treumer F, Roider J, Hillenkamp J (2012) Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovascular AMD with submacular haemorrhage. Br J Ophthalmol 96(5):708–713. https://doi.org/10.1136/bjophthalmol-2011-300655
Van Zeeburg EJT, Van Meurs JC (2012) Literature review of recombinant tissue plasminogen activator used for recent-onset submacular hemorrhage displacement in age-related macular degeneration. Ophthalmologica 229(1):1–14. https://doi.org/10.1159/000343066
Mozaffarieh M, Heinzl H, Sacu S, Wedrich A (2006) In-patient management and treatment satisfaction after intravitreous plasminogen activator injection. Graefe’s Arch Clin Exp Ophthalmol 244(11):1421–1428. https://doi.org/10.1007/s00417-005-0232-z
Chang W, Garg SJ, Maturi R et al (2014) Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol 157(6):1250–1257. https://doi.org/10.1016/j.ajo.2014.02.007
Lewis H (1994) Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol 118(5):559–568. https://doi.org/10.1016/S0002-9394(14)76571-7
Ritzau-Tondrow U, Baraki H, Hoerauf H (2012) Minimally invasive therapy of submacular hemorrhage in exsudative age-related macular degeneration. Ophthalmologe 109(7):670–675
Martel JN, Mahmoud TH (2013) Subretinal pneumatic displacement of subretinal hemorrhage. JAMA Ophthalmol 131(12):1632–1635. https://doi.org/10.1001/jamaophthalmol.2013.5464
Kadonosono K, Arakawa A, Yamane S et al (2015) Displacement of submacular hemorrhages in age-related macular degeneration with subretinal tissue plasminogen activator and air. Ophthalmology 122(1):123–128. https://doi.org/10.1016/j.ophtha.2014.07.027
Juncal VR, Hanout M, Altomare F et al (2018) Surgical management of submacular hemorrhage: experience at an academic Canadian centre. Can J Ophthalmol 53(4):408–414. https://doi.org/10.1016/j.jcjo.2017.10.010
Hattenbach LO, Brieden M, Koch F, Gümbel H (2002) Intravitreale gabe von rt-PA und gas zur behandlung kleinflächiger subfovealer blutungen bei altersbezogener makuladegeneration. Klin Monbl Augenheilkd 219(7):512–518. https://doi.org/10.1055/s-2002-33584
Hattenbach LO, Klais C, Koch FHJ, Gümbel HOC (2001) Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology 108(8):1485–1492. https://doi.org/10.1016/S0161-6420(01)00648-0
Schulze SD, Hesse L (2002) Tissue plasminogen activator plus gas injection in patients with subretinal hemorrahge caused by age-related macular degeneration: Predictive variables for visual outcome. Graefe’s Arch Clin Exp Ophthalmol 240(9):717–720. https://doi.org/10.1007/s00417-002-0516-5
Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ (2007) Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina 27(3):321–328. https://doi.org/10.1097/01.iae.0000237586.48231.75
Tsymanava A, Uhlig CE (2012) Intravitreal recombinant tissue plasminogen activator without and with additional gas injection in patients with submacular haemorrhage associated with age-related macular degeneration. Acta Ophthalmol 90(7):633–638. https://doi.org/10.1111/j.1755-3768.2011.02115.x
Shultz RW, Bakri SJ (2011) Treatment for submacular hemorrhage associated with neovascular age-related macular degeneration. Semin Ophthalmol 26(6):361–371. https://doi.org/10.3109/08820538.2011.585368
De Jong JH, Van Zeeburg EJT, Cereda MG et al (2016) Intravitreal versus Subretinal administration of recombinant tissue Plasminogen activator combined with gas for acute submacular hemorrhages due to age-related macular degeneration. Retina 36(5):914–925. https://doi.org/10.1097/IAE.0000000000000954
Kitagawa Y, Shimada H, Mori R, Tanaka K, Yuzawa M (2016) Intravitreal Tissue Plasminogen Activator, Ranibizumab, and Gas Injection for Submacular Hemorrhage in Polypoidal Choroidal Vasculopathy. Ophthalmology 123(6):1278–1286. https://doi.org/10.1016/j.ophtha.2016.01.035
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors have equally contributed in the design of the study, acquisition and analysis of data, interpretation and preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors report any kind of conflict of interest.
Ethical approval
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All subjects involved in the study have given their written informed consent that their data will be used for research purposes. The study protocol was approved by the institute’s committee on human research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tranos, P., Tsiropoulos, G.N., Koronis, S. et al. Comparison of subretinal versus intravitreal injection of recombinant tissue plasminogen activator with gas for submacular hemorrhage secondary to wet age-related macular degeneration: treatment outcomes and brief literature review. Int Ophthalmol 41, 4037–4046 (2021). https://doi.org/10.1007/s10792-021-01976-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01976-x