Abstract
Background
Cystoid macular edema (CME) is a well-known complication after cataract surgery, and diabetic retinopathy is reported to be an important risk factor for impaired visual recovery. In this prospective study, we compared visual outcome 6 months after surgery in eyes with moderate retinopathy and no previous ME with a control group, and observed the incidence of ME seen on fluorescein angiography (FA) and optical coherence tomography (OCT).
Methods
Thirty-four patients with type-2 diabetes and 35 controls were enrolled. Best-corrected visual acuity (VA) letters ETDRS was measured pre-op, at day 7, week 6 and month 6. FA performed pre-op and at week 6 was divided into three leakage patterns. OCT performed pre-op, at week 6 and month 6 was qualitatively divided into three types. Macular thickness was measured in three circular fields (central subfield, inner and outer circle) from the macular maps.
Results
There was no statistically significant difference in VA before surgery, at day 7 or at 6 months, but at 6 weeks there was a significant difference with lower VA in the diabetic group. Six percent of control and 12% of diabetic eyes developed a clinical CME defined as a loss of >5 letters between day 7 and week 6. Incidence of FA leakage was 23% in control and 76% in diabetic eyes. At 6 weeks, 20% of control and 44% of the diabetic eyes had qualitative changes on OCT. A statistically significant increase in thickness was observed for all three macular areas in both groups, part of it remaining at 6 months. There were, however, no differences in central macular thickness between the groups at any visit. Retinal thickening had poor correlation with VA.
Conclusion
The final visual outcome in eyes with mild to moderate retinopathy, without previous ME, is as good as in normal eyes, but an increased frequency of macular changes may protract recovery of full vision. Changes on OCT or FA are often seen without any obvious effect on VA. OCT is as good as FA at detecting a clinical CME, and is the technique recommended for follow-up before FA is considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cystoid macular edema (CME) sometimes occurs after uncomplicated cataract surgery. A delayed visual recovery can be observed, but the visual prognosis is good [1]. The phenomena is referred to as pseudophakic edema or the “Irvine–Gass syndrome” first described by Irvine in 1953, and later in careful biomicroscopic and angiographic descriptions by Gass and Norton in 1966 [2, 3]. In most cases the edema is transient, but some patients will experience chronic cystic changes in the macula, with permanent visual loss [1, 4, 6].
In patients with diabetes mellitus and retinopathy, macular edema (ME) is the major reason for unfavourable visual outcome after cataract surgery [7, 8]. Duration of the diabetic disease, level of retinopathy, glycaemic control and, above all, ME present at surgery have been reported to increase the risk for postoperative, persistent CME [7–11]. Postoperative CME is also suspected to be more frequent in patients with non-insulin-dependent diabetes mellitus (NIDDM) than in those with insulin-dependent diabetes mellitus (IDDM) [12]. Some authors have reported that retinopathy and macular alterations in diabetics are accelerated by cataract surgery [12–14], while others found that these complications are more likely due to the natural course of the diabetic disease [10, 15, 16]. However, it is often difficult to distinguish cystic macular changes caused by surgery from edema due to pre-existing diabetic maculopathy.
CME can as a rule be identified on biomicroscopic examination, and when associated with a decrease in visual acuity (VA) it is categorized as clinical CME. The incidence of clinical CME after phacoemulsification is reported to be 0.1-2% in healthy populations, [1, 4, 17]. Fluorescein angiography (FA) is the gold standard for confirming ME. In angiographic ME, fluorescein leaks from dilated perifoveal capillaries, and when the hyperfluorescens takes a petaloid pattern in the late phase of the angiogram it is termed cystoid macular edema (CME). Angiographic edema can often be observed without visual impairment, and is then referred to as sub-clinical CME. The incidence of sub-clinical CME in healthy subjects has been reported to be 9 -19% after uncomplicated phacoemulsification [18, 19]. In diabetic patients, the situation is more complex. Different results can be expected between studies on different cohorts with respect to diabetes type, level of retinopathy or previous treatment with laser or intraocular steroid injections [7–9, 11–13]. Thus reported incidence of CME after cataract surgery ranges from 31% to 81% [7, 8, 16].
Even though qualitative evaluation of various leakage patterns observed on FA is possible, quantification of the fluorescence leakage is difficult. Optical coherence tomography (OCT), on the other hand, can be evaluated both qualitatively [20–22] and quantitatively [23, 24]. OCT has also been suggested to be more sensitive for the detection of discrete ME in patients with diabetes compared to a contact lens examination [25, 26].
In the present study, patients with type 2 diabetes scheduled for cataract surgery, with mild to moderate retinopathy, and with no pre-existing ME were included. The patients had not previously been treated with laser. An equally sized control group was also included in this prospective, comparative cohort study. The visual outcome, the incidence and the type of ME on FA and OCT were evaluated after the surgery.
Materials and methods
Subjects
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and the protocol was approved by the local ethics committee. The study participants signed an informed consent, and were examined at the Department of Ophthalmology, Uppsala University Hospital. Sixty-nine patients, average age 71 years, (range 54–82), 26 women and 43 men, were enrolled. The study participants consisted of a consecutive cohort of 34 patients with diabetes mellitus type 2 with mild to moderate retinopathy, and 35 controls that were scheduled for cataract surgery (Table 1). A full medical and ocular history was taken before the cataract surgery. An ocular examination was performed initially, and on each follow-up visit. This included tonometry, slit-lamp examination of the anterior segment and dilated retinal biomicroscopy. Best-corrected visual acuity (BCVA) was determined with the ETDRS chart at 2 meters. A visual acuity score of 85 letters is equivalent to Snellen 20/20. We used a loss of >5 letters between day 7 and week 6 in combination with macular changes on FA and/or OCT as a definition of clinical CME.
The non-diabetic controls were verified with HbA1c taken less than 1 month before surgery. Patients underwent examination preoperatively, at 7 days, 6 weeks and 6 months postoperatively. Exclusion criteria were ocular disease other than diabetes or cataract, pre-proliferative or proliferative retinopathy, previous laser treatment, eyes with a central subfield thickness >250 μm on OCT, subjects with intraoperative complications and eyes with clinically significant diabetic macula edema (CSME) on fundoscopy with 60 diopters lens according to the ETDRS criteria [27]. Eyes with fluorescein leakage in the central macula from perifoveal capillaries were excluded, but small areas of hyperfluorescence from microaneurysms in extrafoveal locations were allowed.
Fundus photography and fluorescein angiography
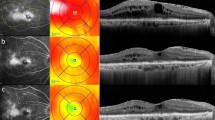
The Topcon digital system camera model TRC-50 IX was used. Each eye underwent 7-field fundus photography with red-free images preoperatively. Angiography was performed preoperatively and at 6 weeks postoperatively. In cases where cataract caused poor angiographic quality and made analysis impossible, the angiography was performed 7 days ± 1 day after the operation and classified as preoperative. This time was chosen on the basis of the study by Klein and Yanuzzi, who reported a very low incidence of angiographic edema within 1 week after cataract surgery [28]. Fluorescein angiographic findings were categorized qualitatively into the following three patterns based on the classification by Kang et al. [21] (see Fig. 1). One masked reviewer (GB) classified the diabetic retinopathy from the red-free images into mild or moderate from the ETDRS scale, [29] and also categorized the angiographic leakage patterns. If more than one pattern was observed in an eye, it was classified as a combination, for example patterns 1 + 2.
The classification into three fluorescein angiographic leakage patterns in the late phase of the angiogram (5 minutes). 1: cystoid leakage with pooling of dye in cystic spaces of the macula. 2: focal leakage with predominantly well-defined focal areas of leakage from micro aneurysm or localized dilated capillaries in diabetes patients marked by circle. 3: diffuse predominantly widespread and ill-defined leakage. Last picture (bottom right) shows a combination of focal (white circle), cystic (black circle)
Optical coherence tomography (OCT)
The Stratus OCT 3 (Carl Zeiss Meditec.Dublin,CA, USA.) with software 4.0.1 was used. The OCT technique has been described elsewhere [30, 31]. Examinations were performed at the baseline visit, 6 weeks, and 6 months after the surgery. The majority of the OCT examinations were performed by one of the authors (UE or EG), and a few by an experienced technician. In a previous study, we found a good repeatability for the central subfield in normal eyes with the macular thickness map scan protocol [33], and we therefore decided to use that protocol in the present study. With this protocol, six 6-mm tomographic scans, each constructed from 512 A-scans, are manually placed over the central macula, and from these measurements the thickness maps are constructed in three circular fields: one centrally located circle with a diameter of 1 mm (central subfield), one intermediate with 3 mm, and an external with 6 mm diameter (Fig. 2). The macular volume within the area covered by the total map, expressed in mm3, was also recorded. OCT scans were repeated when necessary to obtain adequate image quality. Thus, the examiners excluded all scans with artefacts. In cases of artefacts from low reflectivity caused by the cataract, the OCT was performed 6-8 days after surgery and recorded as preoperative status.
OCT data were also analyzed qualitatively. The six OCT single-scans, which build a map, were evaluated separately by an independent observer (GB) and categorized into three types, modified from Otani et al. [20] and Kang et al. [21], see Fig. 3.
The qualitative OCT classification. The top picture is an example of a healthy macula. The three types are: 1 homogenous, “smoothed-out”, thickening of the fovea, 2 sub-foveal low reflective macular detachment, 3 thickening of the fovea with low reflecting cystoid changes in the inner retinal layer. Bottom picture is a combination of types 2 and 3
Surgical procedure
One experienced surgeon (AWM) performed all surgical procedures, which consisted of topical and intracameral anaesthesia, clear cornea small-incision, capsulorhexis, and phacoemulsification. Implantation of a foldable hydrophobic acrylic intraocular lens was performed (AcrySof MA60BM, Acrysof SA60AT or AcrySof SN60AT). At the end of the procedure, 1 mg of cefuroxime was injected into the anterior chamber. Postoperatively topical prednisolone 0.5% was prescribed three times a day, with a tapering schedule over 3 weeks. The prednisolone treatment was moderated individually in cases of increased postoperative inflammation. Clinical CME developed early in a few patients who returned outside the study protocol after day 7 due to poor vision, and in the remaining subjects CME was observed at the 6-week follow up. As in our normal clinical routine, diclofenac 0.1% three times daily, with an individual tapering schedule over 3 weeks, was added. These patients were followed more closely outside the study protocol.
Statistical methods
Analyses, including descriptive statistics, were performed using SPSS version 18.0 (SPSS Inc, Chicago, IL,USA). Letters ETDRS is in an ordinal scale, and when comparing VA between two groups, the Mann–Whitney U-test was used. The intra-individual difference, pre- and postoperatively was analyzed with paired sample t-test, and independent t-test was used when comparing macular thickness between the groups.
Results
Thirty-nine diabetic patients and 36 control patients were enrolled in this study. One diabetic patient and one control patient decided to withdraw from the study. One diabetic patient was excluded due to preoperative ME on FA, and one because of pathological thickening on OCT. One diabetic patient was excluded because of earlier laser treatment which was not noted when the patient was recruited, and one diabetic when corneal opacities made analysis of the angiograms impossible. No subjects were excluded due to surgical complications. Thirty-four patients with type 2 diabetes and 35 controls remained in the study. One patient in the diabetic group died before the postoperative follow-up of 6 months, but was able to attend the 6-week control. For baseline characteristics of the study population see Table 1.
In eight diabetic eyes and four of the control eyes, the FA and OCT examinations were repeated on day 6-8 due to poor preoperative quality caused by the cataract.
Fluorescein angiography
The distributions of fluorescein angiographic patterns at 6 weeks are presented in Table 2. In control eyes, cystoid fluorescein leakage (pattern1) was observed in 23%. No focal or diffuse leakage was seen. In diabetic eyes, increased fluorescein leakage was seen in 76%. Twelve percent showed cystoid leakage on FA (pattern 1), 12% well-defined focal areas of leakage outside the fovea (pattern 2), 18% diffuse leakage (pattern 3) and 35% had a combination of more than one pattern. Figure 4 is an example of a late (5 min) angiogram in a diabetic patient preoperatively and at week 6. On the preoperative image, discrete hyperfluorescent spots can be seen in extrafoveal locations. An increase in hyperfluorescence/leakage can be observed in these areas at week 6, as well as a central cystic leakage from the perifoveal capillaries and a diffuse leakage in a large surrounding area.
A late-phase fluorescein angiogram (5 min) from an eye with diabetic retinopathy. a Pre-operatively, only small areas of hyperfluorescent microaneurysms are seen on the temporal side and there is no leakage in the central macula. b At 6 weeks, an increase in fluorescence from the microaneurysms is observed, as well as cystic leakage from the perifoveal capillaries, and a diffuse leakage in a large area around the central macula
OCT qualitative evaluation
At 6 weeks, seven of 35 eyes in the control group (20%), and 15 of 34 eyes (44%) of diabetics had visible changes on OCT. According to our classification, they were divided into three types (Fig. 3). The distribution is shown in Table 3. Type 1, a homogenous thickening of the fovea, was seen only in diabetic eyes (26%). Type 2 and type 3 OCT changes (cystoid/subfoveal) were equally frequent in eyes with (18%), and without diabetes (20%). These changes were evaluated to be completely resolved in all eyes at 6 months except in three diabetics, where they still were visible but had diminished.
OCT and FA qualitative changes were compared, as seen from Table 4a and b. Six of eight control eyes with cystic changes on FA (pattern 1) also had subfoveal or cystoid changes on OCT (type 2 and/or 3). In the diabetic eyes on the other hand, there was no obvious correspondence between changes on FA and OCT.
OCT quantitative-macular thickness
The data for the average retinal thickness in the central macular subfield (A1), the inner circle, the outer circle, and the total macular volume are presented in Table 5. Preoperatively, there were no significant differences between the groups. At 6 weeks, a statistically significant increase in thickness was observed for all macular areas, as well as macular volume in both groups. The thickness and the volume remained significantly increased at 6 months when compared to preoperative values. There were no differences between diabetic and control eyes for the central subfield or inner circle at any visit. At 6 weeks, however, significantly higher values were recorded in diabetic eyes for the outer circle and the total macula volume (p = 0.005, p = 0.020). This difference remained statistically significant at 6 months (p = 0.013, p = 0.026).
Best-corrected visual acuity
The VA measured at the different visits in diabetic and normal eyes is presented in Fig. 5. There was no statistically significant difference in median VA between the two groups preoperatively (p = 0.06), or at day 7 (p = 0.12) but at week 6 after surgery a significant difference was observed, with lower VA in eyes with diabetic retinopathy (p = 0.004). At 6 months, however, VA in the two groups was almost the same, with no statistically significant difference between them (p = 0.97). Two control eyes (6%) and four diabetic eyes (12%) experienced a loss of >5 letters between day 7 and week 6. This clinical CME was transient in all eyes except in one diabetic eye (2.5%), in which a CSME developed. After focal laser treatment, this eye ended up with a 7 letter loss compared to the preoperative VA.
Box plots of visual acuity in letters ETDRS comparing controls (white boxes) and diabetics (striped boxes) preoperatively, at day 7, week 6, and at month 6. Median VA is drawn as lines. Boxes include interquartile range (25-75% of observations). Outliers are drawn as circles and extremes as triangles. There was no significant difference in median VA between the groups preoperatively (p = 0.06), at day 7 (p = 0.12) or at month 6 (p =0.97). A significant difference was observed at 6 weeks (p = 0.004) (Mann–Whitney U-test)
VA at week 6 vs percent change in central subfield (A1) thickness between baseline and week 6 is plotted in Fig. 6. Most of the control eyes had a good VA, and only a small change in macular thickness, but there were a few eyes with a low VA and corresponding extensive thickening. The diabetic eyes appeared more “scattered”, and revealed some eyes with surprisingly good VA despite a more than 50% thickening of the macula.
Discussion
The present study clearly demonstrates that the visual outcome in diabetic eyes with mild to moderate retinopathy is as good as in the control group 6 months after cataract surgery. However, at 6 weeks there was a significant difference, with lower visual acuity in eyes with diabetic retinopathy combined with a higher frequency of macular changes both on FA and OCT compared to the control group.
Pseudophakic clinical CME is defined differently by various authors. A commonly cited level is a visual acuity of 20/40 or worse in combination with ophthalmoscopic or angiographic CME. In the present study, we have defined clinical CME as a 5 or more letters loss between day 7, when clinical CME is rare [28], and the 6-week control when most CME has developed [1, 7, 8, 16]. The comparison with day 7 VA was chosen in order to avoid any effect of the cataract on VA. There was an incidence of 6% clinical CME in the control eyes and 12% in the diabetic eyes. This is lower than previously reported in diabetic eyes [7, 8, 16], which might be explained by the milder forms of retinopathy in the eyes included with no pre-existing macula edema. The higher incidence in healthy eyes compared to previous studies [1, 4, 18, 19] may be due to our definition of clinical CME, based on a change in VA rather than a certain VA level. This result on clinical CME suggests that a small transient reduction in VA after uncomplicated phacoemulsification might be easily overlooked.
Not surprisingly, macular changes on FA were observed more frequently in diabetic eyes (76%) than in controls (23%). These figures are within the range of previous studies, 9-19% for normal eyes [18, 19] and 31-81% for diabetic eyes [7, 8, 16]. Twice as many diabetics (44%) revealed qualitative OCT changes as compared to the non-diabetics (20.5%) at 6 weeks postoperatively, but the incidence of cystic changes in the macula was of the same order (∼20%) in both groups (Table 3). This suggests that a surgically induced “Irvine–Gass” component affected both groups similarly. However, only two of eight control eyes and four of eight diabetic eyes with cystoid changes had a clinical CME according to our classification. Thus, only 25% of the cystoid changes observed with OCT in control eyes, compared to 50% in eyes with diabetic retinopathy.
Previous studies have demonstrated a correlation between FA and OCT in diabetic patients with CME [21, 34]. In the present study, where eyes had recently developed ME after surgery, such a correlation could not be found. Diabetic eyes with normal OCT or OCT type 1 change, both showed FA of various patterns, mostly containing a diffuse leakage component (Table 4a). More than half of the eyes classified as normal on OCT had a leakage on FA. However, the central subfield in the 11 eyes with FA leakage but a normal OCT was slightly thicker at 6 weeks than in the eight eyes without FA leakage, (247 vs 209 μm, p < 0.01, compared to 217 vs 201 μm at baseline [not significant, Mann–Whitney U-test]), suggesting that qualitative evaluation of OCT images will overlook small, and most likely clinically unimportant, changes in the macula. In healthy subjects, on the other hand, the correspondence between cystoid leakage on FA and OCT type 2 + 3 was good (Table 4b).
Apart from the qualitative analysis, OCT also provides thickness values for the central retina. This offers an opportunity to investigate a possible correlation between VA and retinal thickness. Several authors have reported a correlation between VA and foveal thickness in eyes with diabetic retinopathy, including eyes with a long history of maculopathy and chronic edema [20, 35–39]. The situation after cataract surgery can, however, be different, as the surgical trauma itself induces a rapid increase in macular thickness [11, 41]. This postoperative macular thickening has been reported to occur also in healthy eyes [40–44], where one author found a correlation between VA and macular thickness [40] while others did not [44, 45]. Obviously, other conditions like postoperative corneal edema, anterior segment inflammation or macular ischemia [36] can also impair the vision. Further, as normal macular thicknesses vary widely [33, 46], it is not surprising that there seems to be a poor correlation between macular thickness and VA. Therefore, as in the studies by Kim et al. [11, 47], we choose to evaluate whether the size of the change in macular thickness was related to VA. Kim et al. found that an increase in OCT ‘center point’ thickness of more than 30% in diabetic patients after phacoemulsification was associated with a visual loss at 1 month [11]. In accordance with this, Kim and Bressler recently suggested that the observation of more than 40% increase in retinal thickness from baseline on OCT is a valid method for confirming ‘clinically relevant’ CME after cataract surgery [48]. However, in the present study we did not find that a modest increase in foveal thickness is a strong support for clinically relevant CME, since a VA of ≥75 letters ETDRS (≥ Snellen 20/32) was found in five of nine eyes with an increase in thickness in the central subfield (A1) of more than 40% (Fig. 6). A likely explanation for the discrepancy between the studies is that different types of patients have been included. In the present study, the baseline OCT thickness values were not significantly different between diabetic and normal eyes (Table 5), which is in accordance with the report by Massin et al., who found no differences in macular thickness comparing healthy subjects and diabetics without CSME [49]. ME at the time of surgery, as well as previous laser treatment and, to some extent, the level of retinopathy are important risk factors for developing CME and poor visual outcome after cataract surgery in diabetic eyes [1, 4, 7, 8]. Kim et al. studied patients with various levels of retinopathy, including patients with no retinopathy. Furthermore, some had previously been treated with laser or steroid injections, and the preoperative macular thickness reported in the central subfield was higher than in the present study, 275 μm versus 217 μm. These facts, together with the longer follow-up in the present study, may explain the different outcomes.
In the present study, the thickness of the central macular subfield (A1) increased significantly between the preoperative measurements and the 6-week follow up in both diabetics and controls. There was, however, no significant difference between the two groups (Table 5). A similar finding, with an equal thickening of the central macula in diabetic and non-diabetic subjects 4 weeks after surgery, is also reported by Degenring et al. [41]. In the present study, measurements outside the central macula were also recorded. In these areas, cataract surgery had a more pronounced effect on diabetic than normal eyes. This was also seen in FA patterns, where a majority of the diabetic eyes had an increased leakage from areas with microaneurysms as well as a diffuse widespread leakage, while the control eyes only developed centrally located cystic changes (Table 2). For examples, see Fig. 1, patterns 1 and 3, and Fig. 4. However, parafoveal changes seem to have little effect on VA, and between 6 weeks and 6 months two-thirds of the thickening had been reversed in both diabetic and control eyes (Table 5). This suggests a retained capacity to remove excessive fluid from the retina also in diabetic eyes.
Finally, the present study is one of few reports where attempts have been made to compare (OCT) with the gold standard (FA) on postoperative macular changes [14, 45]. In diabetic eyes, the incidence of macular changes on FA was considerably higher than on qualitative OCT evaluation, and the correspondence between the two techniques was poor. In control eyes, on the other hand, the incidence and type of macular changes on FA were consistent with those found on OCT.
This study demonstrates that clinical post-surgical CME can be detected with OCT as efficiently as with FA. Also, OCT is non-invasive, reproducible and quick to perform. Consequently, OCT is the technique to be recommended in clinical routine. However in cases where laser is indicated, FA is needed for qualitative evaluation and treatment planning.
In conclusion, the final visual outcome for patients with mild to moderate retinopathy without preoperative ME was as good as in healthy patients. The incidence of cystoid changes and thickening in central macula on OCT was in the same order for both groups, suggesting an “Irvine–Gass” component that affected both groups similarly. These changes regressed or disappeared in all the study eyes. Postoperative changes on OCT were often seen without a significant deterioration of the VA, and do therefore not require immediate treatment. A clinical recommendation would be to perform OCT in order to detect CME in cases with unexpectedly low VA after surgery, treat conservatively with topical steroids and NSAID and to follow the visual development together with OCT before FA is considered.
References
Ray S, D’Amico DJ (2002) Pseudophacic cystoid macular edema. Semin Ophthalmol 17:167–180
Irvine SR (1953) A newly defined vitreous syndrome following cataract surgery interpreted according to recent concepts of the structure of the vitreous. Am J Ophthalmol 36:499–619
Gass JD, Norton EW (1966) Cystoid macular edema and papilledema following cataract extraction. A fluorescein fundoscopic and angiographic study. Arch Ophthalmol 76:646–661
Rosetti L, Autelitano A (2000) Cystoid macular edema following cataract surgery. Curr Opin Opthalmol 11(1):65–72
Jampol LM, Sanders DR, Kraff MC (1984) Prophylaxis and therapy of aphakic cystoid macular edema. Surv Ophthalmol 28:535–539
Ruiz RS, Saatci OA (1991) Visual outcome in pseudophacic eyes with clinical cystoid macular edema. Ophthalmic Surg 22:190–193
Pollack A, Leiba H, Buckelman A, Oliver M (1992) Cystoid macular oedema following cataract extraction in patients with diabetes. Br J Ophthalmol 76:221–224
Dowler JFG, Sehmi KS, Hykins PG, Hamilton AM (1999) The natural history of macular edema after cataract surgery in diabetes. Ophthalmology 106:663–668
Henriksson M, Heijl A, Janzon L (1996) Diabetic retinopathy before and after cataract surgery. Br J Ophthalmol 80:789–793
Squirrell D, Bhola BJ, Winder S, Talbot JF (2002) A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br J Ophthalmol 86(5):565–571
Kim SJ, Equi R, Bressler NM (2007) Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology 114:881–889
Flesner P, Sander B, Henning V, Parving H-E, Dornoville de la Cour M, Lund-Andersen H (2002) Cataract surgery on diabetic patients: a prospective evaluation of risk factors and complications. Acta Ophthalmol Scand 80:19–24
Chung J, Kim M-Y, Kim H-S, Yoo J-S, Lee Y-C (2002) Effect of cataract surgery on the progression of diabetic retinopathy. J Cataract Refract Surg 25:626–630
Escarvage GK, Cohen KL, Patel SB, Hartnett E, Armstrong BD, Janowski CM (2006) Quantification of macular and optic disc hyperfluorecence after phacoemulsification in diabetes mellitus. J Cataract Refract Surg 32:803–811
Wagner T, Knaflic D, Rauber M, Mester U (1996) Influence of cataract surgery on the diabetic eye: a prospective study. Ger J Ophthalmol 2:79–83
Krepler K, Biowski R, Schrey S, Jandrasits K, Wedrich A (2002) Cataract surgery in patients with diabetic retinopathy: visual outcome, progression of diabetic retinopathy, and incidence of diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol 240:735–738
Flach AJ (1998) The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 96:557–634
Ursell PG, Spalton DJ, Whitchup SM, Nussenblatt RB (1999) Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg 25:1492–1497
Mentes J, Erakgun T, Afrashi F, Kerci G (2003) Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica 217:408–412
Otani T, Kishi S, Maruyama Y (1999) Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 127:668–693
Kang SW, Park CY, Ham DI (2004) The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol 137(2):313–322
Soliman W, Sander B, Jørgensen TM (2007) Enhanced optical coherence patterns of diabetic macular oedema and their correlation with the pathophysiology. Acta Ophthalmol Scand 85(6):613–617
Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Rutledge B, Schuman JS, Swanson EA, Fujimoto JG (1995) Quantitative assessment of macular oedema with optical coherence tomography. Arch Ophthalmol 113:1019–1029
Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A (2001) Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol 119:1135–1142
Brown JC, Solomon SD, Bressler SB, Schacht AP, DiBernardo C, Bressler NM (2004) Detection of diabetic fovela edema. Contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol 122:330–335
Browning DJ, McOwen MD, Bowen RM, O'Marah TL (2004) Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology 111:712–715
Early Treatment Diabetic Retinopathy Study Research group (1985) Photocoagulation for diabetic macular edema; early treatment diabetic retinopathy study report number 1. Arch Ophthalmol 103:1796–1806
Klein RM, Yannuzzi L (1976) Cystoid macular edema in the first week after cataract extraction. Am J Ophthalmol 81:614–615
Diabetic Retinopathy Study Research Group (1991) Grading diabetic retinopathy from stereoscopic colour fundus photographs—an extension of the modified Airlie House classification ETDRS report number 10. Ophthalmology 98(5 Suppl):786–806
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA (1991) Optical coherence tomography. Science 254:1178–1181
Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, Puliafito CA, Fujimoto JG (1995) Optical coherence tomography of the human retina. Arch Ophthalmol 113:325–332
Polito A, Del Borello M, Isola M, Zemella N, Bandello F (2005) Repeatability and reproducibility of fast macular thickness mapping with Stratus optical coherence tomography. Arch Ophthalmol 123:1330–1337
Eriksson U, Alm A (2009) Repeatability in and interchangeability between the macular and the fast macular thickness protocols: a study on normal eyes with Stratus optical coherence tomography. Acta Ophtahlmol Scand 87(7):725–730
Soliman W, Sander B, Hasler PW, Larsen M (2008) Correlation between intraretinal changes in diabetic macular oedema seen in fluorescein angiography and optical coherence tomography. Acta Ophthalmol Scand 86(1):34–39
Hee MR, Puliafito C, Duker JS, Reichel E, Coker JG, Wilkins JR, Schuman JS, Swanson EA, Fujimoto JG (1998) Topography of diabetic macular edema with optical coherence tomography. Ophthalmology 105(2):360–370
Goebel W, Kretzchmar-Gross T (2002) Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT). Retina 22(6):759–767
Hussain A, Hussain N, Nutheti R (2005) Comparison of mean macular thickness using optical coherence tomography and visual acuity in diabetic retinopathy. Clin Exp Ophthalmol 33(3):240–245
Alkuraya H, Kangave D, Abu El-Asrar AM (2005) The correlation between optical coherence tomographic features and severity of retinopathy, macular thickness and visual acuity in diabetic macular edema. Int Ophthalmol 26(3):93–99
Diabetic Retinopathy Clinical Research Network (2007) Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 114(3):525–536
Nicholas S, Riley A, Patel H, Neveldson B, Purdie G, Wells AP (2006) Correlations between optical coherence tomography measurement of macular thickness and visual acuity after cataract extraction. Clin Exp Ophthalmol 34(2):124–129
Degenring RF, Vey S, Kamppeter B, Budde WM, Jonas JB, Sauder G (2007) Effect of uncomplicated phacoemulsification on the central retina in diabetics and non-diabetic subjects. Graefes Arch Clin Exp Ophthalmol 245:18–23
Sourdille P, Santiago PY (1999) Optical coherence tomography of macular thickness after cataract surgery. J Cataract Refract Surg 25(2):256–261
Ching HY, Wong AC, Wong CC, Woo DC, Chan CW (2006) Cystoid macular oedema and changes in retinal thickness after phacoemulsification with optical coherence tomography. Eye 20(3):297–303
von Jagow B, Ohrloff C, Kohnen T (2007) Macular thickness after uneventful cataract surgery determined by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 245(12):1765–1771
Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG (2004) Macular alterations after small-incision cataract surgery. J Cataract Refract Surg 30(4):752–760
Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS (2006) Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol 124(2):193–198
Kim SJ, Belair M-L, Bressler NM, Dunn JP, Thorne JE, Kedhar SR, Jabs DA (2008) A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 28:870–876
Kim SJ, Bressler NM (2009) Optical coherence tomography and cataract surgery. Curr Opin Ophthalmol 20:46–51
Massin P, Erginay A, Haouchine B, Mehidi AB, Paques M, Gaudric A (2002) Retinal thickness in healthy and diabetic subjects measured using optical coherence tomography mapping software. Eur J Ophthalmol 12(2):102–108
Financial Support
The present study was supported by the Research Fund at the Department of Ophthalmology, Uppsala University Hospital.
Financial Disclosure(s)
None of the authors have any financial interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have full control of all primary data, and agree to allow Graefe´s Archives for Clinical and Experimental Ophthalmology to review this data upon request.
Rights and permissions
About this article
Cite this article
Eriksson, U., Alm, A., Bjärnhall, G. et al. Macular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controls. Graefes Arch Clin Exp Ophthalmol 249, 349–359 (2011). https://doi.org/10.1007/s00417-010-1484-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1484-9