Abstract
Purpose
To study the electroretinographic signals originating in the long-wavelength-sensitive (L) and middle-wavelength-sensitive (M) cone pathways by means of large-field and multifocal cone type-specific electroretinograms (ERGs) in a patient with progressive cone dystrophy.
Methods
A 65-year-old male patient with colour vision disturbances (age at onset 10 years), loss of visual acuity (14 years), and central visual field defects (40 years) was investigated. Large-field flicker-ERG responses to stimuli that exclusively modulated the L-cones or the M-cones, or the two simultaneously (both in-phase and in counter-phase), were measured. Short-wavelength-sensitive (S) cones were not modulated. Multifocal ERGs (mfERGs) were also recorded, with a pattern-reversing display that modulated only the L- or the M-cones at equal cone contrasts and average quantal catches. Genetic analysis of L- and M-pigment genes was performed on genomic DNA isolated from peripheral venous blood.
Results
The patient showed a normal rod-driven ERG but reduced cone-driven electroretinographic amplitudes with normal implicit times in the International Society for Clinical Electrophysiology of Vision (ISCEV) standard ERG. The large-field flicker-ERG responses to pure L-cone modulation were significantly above noise level but were substantially reduced in comparison with both normal trichromatic subjects and (otherwise normal) deuteranopes. The L-cone driven electroretinographic implicit times and phases were within normal limits. The M-cone driven electroretinographic responses were not detectable. A model fit of all the L- and M-cone driven flicker-ERG data revealed that the responses were exclusively driven by the L-cones. Consistently, the cone type-specific mfERGs showed severely reduced but detectable responses to L-cone-isolating stimuli. The M-cone driven multifocal-ERG responses were undistinguishable from noise. The L- and M-pigment gene array consisted of only a single L-pigment gene. The complete coding sequence of this gene was determined and showed no abnormality.
Conclusions
This patient exhibits a coincidence of progressive cone dystrophy and deuteranopia. The molecular genetic data of the L/M-pigment gene array is consistent with the deutan phenotype. It cannot be excluded that the rearrangement of the X-chromosome pigment gene array is responsible for the cone dystrophy in this patient. It is, however, suggested that the dichromacy and the cone dystrophy have different and independent genetic origins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cone dystrophies are a group of inherited retinal disorders that are characterized by bilateral visual acuity loss, colour vision disturbances, central visual field impairment, photophobia, reduced amplitudes of the photopic electroretinogram (ERG) and, sometimes, nystagmus.[5, 10, 22, 30, 36] Genetic studies have revealed numerous genetic subtypes with different modes of genetic transmission (for a review see: http://www.sph.uth.tmc.edu/Retnet/).
Selective functional loss of one cone type caused by mutations of one of the opsin genes may result in selective colour vision deficiencies such as the common forms of X-linked red–green colour vision deficiencies (protan and deutan defects), or the less frequent autosomal dominant inherited blue–yellow (tritan) deficiency.[27, 39, 45] More severe forms of colour vision deficiency involve the degeneration, dysfunction or absence of at least two types of cone photoreceptors, as observed in patients with incomplete or complete achromatopsia [2, 3, 15–17, 47] or severe cone dystrophies [36].
To study whether the different cone types are selectively affected in individual patients, electroretinographic measurements using differently coloured stimuli have been conducted.[13, 14] By means of such colour-stimulated ERGs, several “selective” cone and cone–rod dystrophies have been described: cone–rod dystrophy with blue cone hypersensitivity (also termed enhanced S-cone syndrome) [11, 26], predominant S-cone dystrophy [6, 44], and predominant L-cone dystrophy [15, 29]. However, in none of these studies is a complete separation of responses driven by the different cone types achieved. This is especially true for the differentiation between the L- and M-cone driven pathways, because the absorption spectra of the L- and M-cones overlap considerably [40]. Alternatively, chromatic adaptation procedures have been used in electroretinographic recordings [28, 43]. Although the electroretinographic responses driven by the adapted or desensitized cone type in such procedures may often be very small, they may not be negligible. Moreover, it is almost impossible to compare measurements at different states of adaptation [20].
To overcome these difficulties, a method has been developed that allows one to obtain electroretinographic responses to cone-isolating stimuli and to stimuli in which the L- and M-cones are stimulated simultaneously with known contrasts without changing the overall state of adaptation [21, 41, 42]. This technique has been used to investigate cone type-specific retinal pathways in subjects with normal colour vision and in dichromats [18, 19, 21, 41, 42], retinitis pigmentosa [35], sector retinitis pigmentosa [33], Stargardt macular dystrophy [34], Best macular dystrophy [37], macular dystrophy with protan genotype and phenotype [38] and S-cone monochromacy [32]. (For a review see Scholl [31].) Very recently, a comprehensive study has been performed on a population of patients with cone- and cone–rod dystrophies [36]. In all the studies mentioned it has been found that each of the retinal disorders shows characteristic alterations in certain aspects derived from these cone-driven ERGs. In the present study we report the findings in a patient exhibiting a progressive cone dystrophy with a deutan genotype and phenotype.
Material and methods
A detailed history (including family history), ophthalmological examination (including slit-lamp biomicroscopy, funduscopic evaluation by retinal biomicroscopy and fundus photography), best corrected visual acuity, visual fields (static and kinetic perimetry), dark adaptation thresholds (Tübingen hand perimeter) and colour vision tests (Lanthony D-15 saturated/desaturated and Rayleigh anomaloscope equation) were obtained.
Corneal electroretinographic responses were measured with DTL fibre electrodes which were positioned on the conjunctiva directly beneath the cornea and attached with the two ends at the lateral and nasal canthus. The reference and skin electrodes (gold cup electrodes) were attached to the ipsilateral temple and the forehead, respectively. For all ERG procedures, the pupils were dilated with 0.5% tropicamide and 5% phenylephrine. Scotopic and photopic Ganzfeld ERGs were recorded in accordance with the International Society for Clinical Electrophysiology of Vision (ISCEV) standard [24]. Multifocal-ERG responses to stimuli with 61 hexagonal elements were recorded, using a protocol conforming to the new ISCEV guideline [25].
For the cone type-specific ERGs, the eyes were kept light-adapted for at least 10 min prior to the ERG recording. The methods of L- and M-cone driven large-field and multifocal-ERG recording has been described before [1, 21].
Briefly, for recording the flicker ERG the stimuli were presented on a computer-controlled monitor (BARCO CCID 121) driven at 100 Hz by a VSG 2/3 graphics card (Cambridge Research System). The monitor subtended 124° by 108° at the 10 cm viewing distance. A 30 Hz square wave modulation of the red, green, and blue phosphor was used with predefined Michelson contrasts. The time-averaged luminance of the monitor was 66 cd/m2 (40 cd/m2 for the green phosphor, 20 cd/m2 for the red phosphor, and 6 cd/m2 for the blue phosphor). The time-averaged chromaticity in CIE (1964) large-field coordinates were: x=0.3329, y=0.3181. The excitation in each cone type by the monitor phosphors was calculated by multiplication of the phosphor emission spectra by the psychophysically based fundamentals [40]. The modulation of cone excitation was quantified by the Michelson cone contrast and defined the stimulus strength for each cone type separately. The S-cones were silently substituted in all conditions (S-cone contrast was 0%). The measurements were performed at four conditions with L-cone/M-cone contrast ratios of 1:1, 1:0, 0:1, and -1:1. Twenty-nine subjects with normal colour vision (ages 9–57 years, median 27 years) served as a control. More detailed electroretinographic data on a subpopulation of subjects with normal colour vision have been published previously [21].
To assess the L- and M-cone driven response topography the multifocal ERG was recorded using the VERIS system (version 4.9.1) and a Grass amplifier in combination with the silent substitution technique described above. The average quantal catches of the stimulus were approximately 4.46 log quanta s−1 cone−1 for the L-cone-isolating stimulus and 4.43 log quanta s−1 cone−1 for the M-cone-isolating stimulus, with a Michelson contrast of approximately 47%. Again, the S-cones were silently substituted in both conditions. The stimuli were generated on a flat-screen SONY Trinitron monitor with a resolution of 1,024×768 pixels. It consisted of 103 hexagonal elements that subtended 84°×75° of visual angle at a viewing distance of 18 cm (for details see [1]).
In performing the examinations, we followed the tenets of the Declaration of Helsinki, informed consent was obtained, and approval was given by the Institutional Ethics Committee in Human Experimentation at the University of Tübingen.
Results
The patient was first examined at the age of 28 years and successively on many occasions covering an interval of 38 years. He experienced colour vision disturbances at 10 years of age, loss of visual acuity (at 14 years) and increased glare sensitivity (at 25 years). The major decrease in visual acuity from 20/30 at the age of 29 years to 20/100 at the age of 42 was accompanied by the emergence of a central visual field defect. The following examinations revealed a mild progression of his disease. There were no similarly affected family members, and none were aware of any colour-vision disturbances.
The first Ganzfeld electroretinographic examination was performed when the patient was aged 42 years and revealed normal scotopic and photopic responses. At the same time the Lanthony D-15 saturated colour test revealed a severe red–green deficiency, and the Rayleigh anomaloscope equation (performed on a Nagel type I instrument) showed colour and brightness matches similar to those obtained from complete achromats (see Fig. 1)
Lanthony D-15 test results (left) and the results obtained from the anomaloscope experiments (right) in the patient. The Lanthony D-15 test shows red–green colour vision deficiency. The Rayleigh anomaloscope equation showed colour and brightness matches similar to those obtained from complete achromats
Subsequent Ganzfeld ERG recordings all showed normal scotopic response amplitudes and implicit times for both the dim (24 db) flash and to the intense (0 db) flash. Normal final dark adaptation threshold confirmed normal rod function. The photopic Ganzfeld electroretinographic responses, however, had been reduced in amplitude since the subject had been aged 45 years, but the implicit times were normal.
He was 61 and 65 years old at the time of the detailed electrophysiological examinations. Within this period of 4 years there had been no deterioration of visual function as assessed by history and ophthalmological examination (including visual acuity and visual fields). Best corrected visual acuity varied between 20/400 and 20/200 in both eyes. There was no nystagmus. The anterior segments were normal. Funduscopy showed a central atrophic lesion of approximately 1 disc diameter, with pigment abnormalities within the macular area and temporal pallor of the optic disc in both eyes (see Fig 2). Perimetry revealed absolute and relative central scotomas within the 10° isoptre. Lanthony D-15 desaturated test revealed numerous confusions without a preferential axis; Lanthony D-15 saturated test showed several confusions preferentially along the protan/deutan axis.
Electroretinography according to the ISCEV standard revealed a normal scotopic ERG in both eyes. The oscillatory potentials were reduced in amplitude. The photopic b-wave showed a substantially reduced amplitude (21.7 μV and 20.7 μV for the right and left eye, respectively; 5%–95% percentiles of the normative values: 97–223 μV) with normal implicit times for both eyes (27.8 ms and 28.8 ms for the right and left eye, respectively; 5%–95% percentiles of the normative values: 27.7–32.8 ms). The 30 Hz flicker-ERG responses were also reduced in amplitude (22.2 μV and 19.0 μV for the right and left eye, respectively; 5%–95% percentiles of the normative values: 47–112 μV) with normal implicit times (28.8 ms for both eyes; 5%–95% percentiles of the normative values: 27.2–32.2 ms). Multifocal-ERG (mfERG) responses were averaged according to concentric rings and showed an overall reduction in response amplitude for both eyes. Only the outermost two ring averages (rings 4 and 5) were significantly above noise level, with an amplitude (from trough to peak) of 1.1 μV/deg2 and 1.5 μV/deg2 for rings 4 and 5 of the right eye, respectively, and 0.6 μV/deg2 and 1.1 μV/deg2 for rings 4 and 5 of the left eye, respectively (5%–95% percentiles of the normative values: 6.1–11.9 and 5.8–11.8 μV/deg2 for rings 4 and 5, respectively; Fig. 3). Detailed analysis of implicit times was not possible. The implicit times of the peak amplitude of the two most eccentric ring averages were within normal limits.
Averaged standard mfERG responses to black & white stimulus obtained when the subject was aged 59 years (a) and 66 years (b) from the study eye. Only for the two outermost ring did the averages show waveforms that were distinguishable from noise. The responses were severely reduced in amplitude. They were similar in amplitude at both investigations. The implicit time was within the normal range
Flicker-ERG responses were measured with various combinations of L-cone to M-cone contrast ratios, including L-cone and M-cone type-specific ERGs. The ERG signals to pure L-cone modulation and to pure M-cone modulation for a “normal” trichromat, for a healthy (“normal”) deuteranope, and for the patient are shown in Fig. 4. Substantial responses were measured in the trichromat in both conditions. The deuteranope displayed larger electroretinographic responses to the stimuli isolating the L-cone system than the trichromat. With M-cone-isolating stimuli a residual response was obtained. In the patient, the electroretinographic responses to pure L-cone modulation were substantially above the noise level but significantly reduced in comparison with the normal deuteranope. The M-cone driven ERG was near noise level.
Averaged electroretinographic responses to pure L-cone modulation (left column; 24.7% cone contrast) and pure M-cone modulation (right column; −31.2% cone contrast) for a “normal” trichromat (a), a “normal” deuteranope (b), and the patient with progressive cone dystrophy and deutan genotype and phenotype (c). The ERG signals are 150 ms extracts of 4 s traces that are the averages of 12 runs. Drift components in the electroretinographic responses to the L- and M-cone-isolating stimuli were suppressed by the removal of low frequency components [36]
The electroretinographic responses were Fourier analysed, and the electroretinographic response amplitude and phase were defined as the amplitude and phase of the fundamental component. The electroretinographic responses to pure M-cone modulation were near noise level, whereas those to pure L-cone modulation were substantially above noise level (electroretinographic amplitude to pure L-cone modulation at 24.7% cone contrast was 2.5 μV; mean normal amplitude=6.7 μV; SD=1.7 μV). However, compared with those of an otherwise “normal” deuteranope, the L-cone-driven ERG responses were considerably small (Fig. 4). The L-cone driven ERG phase was within normal limits (−357° at 24.7% cone contrast, corresponding to an implicit time of 33.0 ms; mean normal phase=−376°; SD=16.0°), and the dependency of the response phase on cone contrast was positive.
We found a linear relationship between electroretinographic response amplitude and cone contrast (at all conditions where the electroretinographic responses were above the noise level) in the patient, as has been recently established for subjects with normal colour vision [21, 41]. The slope of the linear regression to the data is the increase in electroretinographic amplitude per percentage increase in cone contrast and was used to define the cone contrast gain. The inverse of the cone contrast gain is the cone contrast increase needed for a 1 μV response increase, [21] which, owing to the linear relationship between amplitude and cone contrast, is equivalent to a threshold. The cone contrast gains and the thresholds were obtained for all ratios of L- to M-cone contrasts.
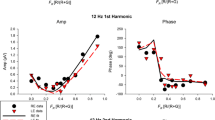
In Fig. 5, the ERG thresholds for different combinations of L- and M-cone modulation are plotted. In (otherwise “normal”) dichromats with red–green colour deficiencies, the best describing functions of the thresholds are straight lines parallel to one of the axes [21]. (The upper right panel shows one typical example.) In the patient, no threshold could be determined for pure M-cone stimulation. As in “normal” deuteranopes, the ERG thresholds at the remaining three ratios of L- to M-cone contrast could be best described by straight lines parallel to the M-cone axis. This indicates that the responses are exclusively L-cone driven, because the thresholds are reached at a constant L-cone contrast. However, compared with the “normal” deuteranope, the patient exhibited significantly increased ERG thresholds (note the different scaling in Fig. 5), indicating a substantially reduced cone-driven ERG sensitivity that is in accordance with a cone dystrophy. Figure 5 also shows the ERG thresholds for a patient with a progressive cone dystrophy carrying a peripherin/RDS mutation (lower left panel; replotted from Fig. 2 of Scholl and Kremers [36]). As can be seen, this patient shows substantially increased thresholds for all conditions, but this decrease in ERG sensitivity is balanced for the L- and M-cone driven responses. The sensitivity decrease in the patient (lower left panel) is similar in magnitude but is not balanced for the L- and M-cone driven electroretinographic pathways.
Threshold contrasts in a “normal” trichromatic subject, a “normal” deuteranope (replotted from Fig. 2a of Kremers et al. [21]), a patient with progressive cone dystrophy carrying a peripherin/RDS mutation (replotted from Fig. 2 in Scholl and Kremers [36]), and the patient exhibiting a progressive cone dystrophy with deutan genotype and phenotype. In the two patients, most ERG thresholds were considerably larger than in the “normal” subjects, corresponding to a decrease in ERG sensitivity (note the different scaling between the different panels). The straight parallel lines are best fits to the data points, indicating the presence of dichromacy (in the dichromat and the patient right panels). However, for the patient with cone dystrophy and deutan geno/phenotype the ERG thresholds were considerably larger than for the otherwise “normal” dichromat and similar to the patient with cone dystrophy carrying a peripherin/RDS mutation. Both indicate substantial loss of electroretinographic sensitivity
Multifocal ERG responses to isolating L- and M-cone stimuli were averaged according to concentric rings after artefact rejection. As was expected from the standard mfERG recordings, for both the L- and M-cone contrast stimuli, the waveforms corresponding to the central retinal area were not different from noise (Fig. 6). For the L-cone driven response the outermost ring average was detectable and showed a waveform that was clearly distinguishable from noise. For the M-cone-isolating condition, even the averaged response from the outermost ring was not distinguishable from noise.
Genetic analysis of L- and M-pigment genes were performed on genomic DNA isolated from peripheral venous blood. The L- and M-pigment gene array consisted of only a single L-pigment gene. The complete coding sequence of this gene was determined and showed no abnormality (J. Nathans, personal communication).
Discussion
Dichromacy is a relatively common phenomenon in healthy males. It is controversial whether the missing cone type in X-linked dichromats is completely [7, 46] or incompletely [4] replaced by the remaining cone type. Recently, we argued for a complete replacement [21, 23]. In previous studies the results of measurements on patients with progressive cone dystrophy [15, 29] or macular dystrophy [38] in combination with a protan phenotype and genotype were presented. A coincidence of cone dystrophy and deuteranopia has never been described before. However, the patient presented above exhibited such a deuteranopic cone dystrophy.
The apparently divergent results of a red–green colour vision disorder found with the Lanthony D-15 test and achromatopsia according to Rayleigh equation anomaloscope experiments may well be explained by retinal dysfunction and are consistent with deuteranopia. While the Lanthony D-15 test is performed under broadband daylight condition (Illuminant D65), which is bright enough to desensitize the rods in the macular area (due to the small pupil size it does not desensitize peripheral rods) and involves all three classes of cones (L-, M- and S-cones) in the colour arrangement task, the monochromatic wavelengths chosen for the Rayleigh equation (549 nm and 666 nm for the red and green half-field, respectively, and 589 nm for the yellow half-field) may not be detected by S-cones (therefore S-cone monochromats and achromats show similar steep brightness matches), and due to the small test field (2° in diameter) it is not sufficient to desensitize rods in the macular area in the case of fixation instability. While deuteranopes are unable to distinguish the colour of the red and green primary, they usually show a characteristic approximately horizontal brightness function (for clarity it is illustrated in Fig. 1). In our patient, however, the absence of M-cone function and the reduced function of the L-cone system precluded a stable fixation combined with almost normal rod sensitivity, and, thus, the match was achieved by brightness matching according to the luminosity function of the rods.
The thresholds of the flicker-ERG to the different ratios of L-cone to M-cone modulation are best described by two straight lines parallel to the M-cone axis, indicating that they are exclusively determined by activity in the L-cones and indicating that only the L-cone is present [21], and, thus, the patient is indeed deuteranopic. Similar results were found in otherwise “normal” deuteranopes [21]. However, compared with such deuteranopes, the patient exhibited considerably increased ERG thresholds, indicating a cone dystrophy. We conclude that the technique of large-field cone type-specific electroretinography is capable of revealing both a deutan phenotype and a cone dystrophy. It appears to be more robust (e.g. less sensitive to noise at low signal amplitudes) than the cone type-specific multifocal electroretinography method, although the results show definite consistency between the two ERG methods, namely reduced but detectable L-cone function and undetectable M-cone function.
The timing of the standard single-flash photopic ERG and the standard 30-Hz flicker-ERG is frequently abnormal in cone dystrophies. However, patients carrying mutations in GUCA1A encoding GCAP1 [8] and peripherin/RDS [9] have been shown to exhibit normal implicit times for the photopic standard electroretinographic responses. Interestingly, the patient described here shares several features that have been described in patients with GUCA1A mutations [8].
Genetic analysis of the L- and M-pigment genes was consistent with the deutan phenotype. It has been speculated that the rearrangement of the X-chromosome pigment gene array may not only cause dichromacy but also a cone dystrophy [3, 15, 16]. Support for this hypothesis came from in vitro studies showing that specific mutations within the X-chromosome pigment gene array can cause disruption of folding, half-life and light activation of the cone opsin molecule and, thus, may cause progressive retinal dystrophies [12]. However, the genetic analysis revealed a normal L-pigment coding gene in this patient. Thus, the simplest explanation would be to assume that the protanopia and the macular dystrophy have different genetic origins, although we cannot exclude that they may be linked.
References
Albrecht J, Jagle H, Hood DC, Sharpe LT (2002) The multifocal electroretinogram (mfERG) and cone isolating stimuli: variation. J Vis 2:543–558
Ayyagari R, Kakuk LE, Bingham EL, Szczesny JJ, Kemp J, Toda Y, Felius J, Sieving PA (2000) Spectrum of color gene deletions and phenotype in patients with blue cone monochromacy. Hum Genet 107:75–82
Ayyagari R, Kakuk LE, Coats CL, Bingham EL, Toda Y, Felius J, Sieving PA (1999) Bilateral macular atrophy in blue cone monochromacy (BCM) with loss of the locus control region (LCR) and part of the red pigment gene. Mol Vis 5:13
Berendschot TTJM, van de Kraats J, van Norren D (1996) Foveal cone mosaic and visual pigment density in dichromats. J Physiol 492(1):307–314
Berson EL, Gouras P, Gunkel RD (1968) Progressive cone–rod degeneration. Arch Ophthalmol 80:68–76
Bresnick GH, Smith VC, Pokorny J (1989) Autosomal dominantly inherited macular cystrophy with preferential short-wavelength cone involvement. Am J Ophthalmol 108:265–276
Cicerone CM, Nerger JL (1989) The density of cones in the fovea centralis of the human dichromat. Vision Res 29:1587–1595
Downes SM, Holder GE, Fitzke FW, Payne AM, Warren MJ, Bhattacharya SS, Bird AC (2001) Autosomal dominant cone and cone–rod dystrophy with mutations in the guanylate cyclase activator 1A gene-encoding guanylate cyclase activating protein-1. Arch Ophthalmol 119:96–105
Fishman GA, Stone EM, Alexander KR, Gilbert LD, Derlacki DJ, Butler NS (1997) Serine-27-phenylalanine mutation within the peripherin/RDS gene in a family with cone dystrophy. Ophthalmology 104:299–306
Goodman G, Ripps H, Siegel IM (1963) Cone dysfunction syndromes. Arch Ophthalmol 70:214–231
Jacobson SG, Marmor MF, Kemp CM, Knighton RW (1990) SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci 31:827–838
Kazmi MA, Sakmar TP, Ostrer H (1997) Mutation of a conserved cysteine in the X-linked cone opsins causes color vision deficiencies by disrupting protein folding and stability. Invest Ophthalmol Vis Sci 38:1074–1081
Kellner U, Foerster MH (1992) Color electroretinography. A method for separation of dysfunctions of cones. Doc Ophthalmol 80:13–23
Kellner U, Foerster MH (1993) Pattern of dysfunction in progressive cone dystrophies—an extended classification. Ger J Ophthalmol 2:170–177
Kellner U, Sadowski B, Zrenner E, Foerster MH (1995) Selective cone dystrophy with protan genotype. Invest Ophthalmol Vis Sci 36:2381–2387
Kellner U, Wissinger B, Tippmann S, Kohl S, Kraus H, Foerster MH (2004) Blue cone monochromatism: clinical findings in patients with mutations in the red/green opsin gene cluster. Graefes Arch Clin Exp Ophthalmol 242:729–735
Kohl S, Marx T, Giddings I, Jagle H, Jacobson SG, Apfelstedt SE, Zrenner E, Sharpe LT, Wissinger B (1998) Total colour blindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet 19:257–259
Kremers J, Scholl HPN (2001) Rod-/L-cone and rod-/M-cone interactions in electroretinograms at different temporal frequencies. Vis Neurosci 18:339–351
Kremers J, Scholl HPN, Knau H, Berendschot TTJM, Usui T, Sharpe LT (2000) L- and M-cone ratios in human trichromats assessed by psychophysics, electroretinography and retinal densitometry. J Opt Soc Am A 17:517–526
Kremers J, Stepien MW, Scholl HPN, Saito CA (2003) Cone selective adaptation influences L- and M-cone driven signals in electroretinography and psychophysics. J Vis 3:146–160
Kremers J, Usui T, Scholl HPN, Sharpe LT (1999) Cone signal contributions to electroretinograms in dichromats and trichromats. Invest Ophthalmol Vis Sci 40:920–930
Krill AE (1977) Hereditary retinal and choroidal diseases. Maryland Harper & Row, Hagerstown
Kurtenbach A, Heine J, Jagle H (2004) Multifocal electroretinogram in trichromat and dichromat observers under cone isolating conditions. Vis Neurosci 21:249–255
Marmor MF, Holder GE, Seeliger MW, Yamamoto S (2004) Standard for clinical electroretinography (2004 update). Doc Ophthalmol 108:107–114
Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y (2003) Guidelines for basic multifocal electroretinography (mfERG). Doc Ophthalmol 106:105–115
Marmor MF, Jacobson SG, Foerster MH, Kellner U, Weleber RG (1990) Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol 110:124–134
Nathans J, Piantanida TP, Eddy RL, Shows TB, Hogness DS (1986) Molecular genetics of inherited variation in human color vision. Science 232:203–210
Padmos P, van Norren D (1971) Cone spectral sensitivity and chromatic adaptation as revealed by human flicker electroretinography. Vision Res 11:27–42
Reichel E, Bruce AM, Sandberg MA, Berson EL (1989) An electroretinographic and molecular genetic study of X-linked cone degeneration. Am J Ophthalmol 108:540–547
Ripps H, Noble KG, Greenstein VC, Siegel IM, Carr RE (1987) Progressive cone dystrophy. Ophthalmology 94:1401–1409
Scholl HPN (2004) Photoreceptor type-specific electroretinography in inherited retinal disorders. Germany Books on Demand GmbH, Norderstedt
Scholl HPN, Kremers J (2001) Electroretinograms in S-cone monochromacy using S-cone and rod isolating stimuli. Color Res Appl 26:S136–S139
Scholl HPN, Kremers J (2003) L- and M-cone driven large-field and multifocal electroretinograms in sector retinitis pigmentosa. Doc Ophthalmol 106:171–181
Scholl HPN, Kremers J, Vonthein R, White K, Weber BHF (2001) L- and M-cone driven electroretinograms in Stargardt's macular dystrophy–fundus flavimaculatus. Invest Ophthalmol Vis Sci 42:1380–1389
Scholl HPN, Kremers J (2000) Large phase differences between L-cone and M-cone driven electroretinograms in retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:3225–3233
Scholl HPN, Kremers J (2003) Alterations of L- and M-cone driven ERGs in cone and cone–rod dystrophies. Vision Res 43:2333–2344
Scholl HPN, Kremers J, Apfelstedt-Sylla E, Zrenner E (2000) L- and M-cone driven ERGs are differently altered in Best's macular dystrophy. Vision Res 40:3159–3168
Scholl HPN, Kremers J, Wissinger B (2001) Macular dystrophy with protan genotype and phenotype studied with cone type specific ERGs. Curr Eye Res 22:221–228
Sharpe LT, Stockman A, Jägle H, Nathans J (1999) Opsin genes, cone photopigments, color vision, and color blindness. In: Gegenfurtner KR, Sharpe LT (eds) Color vision: from molecular genetics to perception. Cambridge University Press, New York, pp 3–51
Stockman A, MacLeod DIA, Johnson NE (1993) Spectral sensitivities of the human cones. J Opt Soc Am A 10:2491–2521
Usui T, Kremers J, Sharpe LT, Zrenner E (1998) Flicker cone electroretinogram in dichromats and trichromats. Vision Res 38:3391–3396
Usui T, Kremers J, Sharpe LT, Zrenner E (1998) Response phase of the flicker electroretinogram (ERG) is influenced by cone excitation strength. Vision Res 38:3247–3251
van Norren D, Padmos P (1973) Human and macaque blue cones studied with electroretinography. Vision Res 13:1241–1254
van Schooneveld MJ, Went LN, Oosterhuis JA (1991) Dominant cone dystrophy starting with blue cone involvement. Br J Ophthalmol 75:332–336
Weitz CJ, Miyake Y, Shinzato K, Montag E, Zrenner E, Went LN, Nathans J (1992) Human tritanopia associated with two amino acid substitutions in the blue-sensitive opsin. Am J Hum Genet 50:498–507
Wesner MF, Pokorny J, Shevell SK, Smith VC (1991) Foveal cone detection statistics in color-normals and dichromats. Vision Res 31:1021–1037
Wissinger B, Gamer D, Jagle H, Giorda R, Marx T, Mayer S, Tippmann S, Broghammer M, Jurklies B, Rosenberg T, Jacobsen SG, Sener EC, Tatlipinar S, Hoyng CB, Castellan C, Bitoun P, Andreasson S, Rudolph G, Kellner U, Lorenz B, Wolff G, Verellen-Dumoulin C, Schwartz M, Cremers FP, Apfelstedt-Sylla E, Zrenner E, Salati R, Sharpe LT, Kohl S (2001) CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet 69:722–737
Acknowledgements
We thank Eckart Apfelstedt-Sylla for his clinical contributions and Jeremy Nathans for providing the genetic data on the L–M pigment gene array in the patient. This work was supported by Deutsche Forschungsgemeinschaft Heisenberg Fellowship SCHO 734/2-1 to H.P.N.S.; by the European Commission FP5, QoL, KA6, Concerted Action Grant QLRT-2001-02494 “PHOTAGE” to H.P.N.S. and J.K., and by a fortüne grant, 1128-0-0, to H.J. and D.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholl, H.P.N., Kremers, J., Besch, D. et al. Progressive cone dystrophy with deutan genotype and phenotype. Graefe's Arch Clin Exp Ophthalmo 244, 183–191 (2006). https://doi.org/10.1007/s00417-005-0022-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0022-7