Abstract
Background and objective
Patients with idiopathic normal pressure hydrocephalus (iNPH) have a higher prevalence of hypertension and diabetes. However, the causal effects of these vascular risk factors on iNPH remain unclear. This study aimed to explore the causal relationship between vascular risk factors (VRFs) and iNPH.
Methods
We conducted the Mendelian randomization (MR) analysis of iNPH. We included nineteen vascular risk factors related to hypertension, diabetes, lipids, obesity, smoking, alcohol consumption, exercise, sleep, and cardiovascular events as exposure factors. We used the inverse-variance weighted method for causal effect estimation and weighted median, maximum likelihood, and MR Egger regression methods for sensitivity analyses.
Results
We found that genetically predicting essential hypertension (OR = 1.608 (1.330–1.944), p = 0.013) and increased sleep duration (OR = 16.395 (5.624–47.799), p = 0.009) were associated with higher odds of iNPH. Type 1 diabetes (OR = 0.869 (0.828–0.913), p = 0.004) was associated with lower odds of iNPH. For the other 16 VRFs, there was no evidence that they were significantly associated with iNPH. Sensitivity analyses showed that essential hypertension and type 1 diabetes were significantly associated with iNPH.
Conclusion
In our MR study on VRFs and iNPH, we found essential hypertension to be a causal risk factor for iNPH. This suggests that hypertension may be involved in the pathophysiological mechanism of iNPH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal pressure hydrocephalus (NPH) is a neurological disease characterized by gait disturbance, cognitive impairment, and urinary incontinence [1]. As the population ages, this disease is gaining attention as reversible dementia. Normal pressure hydrocephalus can be divided into idiopathic and secondary according to etiology. The pathophysiology of idiopathic normal pressure hydrocephalus (iNPH) is currently unknown. However, the population of iNPH is mainly the elderly [2, 3], who often have vascular risk factors (VRFs).
From previous case–control studies, hypertension [4,5,6,7,8,9,10], diabetes [4,5,6, 8,9,10,11], hyperlipidemia [4], abdominal obesity [4], physical inactivity [4], alcohol use disorder [12], and cardiac and cerebrovascular disease [5] are more common in patients with iNPH. Among these VRFs, hypertension is considered to be related to the clinical presentation, imaging, and prognosis of iNPH patients [10, 13]. And a nationwide hospital-based survey found that hypertension was the most common comorbidity in iNPH (40%), followed by diabetes (17.8%) [14]. Therefore, it has been speculated in the past that hypertension may be involved in the mechanism of iNPH, which can increase cerebrospinal fluid (CSF) pressure and pulse pressure, leading to ventriculomegaly [7, 15, 16]. And the ventriculomegaly has now been shown to result from increased CSF pulsatility [17], which is closely regulated by cardiovascular pulsation [18]. In addition, increased blood pressure has been found in mice to reduce the flow of CSF through the perivascular space in the brain, which means a decline in the function of the glymphatic system [19]. The function of the glymphatic system is to facilitate the removal of excess fluid and waste in the central nervous system [20], which has recently been considered one of the pathophysiological mechanisms of iNPH [21, 22]. Moreover, the glymphatic system is closely related to another vascular risk factor: sleep [20]. A previous prospective cohort study found obstructive sleep apnea in 90% (28/31) of patients with iNPH [23]. Sleep apnea is considered to cause iNPH by affecting intracranial venous circulation and glymphatic circulation [24]. In addition to hypertension and sleep apnea, diabetes is also an important vascular factor in iNPH patients. According to a systematic literature review, it occurs more than twice as frequently in iNPH patients as in age-matched controls [11]. And it is considered a risk factor for the development of iNPH along with hypertension and sleep apnea [25]. However, most studies on iNPH and vascular risk factors are observational studies, which cannot directly link the two to observe the impact of vascular risk factors on the occurrence of iNPH. Moreover, the randomized controlled trial (RCT) on iNPH is difficult to implement.
The Mendelian randomization (MR) method is an analysis method based on genetic instrumental variables [26], which can estimate the causal effect of exposure on the outcome. This method directly links exposure to genetic variation, and random segregation of alleles mimics random grouping in RCTs. Therefore, it can theoretically avoid bias from confounding factors between exposures and outcomes.
This study aimed to investigate the causal effect of vascular risk factors on iNPH using Mendelian randomization.
Methods
Study design
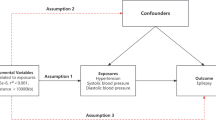
In this study, we used the 2-sample MR design to investigate the causal relationships between the 19 VRFs and iNPH. We made the following assumptions for this MR study evaluation: (1) the instrument is associated with the exposure (relevance). (2) There are no confounders of the instrument and the outcome (exchangeability). (3) The instrument has no direct effect on outcome except through exposure (the exclusion restriction).
Instruments
We obtained the GWAS data of the VRFs and used them as exposure factors. The ancestry of all the GWAS data was European. The included exposure factors were essential hypertension [27], secondary hypertension [27], type 1 diabetes (T1DM) [27], type 2 diabetes (T2DM) [27], low-density lipoprotein cholesterol [28], high-density lipoprotein cholesterol [28], triglycerides [28], body mass index [29], smoking initiation [30], cigarettes smoked per day [30], alcohol intake frequency [29], alcohol dependence [27], moderate to vigorous physical activity levels [31], myocardial infraction [32], coronary artery disease [33], stroke [34], sleep apnea [27], insomnia [29], and sleep duration [29]. Among these exposures, diagnostic criteria for disease-related exposures are presented in supplementary table 1. The selected single nucleotide polymorphisms (SNPs) should meet the criteria that genome-wide significance association with each factor was less than 5 × 10–8. And we clumped the selected SNPs to obtain the SNPs in a threshold of linkage disequilibrium (r2 > 0.01) and a distance of 10000 kb. Later we extracted the SNPs for each exposure factor from the outcome. The effect allele of exposure and outcome datasets were harmonized. Finally, we excluded the SNPs that exit the palindromic sequence.
Data sources for iNPH
The GWAS data of iNPH were obtained from the European cohort: the FinnGen study. The FinnGen study is a global research project that combines genome information with digital health care data [27]. In the FinnGen study round 5, 322 cases defined as NPH and 218,043 controls were included. The endpoint of NPH was defined by the International Classification of Diseases 10th version (ICD-10). The code of NPH in ICD-10 was G91.2.
Statistical analysis
We used the inverse-variance weighted (IVW) method to perform the principal analyses, which combined the SNPs of exposure and the SNPs of the outcome. The Wald ratio (the ratio of genetic association with the outcome to the genetic association with the exposure) was used to estimate the causal effects between exposure and outcome. To test whether the first assumption was satisfied, we calculated the F statistic for all SNPs using the formula. F = Beta2/SE2. F > 10 is considered weak instrument bias is small. And we used the maximum likelihood, the weighted median, and MR Egger regression methods for sensitivity analysis, considering that the IVW method might be affected by pleiotropy. Then MR-PRESSO was used to remove the outlier SNPs, which cause the horizontal pleiotropy. Finally, we searched PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/) for all SNPs included in the primary outcome of the analysis, considering potential confounding factors leading to a violation of the second assumption. We included recurrent traits of non-exposed factors as covariates. We then adjusted for covariates with the main outcome using multivariate Mendelian randomization.
All the statistical analyses were performed using R-4.2.1 with R packages. The R packages for analysis included the TwoSampleMR package, MendelianRandomization package, and MR-PRESSO package. For binary exposure factor variables, we used the odds of the exposure factor to estimate its causal effect on the outcome[35]. P value < 0.05 were considered as potential associations.
Data availability
The sources and information of the GWAS data for all exposures and the outcome involved in this study are presented in supplementary table 4. In addition, all the analysis result data are presented in the paper. All the GWAS data were publicly available through the IEU Open GWAS project online [36, 37]. Link: https://gwas.mrcieu.ac.uk/.
Results
The included exposure factors and IVW estimates of their causal effects with iNPH are presented in Table 1. In the IVW analysis, we found that genetically predicting T1DM (OR = 0.869 (0.828–0.913), p = 0.004), essential hypertension (OR = 1.608 (1.330–1.944), p = 0.013), and sleep duration (OR = 16.395 (5.624–47.799), p = 0.009) were associated with iNPH (Fig. 1). No significant associations were observed in the other 16 exposure factors. After combining the other three methods, all except the MR Egger method showed a significant association with iNPH for both type 1 diabetes and essential hypertension (supplementary table 5). For T1DM and essential hypertension, Fig. 2 shows scatterplots of the effect of SNP on exposure and the effect of SNP on the outcome. However, for sleep duration, only the maximum likelihood method showed that it was associated significantly with iNPH (supplementary table 5).
The association between risk factors and idiopathic normal pressure hydrocephalus (iNPH) using the inverse-variance weighted method. Odds ratios (ORs) represent the association between iNPH and each risk factor. The units of the binary exposure factors are odds: type 1 diabetes; type 2 diabetes; essential hypertension; secondary hypertension; alcohol dependence; myocardial infraction; sleep apnea; sleeplessness/insomnia. The units of the binary exposure factors are logOR: coronary artery disease and stroke. The 1-SD increase is the unit of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, body mass index (BMI), alcohol intake frequency, and moderate to vigorous physical activity levels (MVPA). The unit of smoking initiation is ever smoked regularly compared with never smoked. The unit of cigarettes smoked per day is cigarettes per day
We calculated F statistics for all SNPs used for MR analysis in essential hypertension and type 1 diabetes, and found that they were all > 10 (supplementary table 2; supplementary table 3). In the MR Egger method for the pleiotropy test, potential pleiotropy was only found on body mass index (p = 0.027) (Table 1). Using MR-PRESSO for all exposures, we found no significant difference between the results after removing outliers and the original results. And significant heterogeneity was only presented in the IVW method on sleep apnea (p = 0.049) (Table 1). Then we adjusted systolic blood pressure, diastolic blood pressure, and taking blood pressure medication with essential hypertension. Multivariate MR analysis showed that essential hypertension was associated with iNPH (OR = 2.195(1.520–3.168), p = 0.032) (Fig. 3). In addition, we adjusted for rheumatoid arthritis, autoimmune thyroiditis, and inflammatory bowel disease with type 1 diabetes. Multivariate MR analysis did not show that T1DM was associated with iNPH (OR = 0.841(0.763–0.927), p = 0.074).
The figure shows a multivariate Mendelian randomization analysis for essential hypertension and type 1 diabetes. The 1-SD increase is the unit of systolic blood pressure, diastolic blood pressure, and blood pressure medication. The odds of the binary exposure factor is the unit of rheumatoid arthritis, autoimmune thyroiditis, and inflammatory bowel disease
Discussion
In our MR study on VRFs and iNPH, we found that genetic instruments predicting essential hypertension, and type 1 diabetes were thought to be associated with iNPH. This suggests that they may be vascular factors with a causal effect on iNPH. Essential hypertension increases the risk of iNPH, whereas type 1 diabetes reduces the risk of iNPH. And most sensitivity analyses for essential hypertension and T1DM were consistent with no violation of the MR assumptions. In addition, long sleep duration may be a potential causal risk factor for iNPH. After adjusting for covariates, we found that the causal relationship between essential hypertension and iNPH was attenuated, but still significant. However, after covariate adjustment, there was no significant causal relationship between type 1 diabetes and iNPH. For the other 17 VRFs, there was insufficient evidence for a causal relationship between them and iNPH.
We found that essential hypertension is the only risk factor with a causal effect on iNPH in this study. This suggests that hypertension may be involved in the pathogenesis of iNPH pathophysiology. In previous observational studies, hypertension was often closely associated with ventriculomegaly, which is the primary pathological feature of iNPH. Subsequent studies using monitoring of aqueduct stroke volume [38] confirmed that ventriculomegaly is caused by increased CSF pulsatility, which is regulated by cardiovascular pulsations. This also suggests an association between hypertension and ventriculomegaly to some extent. In addition, we believe that vascular changes caused by hypertension may act to decrease arterial pulsatility, which is a critical process. Furthermore, arterial pulsatility drives the exchange of cerebrospinal fluid and interstitial fluid [39], and decreased exchange indicates impairment of glymphatic system function. A previous study also confirmed that hypertension can induce a decrease in the flow of the perivascular space, which may be related to the stiffening of the arterial wall caused by high blood pressure [19]. We think that this decrease in exchange may result in the deposition of toxic substances in the brain that contribute to cognitive impairment in patients with iNPH. The deposition of toxic solutes in the perivascular space may further aggravate the decrease of arterial pulsatility and fall into a vicious circle. Furthermore, decreased arterial pulsatility may be associated with increased aqueduct stroke volume [40, 41], which reflects increased CSF pulsatility consistent with ventriculomegaly. Previously, it was also believed that arterial pulsation restriction, capillary pulsation increase, and intracranial compliance decrease were the origin of hydrodynamic mechanism of chronic hydrocephalus [42]. In addition, increased pulse pressure as a feature of iNPH has also been considered as a possible mechanism involved [42, 43]. This seems to be supported by our findings that diastolic blood pressure also appears to be significantly associated with iNPH (Fig. 3). In addition to changes in arterial pressure, increased intracranial venous pressure can impede CSF absorption through the arachnoid villi and alter intracranial compliance [44]. However, prospective studies on hypertension and iNPH are still lacking. This article provides evidence for a causal effect of hypertension and iNPH. If hypertension has a causal effect on iNPH, the role of hypertension drugs in patients with iNPH is expected. In addition, of course, we also found that some iNPH patients do not have clinically diagnosed hypertension. In this regard, we believe that hypertension may be a risk factor rather than a decisive factor inducing iNPH. Because some of the iNPH patients are familial iNPH, they are mainly dominated by genetic factors. The link between hypertension and the genetic factors of NPH is also worth considering. In addition, we also considered that the symptoms of iNPH are not specific, and it may be misdiagnosed or overlap with other neurological diseases. And some diseases may have a causal relationship with hypertension, such as vascular dementia, which may bias the results. Therefore, we collected the prevalence of related diseases for the cases of NPH in this study (supplementary table 6). And we consider this bias to be negligible. In addition, secondary hypertension and iNPH are not considered to be related in this study. This may be because secondary hypertension is a secondary diagnosis that can be corrected after the etiology is identified and treated. Therefore, it cannot continuously affect patients like essential hypertension.
Diabetes was discussed separately in this study as T1DM and T2DM. We found that genetically predicting T1DM was associated with iNPH, whereas T2DM was not. Moreover, T1DM appeared to be a causal protective factor for iNPH in this study. We have two explanations for this. First, we concluded that T1DM and iNPH were statistically associated due to survival bias. In the previous studies, most of the studies were on T2DM and iNPH, and there were almost no studies on T1DM and iNPH. The reason is because of the large difference in the age of onset of T1DM and iNPH. The prevalence of iNPH is mainly in the elderly, and in the elderly population, the prevalence of T2DM is remarkably higher than that of T1DM [45]. In the population sample data of our study, the age of onset of T1DM was much younger than that of iNPH (mean age at the first event: 69.76 years old) (supplementary Fig. 1). Compared with the age of iNPH patients, the life expectancy of patients with T1DM is poorer, and they have a high exposure liability to T1DM. In other words, when we select those SNPs that are significantly related to T1DM, the population with higher expression of these SNPs means that they are more susceptible to T1DM. And having T1DM may shorten lifespan, which makes it harder for these populations to reach the age of onset of iNPH. For the protective effect of T1DM, we think that it may be because it is difficult for people with T1DM to reach the age of onset of iNPH. And the causal relationship between T1DM and iNPH dropped dramatically after accounting for other confounding factors. Thus, T1DM presents a pseudo-protective factor effect. Second, we have another conjecture. T1DM is an autoimmune disease, whether there is an association between genetic factors between it and iNPH to present such a result. However, none of the currently known mutated genes that may cause iNPH are associated with susceptibility genes for T1DM. For T2DM, the results of this study showed no causal relationship between it and iNPH. The glymphatic system seems to be considered as the pathway through which T2DM affects iNPH. T2DM has been shown in mice to impair the function of the glymphatic system in the hippocampus and hypothalamus [46], and impairment of the glymphatic system has also been observed in patients with iNPH [21]. However, impairment of the glymphatic system do not appear to be disease specific in iNPH. The impairment can also be seen in aging and Alzheimer's disease states [47, 48]. The performance of impairment of glymphatic system function is considered to be learning and memory impairment, the reason is that the deposition of amyloid-β and tau protein may be closely related [49, 50]. However, the most prominent symptoms in iNPH patients are abnormal gait and ventriculomegaly rather than cognitive impairment, which are the symptoms mainly improved by shunt therapy. In addition, impairment of glymphatic function may not necessarily be the cause of memory impairment. Therefore, we believe that the relationship between type 2 diabetes, the impairment of glymphatic system, and iNPH may be more manifested in the dementia symptoms of iNPH, rather than directly affecting the pathogenesis of iNPH. Although observational studies have found a high prevalence of type 2 diabetes in iNPH patients, this may be because the elderly with iNPH are already at higher risk of type 2 diabetes. Aging is a non-negligible factor in iNPH. In addition, a previous study suggested that diabetes in iNPH patients may be caused by ventriculomegaly and pituitary dysfunction [11]. In this study, we prefer to believe that T1DM does not play a role in the occurrence of iNPH. However, the risk of diabetes in iNPH patients deserves further study.
Regarding the relationship between sleep and iNPH, we included three exposure factors of sleep apnea, insomnia, and sleep duration in this study. Among these three factors, we only found increased sleep duration as a causal risk factor for iNPH confirmed by the IVW method. Although sleep-disordered breathing was found to be more common in iNPH patients in previous studies, comparisons with age-matched older adults were lacking. The sleep-disordered breathing was considered a risk factor for iNPH more because of the observed impairment of glymphatic function [21], which is responsible for clearing the brain of metabolic waste during sleep. However, the impaired glymphatic function is not only seen in patients with iNPH but also in patients with Alzheimer's disease [48]. A previous MR study on obstructive sleep apnea and Alzheimer's disease also showed no apparent causal relationship between obstructive sleep apnea and Alzheimer's disease [51]. Therefore, we think that there is no causal relationship between sleep apnea and iNPH. And impaired glymphatic function may affect the sleep of iNPH patients. A previous study showed that accumulation of β-amyloid in the brain worsens the sleep–wake cycle [52]. Moreover, Alzheimer's disease had a causal effect on sleep patterns in one MR study [53]. Therefore, we think it may be that patients with iNPH have a higher risk of sleep apnea, both of which are affected by the impaired glymphatic function. Interestingly, however, sleep duration was shown to be associated with iNPH in our study. And in a previous MR study on sleep duration and cognition, sleep duration was considered to have a causal relationship with cognition [54]. Moreover, increased sleep duration was found to be associated with increased perivascular space, suggesting that increased sleep duration may be related to the function of the glymphatic system [55, 56]. However, the causal relationship between sleep duration and impairment of glymphatic system function is unclear. The increase of perivascular spaces may result from increased sleep duration and poor sleep quality, while it is also possible that increased sleep duration is a compensatory mechanism for impaired glymphatic system function. However, the sensitivity analysis of this study did not further support the relationship between increased sleep duration and iNPH, which may be related to the violation of the assumptions caused by confounding factors. Therefore, the causal relationship between increased sleep duration and iNPH may require further study to explore.
This study also has limitations. There are few GWAS data on the outcome of this study. The reason is that there are few current GWAS for NPH available. Moreover, we could not exclude the presence of secondary NPH in the NPH cases studied in the present study. Secondary NPH and idiopathic NPH may differ in the application of conclusions. Therefore, this result may be biased when we emphasize iNPH. The main causes of secondary NPH are subarachnoid hemorrhage, traumatic brain injury, and brain malignancy [57]. We collect the prevalence of related diseases in the cases of NPH in supplementary table 6. In addition, causal relationships explained using genetic instruments have limited precision. We could not avoid bias from all confounding factors. And people need to live to a certain age to be included, which can lead to survivor bias. This bias may have affected the results.
In conclusion, we found hypertension to be a causal risk factor for iNPH in this MR study. This suggests that hypertension may be involved in the pathophysiological mechanism of iNPH. How vascular mechanisms play a role in the pathophysiology of iNPH is worth discussing in future studies. For other VRFs, there was no evidence of a causal relationship between them and iNPH.
Abbreviations
- NPH:
-
Normal pressure hydrocephalus
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- VRF:
-
Vascular risk factor
- MR:
-
Mendelian randomization
- GWAS:
-
Genome-wide association study
- SNP:
-
Single nucleotide polymorphisms
- IVW:
-
Inverse-variance weighted
- T1DM:
-
Type 1 diabetes
- T2DM:
-
Type 2 diabetes
- RCT:
-
Randomized controlled trial
- CSF:
-
Cerebrospinal fluid
- ICD:
-
International Classification of Diseases
- OR:
-
Odds ratio
References
Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH (1965) Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure.a treatable syndrome. N Engl J Med 273:117–126. https://doi.org/10.1056/nejm196507152730301
Zaccaria V, Bacigalupo I, Gervasi G, Canevelli M, Corbo M, Vanacore N, Lacorte E (2020) A systematic review on the epidemiology of normal pressure hydrocephalus. Acta Neurol Scand 141:101–114. https://doi.org/10.1111/ane.13182
Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelsø C (2014) Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 82:1449–1454. https://doi.org/10.1212/wnl.0000000000000342
Israelsson H, Carlberg B, Wikkelsö C, Laurell K, Kahlon B, Leijon G, Eklund A, Malm J (2017) Vascular risk factors in INPH: a prospective case-control study (the INPH-CRasH study). Neurology 88:577–585. https://doi.org/10.1212/wnl.0000000000003583
Krauss JK, Regel JP, Vach W, Droste DW, Borremans JJ, Mergner T (1996) Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke 27:24–29. https://doi.org/10.1161/01.str.27.1.24
Pyykkö OT, Nerg O, Niskasaari HM, Niskasaari T, Koivisto AM, Hiltunen M, Pihlajamäki J, Rauramaa T, Kojoukhova M, Alafuzoff I, Soininen H, Jääskeläinen JE, Leinonen V (2018) Incidence, comorbidities, and mortality in idiopathic normal pressure hydrocephalus. World Neurosurg 112:e624–e631. https://doi.org/10.1016/j.wneu.2018.01.107
Graff-Radford NR, Godersky JC (1987) Idiopathic normal pressure hydrocephalus and systemic hypertension. Neurology 37:868–871. https://doi.org/10.1212/wnl.37.5.868
Casmiro M, D’Alessandro R, Cacciatore FM, Daidone R, Calbucci F, Lugaresi E (1989) Risk factors for the syndrome of ventricular enlargement with gait apraxia (idiopathic normal pressure hydrocephalus): a case-control study. J Neurol Neurosurg Psychiatry 52:847–852. https://doi.org/10.1136/jnnp.52.7.847
Eide PK, Pripp AH (2014) Increased prevalence of cardiovascular disease in idiopathic normal pressure hydrocephalus patients compared to a population-based cohort from the HUNT3 survey. Fluids Barrier CNS 11:19. https://doi.org/10.1186/2045-8118-11-19
Jaraj D, Agerskov S, Rabiei K, Marlow T, Jensen C, Guo X, Kern S, Wikkelsø C, Skoog I (2016) Vascular factors in suspected normal pressure hydrocephalus: a population-based study. Neurology 86:592–599. https://doi.org/10.1212/wnl.0000000000002369
Hudson M, Nowak C, Garling RJ, Harris C (2019) Comorbidity of diabetes mellitus in idiopathic normal pressure hydrocephalus: a systematic literature review. Fluids Barriers CNS 16:5. https://doi.org/10.1186/s12987-019-0125-x
Ghaffari-Rafi A, Gorenflo R, Hu H, Viereck J, Liow K (2020) Role of psychiatric, cardiovascular, socioeconomic, and demographic risk factors on idiopathic normal pressure hydrocephalus: a retrospective case-control study. Clin Neurol Neurosurg 193:105836. https://doi.org/10.1016/j.clineuro.2020.105836
Kobayashi E, Kanno S, Kawakami N, Narita W, Saito M, Endo K, Iwasaki M, Kawaguchi T, Yamada S, Ishii K, Kazui H, Miyajima M, Ishikawa M, Mori E, Tominaga T, Tanaka F, Suzuki K (2022) Risk factors for unfavourable outcomes after shunt surgery in patients with idiopathic normal-pressure hydrocephalus. Sci Rep 12:13921. https://doi.org/10.1038/s41598-022-18209-5
Kuriyama N, Miyajima M, Nakajima M, Kurosawa M, Fukushima W, Watanabe Y, Ozaki E, Hirota Y, Tamakoshi A, Mori E, Kato T, Tokuda T, Urae A, Arai H (2017) Nationwide hospital-based survey of idiopathic normal pressure hydrocephalus in Japan: epidemiological and clinical characteristics. Brain Behav 7:e00635. https://doi.org/10.1002/brb3.635
Koto A, Rosenberg G, Zingesser LH, Horoupian D, Katzman R (1977) Syndrome of normal pressure hydrocephalus: possible relation to hypertensive and arteriosclerotic vasculopathy. J Neurol Neurosurg Psychiatry 40:73–79. https://doi.org/10.1136/jnnp.40.1.73
Earnest MP, Fahn S, Karp JH, Rowland LP (1974) Normal pressure hydrocephalus and hypertensive cerebrovascular disease. Arch Neurol 31:262–266. https://doi.org/10.1001/archneur.1974.00490400076009
Wang Z, Zhang Y, Hu F, Ding J, Wang X (2020) Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci Ther 26:1230–1240. https://doi.org/10.1111/cns.13526
Wagshul ME, Eide PK, Madsen JR (2011) The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 8:5. https://doi.org/10.1186/2045-8118-8-5
Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9:4878. https://doi.org/10.1038/s41467-018-07318-3
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024. https://doi.org/10.1016/s1474-4422(18)30318-1
Ringstad G, Vatnehol SAS, Eide PK (2017) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140:2691–2705. https://doi.org/10.1093/brain/awx191
Eide PK, Ringstad G (2019) Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cerebr Blood Flow Metab 39:1355–1368. https://doi.org/10.1177/0271678x18760974
Román GC, Verma AK, Zhang YJ, Fung SH (2018) Idiopathic normal-pressure hydrocephalus and obstructive sleep apnea are frequently associated: a prospective cohort study. J Neurol Sci 395:164–168. https://doi.org/10.1016/j.jns.2018.10.005
Román GC, Jackson RE, Fung SH, Zhang YJ, Verma AK (2019) Sleep-disordered breathing and idiopathic normal-pressure hydrocephalus: recent pathophysiological advances. Curr Neurol Neurosci Rep 19:39. https://doi.org/10.1007/s11910-019-0952-9
Yamada S, Ishikawa M, Nozaki K (2021) Exploring mechanisms of ventricular enlargement in idiopathic normal pressure hydrocephalus: a role of cerebrospinal fluid dynamics and motile cilia. Fluids Barriers CNS 18:20. https://doi.org/10.1186/s12987-021-00243-6
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, Palmer T, Schooling CM, Wallace C, Zhao Q (2022) Mendelian randomization. Nat Rev Methods Primer 2:1–21
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA (2022) FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv
Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, Holmes MV (2020) Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med 17:e1003062. https://doi.org/10.1371/journal.pmed.1003062
Mitchell R, Elsworth B, Mitchell R, Raistrick C, Paternoster L, Hemani G, Gaunt T (2019) MRC IEU UK Biobank GWAS pipeline version 2. data. bris
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X, Choquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga JJ, Huang H, Jang SK, Jansen PR, Ling Y, Mägi R, Matoba N, McMahon G, Mulas A, Orrù V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stančáková A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafò MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51:237–244. https://doi.org/10.1038/s41588-018-0307-5
Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, Alexander GE, Chen Z (2005) Going SB (2018) Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes 42:1161–1176. https://doi.org/10.1038/s41366-018-0120-3
Hartiala JA, Han Y, Jia Q, Hilser JR, Huang P, Gukasyan J, Schwartzman WS, Cai Z, Biswas S, Trégouët DA, Smith NL, Seldin M, Pan C, Mehrabian M, Lusis AJ, Bazeley P, Sun YV, Liu C, Quyyumi AA, Scholz M, Thiery J, Delgado GE, Kleber ME, März W, Howe LJ, Asselbergs FW, van Vugt M, Vlachojannis GJ, Patel RS, Lyytikäinen LP, Kähönen M, Lehtimäki T, Nieminen TVM, Kuukasjärvi P, Laurikka JO, Chang X, Heng CK, Jiang R, Kraus WE, Hauser ER, Ferguson JF, Reilly MP, Ito K, Koyama S, Kamatani Y, Komuro I, Stolze LK, Romanoski CE, Khan MD, Turner AW, Miller CL, Aherrahrou R, Civelek M, Ma L, Björkegren JLM, Kumar SR, Tang WHW, Hazen SL, Allayee H (2021) Genome-wide analysis identifies novel susceptibility loci for myocardial infarction. Eur Heart J 42:919–933. https://doi.org/10.1093/eurheartj/ehaa1040
van der Harst P, Verweij N (2018) Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 122:433–443. https://doi.org/10.1161/circresaha.117.312086
Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, Anderson CD, Chong M, Adams HHH, Ago T, Almgren P, Amouyel P, Ay H, Bartz TM, Benavente OR, Bevan S, Boncoraglio GB, Brown RD Jr, Butterworth AS, Carrera C, Carty CL, Chasman DI, Chen WM, Cole JW, Correa A, Cotlarciuc I, Cruchaga C, Danesh J, de Bakker PIW, DeStefano AL, den Hoed M, Duan Q, Engelter ST, Falcone GJ, Gottesman RF, Grewal RP, Gudnason V, Gustafsson S, Haessler J, Harris TB, Hassan A, Havulinna AS, Heckbert SR, Holliday EG, Howard G, Hsu FC, Hyacinth HI, Ikram MA, Ingelsson E, Irvin MR, Jian X, Jiménez-Conde J, Johnson JA, Jukema JW, Kanai M, Keene KL, Kissela BM, Kleindorfer DO, Kooperberg C, Kubo M, Lange LA, Langefeld CD, Langenberg C, Launer LJ, Lee JM, Lemmens R, Leys D, Lewis CM, Lin WY, Lindgren AG, Lorentzen E, Magnusson PK, Maguire J, Manichaikul A, McArdle PF, Meschia JF, Mitchell BD, Mosley TH, Nalls MA, Ninomiya T, O’Donnell MJ, Psaty BM, Pulit SL, Rannikmäe K, Reiner AP, Rexrode KM, Rice K, Rich SS, Ridker PM, Rost NS, Rothwell PM, Rotter JI, Rundek T, Sacco RL, Sakaue S, Sale MM, Salomaa V, Sapkota BR, Schmidt R, Schmidt CO, Schminke U, Sharma P, Slowik A, Sudlow CLM, Tanislav C, Tatlisumak T, Taylor KD, Thijs VNS, Thorleifsson G, Thorsteinsdottir U, Tiedt S, Trompet S, Tzourio C, van Duijn CM, Walters M, Wareham NJ, Wassertheil-Smoller S, Wilson JG, Wiggins KL, Yang Q, Yusuf S, Bis JC, Pastinen T, Ruusalepp A, Schadt EE, Koplev S, Björkegren JLM, Codoni V, Civelek M, Smith NL, Trégouët DA, Christophersen IE, Roselli C, Lubitz SA, Ellinor PT, Tai ES, Kooner JS, Kato N, He J, van der Harst P, Elliott P, Chambers JC, Takeuchi F, Johnson AD, Sanghera DK, Melander O, Jern C, Strbian D, Fernandez-Cadenas I, Longstreth WT Jr, Rolfs A, Hata J, Woo D, Rosand J, Pare G, Hopewell JC, Saleheen D, Stefansson K, Worrall BB, Kittner SJ, Seshadri S, Fornage M, Markus HS, Howson JMM, Kamatani Y, Debette S, Dichgans M (2018) Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 50:524–537. https://doi.org/10.1038/s41588-018-0058-3
Burgess S, Labrecque JA (2018) Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 33:947–952. https://doi.org/10.1007/s10654-018-0424-6
Elsworth BL, Lyon MS, Alexander T, Liu Y, Hemani G (2020) The MRC IEU OpenGWAS data infrastructure. Cold Spring Harbor Laboratory
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife. https://doi.org/10.7554/eLife.34408
Ringstad G, Emblem KE, Geier O, Alperin N, Eide PK (2015) Aqueductal stroke volume: comparisons with intracranial pressure scores in idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol 36:1623–1630. https://doi.org/10.3174/ajnr.A4340
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33:18190–18199. https://doi.org/10.1523/JNEUROSCI.1592-13.2013
Qvarlander S, Ambarki K, Wåhlin A, Jacobsson J, Birgander R, Malm J, Eklund A (2017) Cerebrospinal fluid and blood flow patterns in idiopathic normal pressure hydrocephalus. Acta Neurol Scand 135:576–584. https://doi.org/10.1111/ane.12636
Greitz D, Hannerz J, Rähn T, Bolander H, Ericsson A (1994) MR imaging of cerebrospinal fluid dynamics in health and disease. On the vascular pathogenesis of communicating hydrocephalus and benign intracranial hypertension. Acta Radiol 35:204–211
Greitz D (2004) Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev 27:145–165. https://doi.org/10.1007/s10143-004-0326-9
Eide PK, Sorteberg W (2010) Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery 66:80–91. https://doi.org/10.1227/01.Neu.0000363408.69856.B8
Oliveira LM, Nitrini R, Román GC (2019) Normal-pressure hydrocephalus: a critical review. Dement Neuropsychol 13:133–143. https://doi.org/10.1590/1980-57642018dn13-020001
Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, Bao W (2018) Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ 362:k1497. https://doi.org/10.1136/bmj.k1497
Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z (2017) Impairment of the glymphatic system after diabetes. J Cerebr Blood Flow Metab 37:1326–1337. https://doi.org/10.1177/0271678x16654702
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. https://doi.org/10.1002/ana.24271
Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, Alper SL, Lundgaard I, Nedergaard M, Kahle KT (2020) Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 26:285–295. https://doi.org/10.1016/j.molmed.2019.11.008
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147. https://doi.org/10.1126/scitranslmed.3003748
Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O’Neill MJ, Wells JA, Lythgoe MF (2020) Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 143:2576–2593. https://doi.org/10.1093/brain/awaa179
Li J, Zhao L, Ding X, Cui X, Qi L, Chen Y (2022) Obstructive sleep apnea and the risk of Alzheimer’s disease and Parkinson disease: a Mendelian randomization study OSA, Alzheimer’s disease and Parkinson disease. Sleep Med 97:55–63. https://doi.org/10.1016/j.sleep.2022.06.004
Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM (2012) Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med 4:150. https://doi.org/10.1126/scitranslmed.3004291
Huang J, Zuber V, Matthews PM, Elliott P, Tzoulaki J, Dehghan A (2020) Sleep, major depressive disorder, and Alzheimer disease: A Mendelian randomization study. Neurology 95:e1963–e1970. https://doi.org/10.1212/wnl.0000000000010463
Henry A, Katsoulis M, Masi S, Fatemifar G, Denaxas S, Acosta D, Garfield V, Dale CE (2019) The relationship between sleep duration, cognition and dementia: a Mendelian randomization study. Int J Epidemiol 48:849–860. https://doi.org/10.1093/ije/dyz071
Ramirez J, Holmes MF, Berezuk C, Kwan D, Tan B, Beaton D, Scott CJM, Ozzoude M, Gao F, Yu D, Swardfager W, Lawrence-Dewar J, Dowlatshahi D, Saposnik G, Boulos MI, Murray BJ, Symons S, Bartha R, Black SE, Swartz RH, Lim A (2021) MRI-visible perivascular space volumes, sleep duration and daytime dysfunction in adults with cerebrovascular disease. Sleep Med 83:83–88. https://doi.org/10.1016/j.sleep.2021.03.043
Siow TY, Toh CH, Hsu JL, Liu GH, Lee SH, Chen NH, Fu CJ, Castillo M, Fang JT (2022) Association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology 98:e829–e838. https://doi.org/10.1212/wnl.0000000000013215
Daou B, Klinge P, Tjoumakaris S, Rosenwasser RH, Jabbour P (2016) Revisiting secondary normal pressure hydrocephalus: does it exist? A review. Neurosurg Focus 41:E6. https://doi.org/10.3171/2016.6.Focus16189
Acknowledgements
We thank the authors and researchers of all GWAS from which we used summary statistics datasets. Special thanks to the researchers and administrators of the IEU GWAS database project for making the GWAS data publicly available and to the participants and researchers of the FinnGen study for providing the GWAS dataset for normal pressure hydrocephalus.
Funding
The work was funded by the National Natural Science Foundation of China, Grant/Award Number: 82201502.
Author information
Authors and Affiliations
Contributions
ZD and HW contributed to the study conception and design. Material preparation and data collection were performed by KH, YC, YL, and YR. The analysis was performed by ZD. The initial manuscript was drafted by ZD and HW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This MR study did not require ethical approval based on the summary data level. All the summary data were obtained from publicly available data sources. No personal information was involved in this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, Z., Wang, H., Huang, K. et al. Association between vascular risk factors and idiopathic normal pressure hydrocephalus: a Mendelian randomization study. J Neurol 270, 2724–2733 (2023). https://doi.org/10.1007/s00415-023-11604-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11604-6